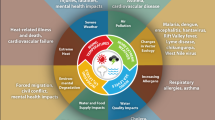
Abstract
The adverse effects of climate change on human health are unfolding in real time. Environmental fragmentation is amplifying spillover of viruses from wildlife to humans. Increasing temperatures are expanding mosquito and tick habitats, introducing vector-borne viruses into immunologically susceptible populations. More frequent flooding is spreading water-borne viral pathogens, while prolonged droughts reduce regional capacity to prevent and respond to disease outbreaks with adequate water, sanitation, and hygiene resources. Worsening air quality and altered transmission seasons due to an increasingly volatile climate may exacerbate the impacts of respiratory viruses. Furthermore, both extreme weather events and long-term climate variation are causing the destruction of health systems and large-scale migrations, reshaping health care delivery in the face of an evolving global burden of viral disease. Because of their immunological immaturity, differences in physiology (e.g., size), dependence on caregivers, and behavioral traits, children are particularly vulnerable to climate change. This investigation into the unique pediatric viral threats posed by an increasingly inhospitable world elucidates potential avenues of targeted programming and uncovers future research questions to effect equitable, actionable change.
Impact
-
A review of the effects of climate change on viral threats to pediatric health, including zoonotic, vector-borne, water-borne, and respiratory viruses, as well as distal threats related to climate-induced migration and health systems.
-
A unique focus on viruses offers a more in-depth look at the effect of climate change on vector competence, viral particle survival, co-morbidities, and host behavior.
-
An examination of children as a particularly vulnerable population provokes programming tailored to their unique set of vulnerabilities and encourages reflection on equitable climate adaptation frameworks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
NASA Goddard Institute for Space Studies. GISS Surface Temperature Analysis (GISTEMP). National Aeronautics and Space Administration https://data.giss.nasa.gov/gistemp/ (2023).
Bernstein, A. S. Climate Change and Infectious Disease. in Harrison’s Principles of Internal Medicine (eds. Loscalzo, J. et al.) (McGraw-Hill Education, 2022).
Mora, C. et al. Over half of known human pathogenic diseases can be aggravated by climate change. Nat. Clim. Change 12, 869–875 (2022).
Hayhoe, K. Greater Emissions Lead to Significantly More Warming. (2017).
Martens, W. J., Niessen, L. W., Rotmans, J., Jetten, T. H. & McMichael, A. J. Potential impact of global climate change on malaria risk. Environ. Health Perspect. 103, 458–464 (1995).
Gona, P. N. & More, A. F. Bacterial pathogens and climate change. Lancet 400, 2161–2163 (2022).
Global Climate Change and Human Health: From Science to Practice, 2nd Edition. (Jossey-Bass, 2021).
IPCC. Climate change 2022: impacts, adaptation and vulnerability. https://www.ipcc.ch/report/ar6/wg2/ (2022).
UNICEF. The Climate Crisis is a Child Rights Crisis: Introducing the Children’s Climate Risk Index. https://www.unicef.org/media/105376/file/UNICEF-climate-crisis-child-rights-crisis.pdf (2021).
Ahdoot, S. et al. Global climate change and children’s health. Pediatrics 136, e1468–e1484 (2015).
Di Cicco, M. E. et al. Climate change and childhood respiratory health: a call to action for paediatricians. Int. J. Environ. Res. Public. Health 17, 5344 (2020).
Perin, J. et al. Global, regional, and national causes of under-5 mortality in 2000–19: an updated systematic analysis with implications for the sustainable development goals. Lancet Child Adolesc. Health 6, 106–115 (2022).
Moya, J., Bearer, C. F. & Etzel, R. A. Children’s behavior and physiology and how it affects exposure to environmental contaminants. Pediatrics 113, 996–1006 (2004).
Kim, J. Ambient air pollution: health hazards to children. Pediatrics 114, 1699–1707 (2004).
DeVincenzo, J. P. Factors predicting childhood respiratory syncytial virus severity: what they indicate about pathogenesis. Pediatr. Infect. Dis. J. 24, S177 (2005).
