Abstract
Background
Despite advancements in neonatal care, germinal matrix-intraventricular hemorrhage impacts 20% of very preterm infants, exacerbating their neurological prognosis. Understanding its complex, multifactorial pathophysiology and rapid onset remains challenging. This study aims to link specific cord blood biomolecules at birth with post-natal germinal matrix-intraventricular hemorrhage onset.
Methods
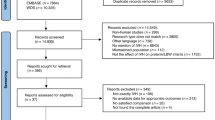
A monocentric, prospective case-control study was conducted at Rouen University Hospital from 2015 to 2020. Premature newborns ( < 30 gestational age) were included and cord blood was sampled in the delivery room. A retrospective matching procedure was held in 2021 to select samples for proteomic and metabolomic analysis of 370 biomolecules.
Results
26 patients with germinal matrix-intraventricular hemorrhage cases and 60 controls were included. Clinical differences were minimal, except for higher invasive ventilation rates in the germinal matrix-intraventricular hemorrhage group. Germinal matrix-intraventricular hemorrhage newborns exhibited lower phosphatidylcholine levels and elevated levels of four proteins: BOC cell adhesion-associated protein, placental growth factor, Leukocyte-associated immunoglobulin-like receptor 2, and tumor necrosis factor-related apoptosis-inducing ligand receptor 2.
Conclusion
This study identifies biomolecules that may be linked to subsequent germinal matrix-intraventricular hemorrhage, suggesting heightened vascular disruption risk as an independent factor. These results need further validation but could serve as early germinal matrix-intraventricular hemorrhage risk biomarkers for future evaluations.
Impact
-
Decrease in certain phosphatidylcholines and increase in four proteins in cord blood at birth may be linked to subsequent germinal matrix-intraventricular hemorrhage in premature newborns.
-
The four proteins are BOC cell adhesion-associated protein, placental growth factor, leukocyte-associated immunoglobulin-like receptor 2, and TNF-related apoptosis-inducing ligand receptor 2.
-
This biological imprint could point toward higher vascular disruption risk as an independent risk factor for this complication and with further validations, could be used for better stratification of premature newborns at birth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data is available and can be found in the supplementary tables.
References
Patel, R. Short- and long-term outcomes for extremely preterm infants. Am. J. Perinatol. 33, 318–328 (2016).
Hollebrandse, N. L. et al. School-age outcomes following intraventricular haemorrhage in infants born extremely preterm. Arch. Dis. Child Fetal Neonatal Ed. 106, 4–8 (2021).
Egesa, W. I. et al. Germinal matrix-intraventricular hemorrhage: a tale of preterm infants. Int. J. Pediatr. 2021, 1–14 (2021).
Raybaud, C., Ahmad, T., Rastegar, N., Shroff, M. & Al Nassar, M. The premature brain: developmental and lesional anatomy. Neuroradiology 55, 23–40 (2013).
Vogel, J. P. et al. Updated who recommendations on antenatal corticosteroids and tocolytic therapy for improving preterm birth outcomes. Lancet Glob. Health 10, e1707–e1708 (2022).
Doyle, L. W., Crowther, C. A., Middleton, P. & Marret, S. Antenatal magnesium sulfate and neurologic outcome in preterm infants: a systematic review. Obstet. Gynecol. 113, 1327–1333 (2009).
Crowther, C. A. et al. Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: an individual participant data meta-analysis. PLOS Med. 14, e1002398 (2017).
Ancel, P.-Y. et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011. JAMA Pediatr. 169, 230 (2015).
Mccormick, M. C., Litt, J. S., Smith, V. C. & Zupancic, J. A. F. Prematurity: an overview and public health implications. Annu. Rev. Public Health 32, 367–379 (2011).
Committee on Understanding Premature Birth and Assuring Healthy Outcomes. Preterm Birth: Causes, Consequences, and Prevention (National Academies Press, 2007).
Ballabh, P. Pathogenesis and prevention of intraventricular hemorrhage. Clin. Perinatol. 41, 47–67 (2014).
Limperopoulos, C. et al. Cerebral hemodynamic changes during intensive care of preterm Infants. Pediatrics 122, e1006–e1013 (2008).
Ballabh, P., Braun, A. & Nedergaard, M. Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr. Res 56, 117–124 (2004).
Ballabh, P. et al. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat. Med 13, 477–485 (2007).
Papile, L. A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 Gm. J. Pediatr. 92, 529–534 (1978).
Gilard, V. et al. Post Hemorrhagic hydrocephalus and neurodevelopmental outcomes in a context of neonatal intraventricular hemorrhage: an institutional experience in 122 preterm children. BMC Pediatr. 18, 288 (2018).
McCrea, H. J. & Ment, L. R. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin. Perinatol. 35, 777–792 (2008). vii.
