Abstract
Objective:
To determine the prognostic value of serum chromogranin-A (CGA) in a two-cohort study of men with metastatic castrate resistant prostate cancer (mCRPC) and to compare with circulating tumor cells (CTCs)-based prognosis.
Patients and methods:
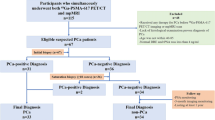
A two-cohort-based evaluation for serum CGA for prognostication in CRPC stage was performed using a screening cohort of 256 men with mCRPC and an independent validation cohort of 92 men with mCRPC. In both cohorts, men receiving proton pump inhibitors and those with non-castrate levels of testosterone (>50 ng/dl) were excluded. Serum CGA was measured in a homogeneous automated immunofluorescent assay using time-resolved amplified cryptate emission. In the validation cohort, CTC enumeration was also performed using the FDA-cleared CELLSEARCH® CTC test. Cox proportional hazard regression models were used for prognostic association of serum CGA and CTC counts with overall survival.
Results:
In the screening cohort, 200 men were eligible for analysis. The median serum CGA was 100.3 ng/mL (interquartile range: 67–161.3) and 34/200 were above the reference range. In the subset of men with Gleason scores ≥ 8, elevated CGA was associated with shorter overall survival [hazard ratio (HR) 2.19, p = 0.017]. In the validation cohort for 71 men eligible for analysis, the median serum CGA was 90 ng/mL (interquartile range: 55–156) and 31/71 patients had an elevated CGA. 51% of patients had a Gleason score ≥ 8 and 66/71 patients had CTCs enumerated with 26/66 with a CTC count ≥ 5 per 7.5 ml blood sample (unfavorable). Both elevated serum CGA (HR: 1.91, p = 0.043) and unfavorable CTC counts (HR: 2.97, p = 0.0012) were adversely associated with overall survival and patients with ≥ 5 CTCs and elevated serum CGA had the shortest overall survival (HR: 3.76, p = 0.008).
Conclusion:
Elevated serum CGA was negatively associated with OS in men with mCRPC. Serum CGA represents a prognostic biomarker that may complement CTC enumeration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wang HT, Yao YH, Li BG, Tang Y, Chang JW, Zhang J. Neuroendocrine Prostate Cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J Clin Oncol. 2014;32:3383–90.
Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28.
Jiborn T, Bjartell A, Abrahamsson P-A. Neuroendocrine differentiation in prostatic carcinoma during hormonal treatment. Urology. 1998;51:585–9.
Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. 2004;45:586–92.
Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1:487–95.
Gkolfinopoulos S, Tsapakidis K, Papadimitriou K, Papamichael D, Kountourakis P. Chromogranin A as a valid marker in oncology: Clinical application or false hopes? World J Methodol. 2017;7:9–15.
Krijnen J, Janssen P, Ruizeveld de Winter J, Krimpen H, Schröder F, Kwast T. Do neuroendocrine cells in human prostate cancer express androgen receptor? Histochem Cell Biol. 1993;100:393–8.
Bonkhoff H, Neuroendocrine differentiation in human prostate cancer. Morphogenesis, proliferation and androgen receptor status. Ann Oncol. 2001;12:S141–4.
Angelsen A, Syversen U, Haugen OA, Stridsberg M, Mjølnerød OK, Waldum HL. Neuroendocrine differentiation in carcinomas of the prostate: do neuroendocrine serum markers reflect immunohistochemical findings? Prostate. 1997;30:1–6.
Cabrespine A, Guy L, Gachon F, Cure H, Chollet P, Bay JO. Circulating chromogranin a and hormone refractory prostate cancer chemotherapy. J Urol. 2006;175:1347–52.
Berruti A, Mosca A, Tucci M, Terrone C, Torta M, Tarabuzzi R, et al. Independent prognostic role of circulating chromogranin A in prostate cancer patients with hormone-refractory disease. Endocr Relat Cancer. 2005;12:109–17.