Javouhey, E., Pouyau, R. & Massenavette, B. Pathophysiology of acute respiratory failure in children with bronchiolitis and effect of CPAP. Noninvasive Vent. High-Risk Infect. Mass Casualty Events 233–249, https://doi.org/10.1007/978-3-7091-1496-4_27 (2013).
Hartman, R. M. et al. Risk factors for mortality among children younger than age 5 years with severe diarrhea in low- and middle-income countries: findings from the World Health Organization-coordinated Global Rotavirus and Pediatric Diarrhea Surveillance Networks. Clin. Infect. Dis. 76, e1047–e1053 (2023).
Landigran, P. & Garg, A. Children are not little adults. In Children’s health and the environment (World Health Organization, 2004).
Bado, A. R., Susuman, A. S. & Nebie, E. I. Trends and risk factors for childhood diarrhea in sub-Saharan countries (1990–2013): assessing the neighborhood inequalities. Glob. Health Action 9, https://doi.org/10.3402/gha.v9.30166 (2016).
Acácio, S. et al. Risk factors for death among children 0–59 months of age with moderate-to-severe diarrhea in Manhiça district, southern Mozambique. BMC Infect. Dis. 19, 322 (2019).
Bennett, C. M. & Friel, S. Impacts of climate change on inequities in child health. Children 1, 461–473 (2014).
Gao, J., Sun, Y., Lu, Y. & Li, L. Impact of ambient humidity on child health: a systematic review. PLOS ONE 9, e112508 (2014).
Xu, Z. et al. The impact of heat waves on children’s health: a systematic review. Int. J. Biometeorol. 58, 239–247 (2014).
Lindsey, B., Kampmann, B. & Jones, C. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr. Opin. Infect. Dis. 26, 248–253 (2013).
Silasi, M. et al. Viral infections during pregnancy. Am. J. Reprod. Immunol. 73, 199–213 (2015).
Carsetti, R. et al. The immune system of children: the key to understanding SARS-CoV-2 susceptibility? Lancet Child Adolesc. Health 4, 414–416 (2020).
Hanson, L. A. & Söderström, T. Human milk: Defense against infection. Prog. Clin. Biol. Res. 61, 147–159 (1981).
Hanson, L. A. Breastfeeding provides passive and likely long-lasting active immunity. Ann. Allergy Asthma Immunol. 81, 523–534 (1998).
Sato, H., Albrecht, P., Reynolds, D. W., Stagno, S. & Ennis, F. A. Transfer of measles, mumps, and rubella antibodies from mother to infant. Its effect on measles, mumps, and rubella immunization. Am. J. Dis. Child. 1960 133, 1240–1243 (1979).
Rijkers, G. T., Sanders, E. A. M., Breukels, M. A. & Zegers, B. J. M. Responsiveness of infants to capsular polysaccharides: implications for vaccine development. Res. Med. Microbiol. 7, 3 (1996).
Simon, A. K., Hollander, G. A. & McMichael, A. Evolution of the immune system in humans from infancy to old age. Proc. R. Soc. B Biol. Sci. 282, 20143085 (2015).
Bridy-Pappas, A. E., Margolis, M. B., Center, K. J. & Isaacman, D. J. Streptococcus pneumoniae: Description of the pathogen, disease epidemiology, treatment, and prevention. Pharmacother. J. Hum. Pharmacol. Drug Ther. 25, 1193–1212 (2005).
Daniels, C. C., Rogers, P. D. & Shelton, C. M. A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J. Pediatr. Pharmacol. Ther. JPPT 21, 27–35 (2016).
Albrecht, M. & Arck, P. C. Vertically transferred immunity in neonates: mothers, mechanisms and mediators. Front. Immunol. 11, 555 (2020).
Heymann, A., Chodick, G., Reichman, B., Kokia, E. & Laufer, J. Influence of school closure on the incidence of viral respiratory diseases among children and on health care utilization. Pediatr. Infect. Dis. J. 23, 675 (2004).