Leijser, L. M. & de Vries, L. S. Preterm brain injury: germinal matrix-intraventricular hemorrhage and post-hemorrhagic ventricular dilatation. Handb. Clin. Neurol. 162, 173–199 (2019).
Gilard, V., Tebani, A., Bekri, S. & Marret, S. Intraventricular hemorrhage in very preterm infants: a comprehensive review. J. Clin. Med. 9, 2447 (2020).
de Graaf, M. A., Jager, K. J., Zoccali, C. & Dekker, F. W. Matching, an appealing method to avoid confounding? Nephron Clin. Pr. 118, c315–318 (2011).
R Core Team. R.: A language and environment for statistical computing. R Foundation for Statistical Computing (R Core Team, 2020).
Makowski, D. L. {Performance}: an {R} package for assessment, comparison and testing of Statistical Models. J. Open Source Softw. 6, 3139 (2021).
Hastie, T., Tibshirani, R., Narasimhan, B. & Chu, G. Impute: Imputation for Microarray Data. https://git.bioconductor.org/packages/impute (2022).
Stacklies, W., Redestig, H., Scholz, M., Walther, D. & Selbig, J. Pcamethods-a bioconductor package providing PCA methods for incomplete data. Bioinformatics 23, 1164–1167 (2007).
Wright, M. N. & Ziegler, A. Ranger: a fast implementation of random forests for high dimensional data in C++ and R. J. Stat. Softw. 77, 1–17 (2017).
Nembrini, S., König, I. R. & Wright, M. N. The revival of the gini importance? Bioinformatics 34, 3711–3718 (2018).
Janitza, S., Celik, E. & Boulesteix, A.-L. A computationally fast variable importance test for random forests for high-dimensional. Adv. Data Anal. Classification 12, 885–915 (2018).
Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research https://CRAN.R-project.org/package=psych (2023).
Benjamini, Y. & Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 29, 1165–1188 (2001).
WHO. Who Recommendations on Antenatal Corticosteroids for Improving Preterm Birth Outcomes. (WHO, 2022).
Jayaram, P. M., Mohan, M. K., Farid, I. & Lindow, S. Antenatal magnesium sulfate for fetal neuroprotection: a critical appraisal and systematic review of clinical practice guidelines. J. Perinat. Med. 47, 262–269 (2019).
Helwich, E., Rutkowska, M., Bokiniec, R., Gulczynska, E. & Hozejowski, R. Intraventricular hemorrhage in premature infants with respiratory distress syndrome treated with surfactant: incidence and risk factors in the Prospective Cohort Study. Dev. Period Med 21, 328–335 (2017).
Mian, Q. et al. Impact of delivered tidal volume on the occurrence of intraventricular haemorrhage in preterm infants during positive pressure ventilation in the delivery room. Arch. Dis. Child Fetal Neonatal Ed. 104, F57–F62 (2019).
Aly, H., Hammad, T. A., Essers, J. & Wung, J. T. Is mechanical ventilation associated with intraventricular hemorrhage in preterm infants? Brain Dev. 34, 201–205 (2012).
Furse, S. & De Kroon, A. I. P. M. Phosphatidylcholine’s functions beyond that of a membrane brick. Mol. Membr. Biol. 32, 117–119 (2015).
Cui, Z. & Houweling, M. Phosphatidylcholine and cell death. Biochim. Biophys. Acta 1585, 87–96 (2002).
Wright, M. M., Howe, A. G. & Zaremberg, V. Cell membranes and apoptosis: role of cardiolipin, phosphatidylcholine, and anticancer lipid analogues. Biochem. Cell Biol. 82, 18–26 (2004).
Morita, S. Y. & Ikeda, Y. Regulation of membrane phospholipid biosynthesis in mammalian cells. Biochem. Pharm. 206, 115296 (2022).
Cole, L. K., Vance, J. E. & Vance, D. E. Phosphatidylcholine biosynthesis and lipoprotein metabolism. Biochim. Biophys. Acta 1821, 754–761 (2012).
Neale Ridgway, Roger McLeod. Biochemistry of Lipids, Lipoproteins and Membranes. 6th edn. (Elsevier, Amsterdam, 2016).
Herrera, E. & Desoye, G. Maternal and fetal lipid metabolism under normal and gestational diabetic conditions. Horm. Mol. Biol. Clin. Investig. 26, 109–127 (2016).
Chao De La Barca, J. M. et al. A metabolomic profiling of intra-uterine growth restriction in placenta and cord blood points to an impairment of lipid and energetic metabolism. Biomedicines 10, 1411 (2022).
Cao, Y., Linden, P., Shima, D., Browne, F. & Folkman, J. In vivo angiogenic activity and hypoxia induction of heterodimers of placenta growth factor/vascular endothelial growth factor. J. Clin. Investig. 98, 2507–2511 (1996).
d’Audigier, C. et al. Targeting Vegfr1 on endothelial progenitors modulates their differentiation potential. Angiogenesis 17, 603–616 (2014).
