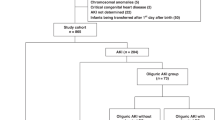
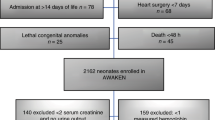
Abstract
Background
We evaluated time-varying perinatal risk factors associated with early (≤7 post-natal days) and late (>7 post-natal days) severe acute kidney injury (AKI) occurrence and duration.
Methods
A secondary analysis of Preterm Erythropoietin Neuroprotection Trial data. We defined severe AKI (stage 2 or 3) per neonatal modified Kidney Disease: Improving Global Outcomes criteria.
Adjusted Cox proportional hazards models were conducted with exposures occurring at least 72 h before severe AKI. Adjusted negative binomial regression models were completed to evaluate risk factors for severe AKI duration.
Results
Of 923 participants, 2% had early severe AKI. In the adjusted model, gestational diabetes (adjusted HR (aHR) 5.4, 95% CI 1.1–25.8), non-steroidal anti-inflammatory drugs (NSAIDs) (aHR 3.2, 95% CI 1.0–9.8), and vancomycin (aHR 13.9, 95% CI 2.3–45.1) were associated with early severe AKI.
Late severe AKI occurred in 22% of participants. Early severe AKI (aHR 2.5, 95% CI 1.1–5.4), sepsis (aHR 2.5, 95% CI 1.4–4.4), vasopressors (aHR 2.9, 95% CI 1.8–4.6), and diuretics (aHR 2.6, 95% CI 1.9–3.6) were associated with late severe AKI.
Participants who had necrotizing enterocolitis or received NSAIDs had longer severe AKI duration.
Conclusion
We identified major risk factors for severe AKI that can be the focus of future research.
Impact statement
-
Time-dependent risk factors for severe acute kidney injury (AKI) and its duration are not well defined among infants born <28 weeks’ gestation.
-
Over 1 in 5 infants born <28 weeks’ gestation experienced severe AKI, and this study identified several major time-dependent perinatal risk factors occurring within 72 h prior to severe AKI.
-
This study can support efforts to develop risk stratification and clinical decision support to help mitigate modifiable risk factors to reduce severe AKI occurrence and duration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data for this secondary analysis was analyzed from the publicly available dataset from the Preterm Erythropoietin Neuroprotection Trial supported per policy by the NIH National Institute of Neurological Disorders and Stroke and National Institute of Diabetes and Digestive and Kidney Diseases. Data can be requested via the NINDS site at: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.ninds.nih.gov/sites/default/files/migrate-documents/sig_form_revised_508c.pdf.
References
Askenazi, D. J. et al. Prevalence of acute kidney injury (AKI) in extremely low gestational age neonates (ELGAN). Pediatr. Nephrol. (Berl., Ger.). 35, 1737–1748 (2020).
Aziz, K. B., Schles, E. M., Makker, K. & Wynn, J. L. Frequency of acute kidney injury and association with mortality among extremely preterm infants. JAMA Netw. Open. 5, e2246327 (2022). National Institutes of Health during the study but outside the submitted work. No other disclosures were reported.
Charlton, J. R. et al. Incidence and risk factors of early onset neonatal AKI. Clin. J. Am. Soc. Nephrol. : Cjasn. 14, 184–195 (2019).
Charlton, J. R. et al. Late onset neonatal acute kidney injury: results from the AWAKEN Study. Pediatr. Res. 85, 339–348 (2019).
Hingorani, S. et al. Prevalence and risk factors for kidney disease and elevated BP in 2-year-old children born extremely premature. Clin. J. Am. Soc. Nephrology. 17, 1129–1138 (2022).
Selewski, D. T. et al. Neonatal acute kidney injury. Pediatrics 136, e463–e473 (2015).
Bruel, A. et al. Renal outcome in children born preterm with neonatal acute renal failure: IRENEO-a prospective controlled study. Pediatr. Nephrol. (Berl., Ger.). 31, 2365–2373 (2016).
Abitbol, C. L. et al. Long-term follow-up of extremely low birth weight infants with neonatal renal failure. Pediatr. Nephrol. (Berl., Ger.). 18, 887–893 (2003).
Harer, M. W., Pope, C. F., Conaway, M. R. & Charlton, J. R. Follow-up of acute kidney injury in neonates during childhood years (FANCY): a prospective cohort study. Pediatr. Nephrol. (Berl., Ger.). 32, 1067–1076 (2017).
Daga, A., Dapaah-Siakwan, F., Rajbhandari, S., Arevalo, C. & Salvador, A. Diagnosis and risk factors of acute kidney injury in very low birth weight infants. Pediatrics Neonatol. 58, 258–263 (2017).
Sethi, S. K. et al. Risk factors and outcomes of neonates with acute kidney injury needing peritoneal dialysis: Results from the prospective TINKER (The Indian PCRRT-ICONIC Neonatal Kidney Educational Registry) study. Perit. Dialysis Int. 42, 460–469 (2022).