Sheffield, P. E. & Landrigan, P. J. Global climate change and children’s health: threats and strategies for prevention. Environ. Health Perspect. 119, 291–298 (2011).
Xu, Z. et al. Climate change and children’s health—a call for research on what works to protect children. Int. J. Environ. Res. Public. Health 9, 3298–3316 (2012).
Oner, A. F. et al. H5N1 Avian Influenza in children. Clin. Infect. Dis. 55, 26–32 (2012).
WFP. A generation at risk: nearly half of global food crisis hungry are children, say WFP, African Union Development Agency NEPAD, The Education Commission and education partners | World Food Programme. https://www.wfp.org/news/generation-risk-nearly-half-global-food-crisis-hungry-are-children-say-wfp-african-union (2022).
Black, R. E. et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382, 427–451 (2013).
Victora, C. G. et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371, 340–357 (2008).
Dipasquale, V., Cucinotta, U. & Romano, C. Acute malnutrition in children: pathophysiology, clinical effects and treatment. Nutrients 12, 2413 (2020).
UNICEF, The World Bank & WHO. Levels and trends in child malnutrition. (2021).
Guerrant, R. L., Schorling, J. B., McAuliffe, J. F. & Souza, M. A. D. Diarrhea as a cause and an effect of malnutrition: diarrhea prevents catch-up growth and malnutrition increases diarrhea frequency and duration. Am. J. Trop. Med. Hyg. 47, 28–35 (1992).
Brundtland, G. H. Nutrition and infection: Malnutrition and mortality in public health. Nutr. Rev. 58, S1–S4 (2000). ; discussion S63-73.
Bhaskaram, P. Micronutrient malnutrition, infection, and immunity: an overview. Nutr. Rev. 60, S40–S45 (2002).
Molla, A., Molla, A. M., Sarker, S. A., Khatoon, M. & Mujibur Rahaman, M. Effects of acute diarrhea on absorption of macronutrients during disease and after recorvery. In Diarrhea and Malnutrition 143–155 (Plenum Publishing Corporation, 1983).
Martorell, R. & Yarbrough, C. The energy cost of diarrheal diseases and other common illnesses in children. in Diarrhea and Malnutrition 125–143 (Plenum Publishing Corporation, 1983).
United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2010 Revision. https://www.un.org/en/development/desa/publications/world-population-prospects-the-2010-revision.html (2011).
Carlson, C. J. et al. Climate change increases cross-species viral transmission risk. Nature 607, 555–562 (2022).
Curseu, D., Popa, M., Sirbu, D. & Stoian, I. Potential Impact of Climate Change on Pandemic Influenza Risk. Glob. Warm. 643–657, https://doi.org/10.1007/978-1-4419-1017-2_45 (2009).
Morin, C. W. et al. Avian influenza virus ecology and evolution through a climatic lens. Environ. Int. 119, 241–249 (2018).
Escudero-Pérez, B., Lalande, A., Mathieu, C. & Lawrence, P. Host–pathogen interactions influencing zoonotic spillover potential and transmission in humans. Viruses 15, 599 (2023).
Gils & van, J. A. et al. Hampered foraging and migratory performance in swans infected with low-pathogenic avian influenza a virus. PLOS ONE 2, e184 (2007).
Olival, K. J. et al. Host and viral traits predict zoonotic spillover from mammals. Nature 546, 646–650 (2017).
Leroy, E. M. et al. Fruit bats as reservoirs of Ebola virus. Nature 438, 575–576 (2005).
Leendertz, S. A. J., Gogarten, J. F., Düx, A., Calvignac-Spencer, S. & Leendertz, F. H. Assessing the evidence supporting fruit bats as the primary reservoirs for Ebola viruses. EcoHealth 13, 18–25 (2016).
Redding, D. W. et al. Impacts of environmental and socio-economic factors on emergence and epidemic potential of Ebola in Africa. Nat. Commun. 10, 4531 (2019).