Uemura, A. et al. Vegfr1 signaling in retinal angiogenesis and microinflammation. Prog. Retin. Eye Res. 84, 100954 (2021).
Apte, R. S., Chen, D. S. & Ferrara, N. Vegf in signaling and disease: beyond discovery and development. Cell 176, 1248–1264 (2019).
Freitas-Andrade, M., Carmeliet, P., Charlebois, C., Stanimirovic, D. B. & Moreno, M. J. Plgf knockout delays brain vessel growth and maturation upon systemic hypoxic challenge. J. Cereb. Blood Flow. Metab. 32, 663–675 (2012).
Yano, K. et al. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J. Exp. Med. 203, 1447–1458 (2006).
Yang, D. et al. Overexpression of vascular endothelial growth factor in the germinal matrix induces neurovascular proteases and intraventricular hemorrhage. Sci. Transl. Med 5, 193ra190 (2013).
Lecuyer, M. et al. Plgf, a placental marker of fetal brain defects after in utero alcohol exposure. Acta Neuropathol. Commun. 5, 44 (2017).
Okada, A. et al. Boc is a receptor for sonic hedgehog in the guidance of commissural axons. Nature 444, 369–373 (2006).
Sanchez-Arrones, L., Cardozo, M., Nieto-Lopez, F. & Bovolenta, P. Cdon and Boc: two transmembrane proteins implicated in cell-cell communication. Int J. Biochem Cell Biol. 44, 698–702 (2012).
Siegenthaler, J. A., Sohet, F. & Daneman, R. ‘Sealing Off the Cns’: cellular and molecular regulation of blood–brain barriergenesis. Curr. Opin. Neurobiol. 23, 1057–1064 (2013).
Chapouly, C., Guimbal, S., Hollier, P.-L. & Renault, M.-A. Role of hedgehog signaling in vasculature development, differentiation, and maintenance. Int. J. Mol. Sci. 20, 3076 (2019).
Cheng, B. et al. Shh activation restores interneurons and cognitive function in newborns with intraventricular haemorrhage. Brain 146, 629–644 (2023).
Lebbink, R. J. et al. The soluble leukocyte-associated Ig-like receptor (Lair)-2 antagonizes the collagen/lair-1 inhibitory immune interaction. J. Immunol. 180, 1662–1669 (2008).
Lenting, P. J., Westerlaken, G. H. A., Denis, C. V., Akkerman, J. W. & Meyaard, L. Efficient inhibition of collagen-induced platelet activation and adhesion by Lair-2, a soluble Ig-like receptor family member. PLoS ONE 5, e12174 (2010).
Olde Nordkamp, M. J. M. et al. Inhibition of the classical and lectin pathway of the complement system by recombinant lair-2. J. Innate Immun. 6, 284–292 (2014).
Lemke, J., Von Karstedt, S., Zinngrebe, J. & Walczak, H. Getting trail back on track for cancer therapy. Cell Death Differ. 21, 1350–1364 (2014).
Wilson, S. et al. Proapoptotic activation of death receptor 5 on tumor endothelial cells disrupts the vasculature and reduces tumor growth. Cancer Cell 22, 80–90 (2012).
Léger, C. et al. Glutamate controls vessel-associated migration of GABA interneurons from the pial migratory route via NMDA receptors and endothelial protease activation. Cell. Mol. Life Sci. 77, 1959–1986 (2020).
Acknowledgements
We thank Nathalie Mestre for data capture and monitoring. We thank the medical team and the midwives for their implication to this study. We thank the parents for consenting to have their child participate in this study. The promoter of the study is the Delegation for Clinical Research and Innovation of the Rouen University Hospital. This research received the favorable opinion of the ethical comity “Comité de Protection des Personnes”—CPP and the authorization of the “Agence Nationale de Sécurité du Medicament”—ANSM on 11/07/2014. This research is registered on the clinicaltrials.gov website with the identifier NCT02400853. The collection of biological samples carried out within the framework of this research was declared to the ANSM at the same time as the request for authorization of the research and will be declared at the end of the study to the Minister in charge of research and the Director of the Regional Health Agency.
Funding
This study received intramural financial support from the Rouen University Hospital.
Author information
Authors and Affiliations
Contributions
S.M., S.B., A.T. conceptualized and designed the research, and collected and analyzed data. S.M., A.T., S.B., and F.D. drafted the initial manuscript. F.D., C.P., T.P., S.S., L.A.D., C.L.C. acquired the data. F.D., A.T. performed the statistical analysis. All authors reviewed, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written parental consent was needed and obtained as soon as possible after birth following a clear explanation of the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ducatez, F., Tebani, A., Abily-Donval, L. et al. New insights and potential biomarkers for intraventricular hemorrhage in extremely premature infant, case-control study. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03111-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03111-9
This article is cited by
-
Could we detect intraventricular hemorrhage before it happens?
Pediatric Research (2024)