Uemura, O. et al. Perinatal factors contributing to chronic kidney disease in a cohort of Japanese children with very low birth weight. Pediatr. Nephrol. (Berl., Ger.). 36, 953–960 (2021).
Hidalgo, G. et al. Association of income level with kidney disease severity and progression among children and adolescents with CKD: a report from the Chronic Kidney Disease in Children (CKiD) Study. Am. J. Kidney Dis. 62, 1087–1094 (2013).
Rhone, E. T., Carmody, J. B., Swanson, J. R. & Charlton, J. R. Nephrotoxic medication exposure in very low birth weight infants. J. Matern.-fetal Neonatal Med. 27, 1485–1490 (2014).
Khor, C.-S. & Wang, W.-J. The role of acute kidney injury duration in clinical practice. Annals Transl. Med. 7, S88 1–3 (2019).
Han, S. S. et al. Duration of acute kidney injury and mortality in critically ill patients: a retrospective observational study. BMC Nephrol. 14, 1–7 (2013).
Brown, J. R., Kramer, R. S., Coca, S. G. & Parikh, C. R. Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann. Thorac. Surg. 90, 1142–1148 (2010).
Moffett, B. S. & Arikan, A. A. Trajectory of AKI in hospitalized pediatric patients-impact of duration and repeat events. Nephrol. Dialysis Transpl. 37, 1443–1450 (2022). Epub 2021/07/11. PubMed PMID: 34245299.
Juul, S. E. et al. A randomized trial of erythropoietin for neuroprotection in preterm infants. N. Engl. J. Med. 382, 233–243 (2020).
Juul, S. E., Mayock, D. E., Comstock, B. A. & Heagerty, P. J. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Matern. Health Neonatol. Perinatol. 1, 1–9 (2015).
Lazarus, B., Davies, M. R. P., Trubiano, J. A. & Pellicano, R. Time to acute kidney injury in β-lactam-induced acute interstitial nephritis. Kidney Int. Rep. 5, 1068–1070 (2020).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc. Health 1, 184–194 (2017).
Kaddourah, A., Basu, R. K., Bagshaw, S. M. & Goldstein, S. L. Epidemiology of acute kidney injury in critically Ill children and young adults. N. Engl. J. Med. 376, 11–20 (2017).
Salerno, S. N. et al. Association between nephrotoxic drug combinations and acute kidney injury in the neonatal intensive care unit. J. Pediatr. 228, 213–219 (2021).
Mohamed, T. H., Klamer B., Mahan J. D., Spencer J. D., & Slaughter, J. L. Diuretic therapy and acute kidney injury in preterm neonates and infants. Pediatric nephrology (Berlin, Germany). 2021. https://doi.org/10.1007/s00467-021-05132-6.
Cerqueira, D. M. et al. In utero exposure to maternal diabetes impairs nephron progenitor differentiation. Am. J. Physiol. Ren. Physiol. 317, F1318–f30 (2019).
Aliou, Y. et al. Post-weaning high-fat diet accelerates kidney injury, but not hypertension programmed by maternal diabetes. Pediatr. Res. 79, 416–424 (2016).
Callaway, D. A. et al. Prematurity disrupts glomeruli development, whereas prematurity and hyperglycemia lead to altered nephron maturation and increased oxidative stress in newborn baboons. Pediatr. Res. 83, 702–711 (2018).
Dyck, R. F. et al. Congenital anomalies of the kidney and urinary tract (CAKUT): An emerging relationship with pregestational diabetes mellitus among first nations and non-first nations people in Saskatchewan-results from the DIP: ORRIIGENSS project. Can. J. Diabetes 45, 346–54.e1 (2021).
Venkatesh, K. K. et al. Risk of adverse pregnancy outcomes among pregnant individuals with gestational diabetes by race and ethnicity in the United States, 2014–2020. JAMA 327, 1356–1367 (2022) The National Institutes of Health outside the submitted work. No other disclosures were reported.
Smythe, C. M., Nickel, J. F. & Bradley, S. E. The effect of epinephrine (USP), l-epinephrine, and l-norepinephrine on glomerular filtration rate, renal plasma flow, and the urinary excretion of sodium, potassium, and water in normal man. J. Clin. Invest. 31, 499–506 (1952).
Hoogenberg, K., Smit, A. J. & Girbes, A. R. J. Effects of low-dose dopamine on renal and systemic hemodynamics during incremental norepinephrine infusion in healthy volunteers. Crit. Care Med. 26, 260–265 (1998).
Blatt, N. B., Srinivasan, S., Mottes, T., Shanley, M. M. & Shanley, T. P. Biology of sepsis: Its relevance to pediatric nephrology. Pediatr. Nephrol. (Berl., Ger.). 29, 2273–2287 (2014).
Sakr, Y., Dubois, M. J., De Backer, D., Creteur, J. & Vincent, J. L. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit. Care Med. 32, 1825–1831 (2004).