Kupferschmidt, K. This bat species may be the source of the Ebola epidemic that killed more than 11,000 people in West Africa. Science https://www.science.org/content/article/bat-species-may-be-source-ebola-epidemic-killed-more-11000-people-west-africa (2019).
Memish, Z. A. et al. Middle East Respiratory Syndrome Coronavirus in Bats, Saudi Arabia. Emerg. Infect. Dis. 19, 1819–1823 (2013).
Anthony, S. J. et al. Further Evidence for bats as the evolutionary source of Middle East Respiratory Syndrome Coronavirus. mBio 8, e00373–17 (2017).
Li, W. et al. Bats are natural reservoirs of SARS-like Coronaviruses. Science 310, 676–679 (2005).
Cui, J., Li, F. & Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 17, 181–192 (2019).
Beyer, R. M., Manica, A. & Mora, C. Shifts in global bat diversity suggest a possible role of climate change in the emergence of SARS-CoV-1 and SARS-CoV-2. Sci. Total Environ. 767, 145413 (2021).
Horta, M. A., Ledesma, L. A., Moura, W. C. & Lemos, E. R. S. From dogs to bats: Concerns regarding vampire bat-borne rabies in Brazil. PLoS Negl. Trop. Dis. 16, e0010160 (2022).
Martin, G. et al. Climate change could increase the geographic extent of Hendra Virus spillover risk. EcoHealth 15, 509–525 (2018).
Yuen, K. Y. et al. Hendra virus: Epidemiology dynamics in relation to climate change, diagnostic tests and control measures. One Health 12, 100207 (2021).
Eby, P. et al. Pathogen spillover driven by rapid changes in bat ecology. Nature 613, 340–344 (2023).
Daszak, P. et al. Interdisciplinary approaches to understanding disease emergence: The past, present, and future drivers of Nipah virus emergence. Proc. Natl Acad. Sci. 110, 3681–3688 (2013).
Hjelle, B. & Glass, G. E. Outbreak of Hantavirus Infection in the Four Corners Region of the United States in the Wake of the 1997–1998 El Nino—Southern Oscillation. J. Infect. Dis. 181, 1569–1573 (2000).
Patz, J. A., Epstein, P. R., Burke, T. A. & Balbus, J. M. Global climate change and emerging infectious diseases. JAMA 275, 217–223 (1996).
Bonwitt, J. et al. At home with Mastomys and Rattus: Human-rodent interactions and potential for primary transmission of Lassa Virus in domestic spaces. Am. J. Trop. Med. Hyg. 96, 935–943 (2017).
Redding, D. W. et al. Geographical drivers and climate-linked dynamics of Lassa fever in Nigeria. Nat. Commun. 12, 5759 (2021).
Zhou, J. et al. Biological features of novel avian influenza A (H7N9) virus. Nature 499, 500–503 (2013).
Tian, H. et al. Climate change suggests a shift of H5N1 risk in migratory birds. Ecol. Model. 306, 6–15 (2015).
Yang, R. et al. Human infection of avian influenza A H3N8 virus and the viral origins: a descriptive study. Lancet Microbe 3, e824–e834 (2022).
CDC. Human Infection with Avian Influenza A(H3N8) Virus Reported by China. Centers for Disease Control and Prevention https://www.cdc.gov/flu/avianflu/spotlights/2022-2023/avian-influenza-h3n8-china.htm (2023).
Nyamwaya, D. K., Thumbi, S. M., Bejon, P., Warimwe, G. M. & Mokaya, J. The global burden of Chikungunya fever among children: A systematic literature review and meta-analysis. PLOS Glob. PLOS Glob. Public Health 2, e0000914 (2022).
Jones, B. A. et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl Acad. Sci. 110, 8399–8404 (2013).
Herrero, M. et al. Livestock and the environment: what have we learned in the past decade? Annu. Rev. Environ. Resour. 40, 177–202 (2015).
Graham, J. P. et al. The animal-human interface and infectious disease in industrial food animal production: rethinking biosecurity and biocontainment. Public Health Rep. Wash. DC 1974 123, 282–299 (2008).