Heck MM, Thaler MA, Schmid SC, Seitz AK, Tauber R, Kubler H, et al. Chromogranin A and neurone-specific enolase serum levels as predictors of treatment outcome in patients with metastatic castration-resistant prostate cancer undergoing abiraterone therapy. BJU Int. 2017;119:30–7.
Korse CM, Muller M, Taal BG. Discontinuation of proton pump inhibitors during assessment of chromogranin A levels in patients with neuroendocrine tumours. Br J Cancer. 2011;105:1173–5.
De Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9.
Hulsen T, de Vlieg J, Alkema W. BioVenn–a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genom. 2008;9:488.
Tan M, Karaoğlan Ü, Celik B, Ataoğlu Ö, Biri H, Bozkirli I. Prostate cancer and neuroendocrine differentiation. Int Urol Nephrol. 1999;31:75–82.
Bollito E,Berruti A,Bellina M,Mosca A,Leonardo E,Tarabuzzi R, et al. Relationship between neuroendocrine features and prognostic parameters in human prostate adenocarcinoma. Ann Oncol. 2001;12:S159–64.
Beltran H, Jendrisak A, Landers M, Mosquera JM, Kossai M, Louw J et al. The initial detection and partial characterization of circulating tumor cells in neuroendocrine prostate cancer. Clin Cancer Res. 2015;22:1510–9.
Taplin M-E, George DJ, Halabi S, Sanford B, Febbo PG, Hennessy KT, et al. Prognostic significance of plasma chromogranin a levels in patients with hormone-refractory prostate cancer treated in Cancer and Leukemia Group B 9480 study. Urology. 2005;66:386–91.
Conteduca V, Aieta M, Amadori D, De Giorgi U. Neuroendocrine differentiation in prostate cancer: current and emerging therapy strategies. Crit Rev Oncol Hematol. 2014;92:11–24.
Conteduca V, Burgio SL, Menna C, Carretta E, Rossi L, Bianchi E, et al. Chromogranin A is a potential prognostic marker in prostate cancer patients treated with enzalutamide. Prostate. 2014;74:1691–6.
Burgio SL, Conteduca V, Menna C, Carretta E, Rossi L, Bianchi E, et al. Chromogranin A predicts outcome in prostate cancer patients treated with abiraterone. Endocr Relat Cancer. 2014;21:487–93.
Hvamstad T, Jordal A, Hekmat N, Paus E, Fosså SD. Neuroendocrine serum tumour markers in hormone-resistant prostate cancer. Eur Urol. 2003;44:215–21.
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, et al. REporting recommendations for tumour MARKer prognostic studies (REMARK). Eur J Cancer. 2005;41:1690–6.
Acknowledgements
This study is supported in part by R01 CA21209 to MK; Joseph and Gail Gassner support to MK; and for the prospective clinical trial that performed the CTC collections by the Mayo Clinic Center for Individualized Medicine with support for MK, DH, RC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Giridhar, K.V., Sanhueza, C., Hillman, D.W. et al. Serum chromogranin-A-based prognosis in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 21, 431–437 (2018). https://doi.org/10.1038/s41391-018-0046-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-018-0046-9
This article is cited by
-
Association between concomitant proton pump inhibitor use and survival of patients with metastatic prostate cancer receiving abiraterone acetate: a post-hoc analysis of pooled data from three randomized controlled trials
Prostate Cancer and Prostatic Diseases (2023)
-
Chromogranin A: a useful biomarker in castration-resistant prostate cancer
World Journal of Urology (2022)
-
Resistance to the Androgen Receptor Centred Therapies: Biology and Management
SN Comprehensive Clinical Medicine (2021)
-
The deleterious association between proton pump inhibitors and prostate cancer-specific mortality – a population-based cohort study
Prostate Cancer and Prostatic Diseases (2020)