Venkatachalam, M. A. & Weinberg, J. M. The tubule pathology of septic acute kidney injury: A neglected area of research comes of age. Kidney Int. 81, 338–340 (2012).
Adegboyega, O. O., Singh, Y., Bhutada, A., Kupferman, J. C. & Rastogi, S. Recurrent acute kidney injury in preterm neonates is common and associated with worse outcomes and higher mortality. Pediatr. Res. 92, 284–290 (2022).
Daraskevicius, J. et al. Phenotypes and baseline risk factors of acute kidney injury in children after allogeneic hematopoietic stem cell transplantation. Front Pediatr. 8, 499 (2020).
Siew, E. D. et al. Predictors of recurrent AKI. J. Am. Soc. Nephrology 27, 1190–1200 (2016).
DeFreitas M. J. et al. Maternal hypertension disorders and neonatal acute kidney injury: Results from the AWAKEN Study. Am. J. Perinatol. 2022. https://doi.org/10.1055/a-1780-2249.
Garg P. M. et al. Severe acute kidney injury in neonates with necrotizing enterocolitis: Risk factors and outcomes. Pediatric Res. 2021. https://doi.org/10.1038/s41390-020-01320-6.
Criss, C. N. et al. Acute kidney injury in necrotizing enterocolitis predicts mortality. Pediatr. Nephrol. (Berl., Ger.). 33, 503–510 (2018).
Garg P. M. et al. Gestational age-specific clinical correlates of acute kidney injury in preterm infants with necrotizing enterocolitis. Pediatric Res. https://doi.org/10.1038/s41390-023-02736-6. (2023).
Westreich, D. & Greenland, S. The Table 2 fallacy: Presenting and interpreting confounder and modifier coefficients. Am. J. Epidemiol. 177, 292–298 (2013).
Iacobelli, S. & Guignard, J. P. Maturation of glomerular filtration rate in neonates and infants: an overview. Pediatr. Nephrol. (Berl., Ger.). 36, 1439–1446 (2021).
Acknowledgements
The following individuals served as collaborators and investigators for the ALMOND studies. They collaborated in protocol development and review, data analysis, and participated in drafting or review of the manuscript, and their names should be citable by PubMed.
Funding
The primary author’s research efforts in this publication are supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under award numbers K23DK131289 and L40DK130155. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This study is part of the Assessing Longitudinal Micropremie Outcomes in Infants at risk for renal Disease (ALMOND) study coordinated by the NKC. Statistical support for this study is provided by Nuwellis. The original study, the Preterm Erythropoietin Neuroprotection Trial, was funded by grants U01 NS077953 and U01 NS077955 from the National Institute of Neurological Disorders and Stroke. Kidney-specific data collection was funded by grant R01 DK103608 from the NIDDK as part of the Recombinant Erythropoietin for Protection of Infant Renal Disease (REPaIReD) Study on behalf of co-author DJA. JRC receives funding from the NIH/NIDDK: R56DK110622, 1R41DK129138, 2P50DK096373, and 1R21DK134104. AMS reports funding from NIH National Heart, Lung, and Blood Institute K23HL148394, L40HL148910-2, R01HL146818, and R01HL164434. MJD reports funding from NIH 5KL2TR002737-04 and the Micah Batchelor Foundation.
Author information
Authors and Affiliations
Consortia
Contributions
KRS and DJA made substantial contributions to the conceptualization of study, research methodology, formal analysis, writing of the initial manuscript, reviewing or editing of the manuscript, and gave final approval for this version to be published. DJA made substantial contributions to the conceptualization of study, research methodology, formal analysis, reviewing or editing of the manuscript, and gave final approval for this version to be published. RG and NA made substantial contributions to the conceptualization of study, formal analysis, reviewing or editing of the manuscript, and gave final approval for this version to be published. AMS, JRS, MZ, HJS, MJD, and JRC made substantial contributions to the conceptualization of study, reviewing, or editing of the manuscript, and gave final approval for this version to be published.
Corresponding author
Ethics declarations
Competing interests
For full disclosure, we provide here an additional list of other author’s commitments and funding sources that are not directly related to this study: DJA is a consultant for Baxter, Nuwellis, Seastar, and Bioporto. Over the last 24 months, his institution has received funding for education and research that is not related to this project from NIH, Baxter, Nuwellis, Medtronic, Bioporto, Portero, Lediant and Seastar. He has financial interests in patent/innovations in the area of kidney support therapies and urine collection devices. He is the Founder and Chief Scientific Officer for Zorro-Flow Inc. JRC is a consultant for Medtronics, investor in Zorro-Flow, and co-owner of Sindri Technologies, LLC. She serves on the Board of the Neonatal Kidney Collaborative (NKC).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sanderson, K., Griffin, R., Anderson, N. et al. Perinatal risk factors associated with acute kidney injury severity and duration among infants born extremely preterm. Pediatr Res (2024). https://doi.org/10.1038/s41390-024-03102-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-024-03102-w