Drew, T. W. The emergence and evolution of swine viral diseases: to what extent have husbandry systems and global trade contributed to their distribution and diversity? Rev. Sci. Tech. Int. Epizoot. 30, 95–106 (2011).
Looi, L.-M. & Chua, K.-B. Lessons from the Nipah virus outbreak in Malaysia. Malays. J. Pathol. 29, 63–67 (2007).
Gilbert, M., Xiao, X. & Robinson, T. P. Intensifying poultry production systems and the emergence of avian influenza in China: a ‘One Health/Ecohealth’ epitome. Arch. Public Health 75, 48 (2017).
Gibb, R., Franklinos, L. H. V., Redding, D. W. & Jones, K. E. Ecosystem perspectives are needed to manage zoonotic risks in a changing climate. BMJ m3389 (2020) https://doi.org/10.1136/bmj.m3389.
Montgomery, J. M. et al. Risk factors for Nipah Virus Encephalitis in Bangladesh. Emerg. Infect. Dis. 14, 1526–1532 (2008).
Warrell, M. J. Emerging aspects of rabies infection: with a special emphasis on children. Curr. Opin. Infect. Dis. 21, 251 (2008).
Lunney, M. et al. Knowledge, attitudes and practices of rabies prevention and dog bite injuries in urban and peri-urban provinces in Cambodia, 2009. Int. Health 4, 4–9 (2012).
Morin, C. W., Comrie, A. C. & Ernst, K. Climate and Dengue transmission: evidence and implications. Environ. Health Perspect. 121, 1264–1272 (2013).
Kraemer, M. U. G. et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 4, 854–863 (2019).
Messina, J. P. et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 4, 1508–1515 (2019).
Greer, A., Ng, V. & Fisman, D. Public health: Climate change and infectious diseases in North America: the road ahead. CMAJ Can. Med. Assoc. J. 178, 715 (2008).
Rocklöv, J. & Dubrow, R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 21, 479–483 (2020).
Gage, K. L., Burkot, T. R., Eisen, R. J. & Hayes, E. B. Clim. Vectorborne Dis. Am. J. Prev. Med. 35, 436–450 (2008).
El-Sayed, A. & Kamel, M. Climatic changes and their role in emergence and re-emergence of diseases. Environ. Sci. Pollut. Res. Int. 27, 22336–22352 (2020).
Daniel, M., Danielova, Kriz, B., Jirsa, A. & Nozicka, J. Shift of the tick Ixodes ricinus and tick-borne encephalitis to higher altitudes in Central Europe. Eur. J. Clin. Microbiol. Infect. Dis. 22, 327–328 (2003).
Daniel, M., Danielová, V., Kříž, B. & Kott, I. An attempt to elucidate the increased incidence of tick-borne encephalitis and its spread to higher altitudes in the Czech Republic. Int. J. Med. Microbiol. 293, 55–62 (2004).
Taylor, D. et al. Environmental change and Rift Valley fever in eastern Africa: projecting beyond HEALTHY FUTURES. Geospatial Health 11, 115–128 (2016).
Campbell, L. P. et al. Climate change influences on global distributions of dengue and chikungunya virus vectors. Philos. Trans. R. Soc. B Biol. Sci. 370, 1–9 (2015).
Ebi, K. L. & Paulson, J. A. Climate change and child health in the United States. Curr. Probl. Pediatr. Adolesc. Health Care 40, 2–18 (2010).
Paz, S. Climate change impacts on West Nile virus transmission in a global context. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 370, 20130561 (2015).
Liu-Helmersson, J., Stenlund, H., Wilder-Smith, A. & Rocklöv, J. Vectorial Capacity of Aedes aegypti: Effects of temperature and implications for global Dengue Epidemic Potential. PLOS ONE 9, e89783 (2014).
Tran, B.-L., Tseng, W.-C., Chen, C.-C. & Liao, S.-Y. Estimating the threshold effects of climate on Dengue: A case study of Taiwan. Int. J. Environ. Res. Public. Health 17, 1392 (2020).
Colón-González, F. J., Fezzi, C., Lake, I. R. & Hunter, P. R. The effects of weather and climate change on Dengue. PLoS Negl. Trop. Dis. 7, e2503 (2013).
McMichael, C., Barnett, J. & McMichael, A. J. An Ill Wind? Climate change, migration, and health. Environ. Health Perspect. 120, 646–654 (2012).
Watts, N. et al. The 2019 report of The Lancet Countdown on health and climate change: ensuring that the health of a child born today is not defined by a changing climate. Lancet 394, 1836–1878 (2019).
Gubler, D. J. Dengue, urbanization and globalization: the unholy trinity of the 21st Century. Trop. Med. Health 39, S3–S11 (2011).
Lowe, R. et al. Combined effects of hydrometeorological hazards and urbanisation on dengue risk in Brazil: a spatiotemporal modelling study. Lancet Planet. Health 5, e209–e219 (2021).
Thomson, M. C. & Stanberry, L. R. Climate change and vectorborne diseases. N. Engl. J. Med. 387, 1969–1978 (2022).
WHO. Pakistan Floods Situation Report. https://cdn.who.int/media/docs/default-source/documents/emergencies/sitrep-flood-situation-14th-oct-2022-ml-aa.pdf?sfvrsn=9f42ba18_1&download=true (2022).
UNICEF. Devastating floods in Pakistan. United Nations Childrens Fund https://www.unicef.org/emergencies/devastating-floods-pakistan-2022 (2022).
Mondal, N. The resurgence of dengue epidemic and climate change in India. Lancet 401, 727–728 (2023).
Khan, J., Khan, I., Ghaffar, A. & Khalid, B. Epidemiological trends and risk factors associated with dengue disease in Pakistan (1980–2014): a systematic literature search and analysis. BMC Public Health 18, 745 (2018).
Xuan, L. T. T., Van Hau, P., Thu, D. T. & Toan, D. T. T. Estimates of meteorological variability in association with dengue cases in a coastal city in northern Vietnam: an ecological study. Glob. Health Action 7, 52–58 (2014).
Phung, D. et al. Identification of the prediction model for dengue incidence in Can Tho city, a Mekong Delta area in Vietnam. Acta Trop. 141, 88–96 (2015).
Mala, S. & Jat, M. K. Implications of meteorological and physiographical parameters on dengue fever occurrences in Delhi. Sci. Total Environ. 650, 2267–2283 (2019).
Carvajal, T. M. et al. Machine learning methods reveal the temporal pattern of dengue incidence using meteorological factors in metropolitan Manila, Philippines. BMC Infect. Dis. 18, 183 (2018).
Alkhaldy, I. Modelling the association of dengue fever cases with temperature and relative humidity in Jeddah, Saudi Arabia—A generalised linear model with break-point analysis. Acta Trop. 168, 9–15 (2017).
Almeida, A. P. G. et al. Bioecology and vectorial capacity of Aedes albopictus (Diptera: Culicidae) in Macao, China, in relation to Dengue Virus Transmission. J. Med. Entomol. 42, 419–428 (2005).
Bai, L., Morton, L. C. & Liu, Q. Climate change and mosquito-borne diseases in China: a review. Glob. Health 9, 10 (2013).
Kulkarni, M. A., Duguay, C. & Ost, K. Charting the evidence for climate change impacts on the global spread of malaria and dengue and adaptive responses: a scoping review of reviews. Glob. Health 18, 1 (2022).
Giesen, C. et al. The impact of climate change on mosquito-borne diseases in Africa. Pathog. Glob. Health 114, 287–301 (2020).
Watts, N. et al. The 2020 report of The Lancet Countdown on health and climate change: responding to converging crises. Lancet 397, 129–170 (2021).
Taylor, L. Dengue and chikungunya cases surge as climate change spreads arboviral diseases to new regions. BMJ Online 380, 717–717 (2023).
Proestos, Y. et al. Present and future projections of habitat suitability of the Asian tiger mosquito, a vector of viral pathogens, from global climate simulation. Philos. Trans. R. Soc. B Biol. Sci. 370, 1–16 (2015).
Baylis, M. Potential impact of climate change on emerging vector-borne and other infections in the UK. Environ. Health 16, 112 (2017).
Zeller, H., Van Bortel, W. & Sudre, B. Chikungunya: Its history in Africa and Asia and its spread to new regions in 2013–2014. J. Infect. Dis. 214, S436–S440 (2016).
Tjaden, N. B. et al. Modelling the effects of global climate change on Chikungunya transmission in the 21st century. Sci. Rep. 7, 3813 (2017).
Oliveira, E. Cde et al. Short report: Introduction of chikungunya virus ECSA genotype into the Brazilian Midwest and its dispersion through the Americas. PLoS Negl. Trop. Dis. 15, e0009290 (2021).
Souza & de, W. M. et al. Spatiotemporal dynamics and recurrence of chikungunya virus in Brazil: an epidemiological study. Lancet Microbe 4, e319–e329 (2023).
Rezza, G. Dengue and chikungunya: long-distance spread and outbreaks in naïve areas. Pathog. Glob. Health 108, 349–355 (2014).
Tjaden, N. B., Cheng, Y., Beierkuhnlein, C. & Thomas, S. M. Chikungunya beyond the tropics: where and when do we expect disease transmission in Europe? Viruses 13, 1024 (2021).
Fischer, D. et al. Climate change effects on Chikungunya transmission in Europe: geospatial analysis of vector’s climatic suitability and virus’ temperature requirements. Int. J. Health Geogr. 12, 51–51 (2013).
WHO. Zika virus. https://www.who.int/news-room/fact-sheets/detail/zika-virus.
Wen, Z., Song, H. & Ming, G. How does Zika virus cause microcephaly? Genes Dev. 31, 849–861 (2017).
Tesla, B. et al. Temperature drives Zika virus transmission: evidence from empirical and mathematical models. Proc. R. Soc. B Biol. Sci. 285, 20180795 (2018).
Antoniou, E. et al. Zika Virus and the risk of developing microcephaly in infants: a systematic review. Int. J. Environ. Res. Public. Health 17, 3806 (2020).
Adams Waldorf, K. M., Olson, E. M., Nelson, B. R., Little, M.-T. E. & Rajagopal, L. The aftermath of Zika: Need for long-term monitoring of exposed children. Trends Microbiol 26, 729–732 (2018).
Shapiro-Mendoza, C. K. et al. Pregnancy outcomes after maternal Zika Virus infection during pregnancy–U.S. Territories, January 1, 2016–April 25, 2017. MMWR Morb. Mortal. Wkly. Rep. 66, 615–621 (2017).
Hoen, B. et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N. Engl. J. Med. 378, 985–994 (2018).
Costa, M. C. et al. Case fatality rate related to microcephaly congenital Zika Syndrome and associated factors: a nationwide retrospective study in Brazil. Viruses 12, 1228 (2020).
Ali, S. et al. Environmental and social change drive the explosive emergence of Zika Virus in the Americas. PLoS Negl. Trop. Dis. 11, e0005135 (2017).
Sadeghieh, T. et al. Zika virus outbreak in Brazil under current and future climate. Epidemics 37, 100491 (2021).
Ngonghala, C. N. et al. Effects of changes in temperature on Zika dynamics and control. J. R. Soc. Interface 18, 20210165 (2021).
Ryan, S. J. et al. Warming temperatures could expose more than 1.3 billion new people to Zika virus risk by 2050. Glob. Change Biol. 27, 84–93 (2021).
Paz, S. The west nile virus outbreak in Israel (2000) from a new perspective: The regional impact of climate change. Int. J. Environ. Health Res. 16, 1–13 (2006).
Pradier, S., Lecollinet, S. & Leblond, A. West Nile virus epidemiology and factors triggering change in its distribution in Europe. Rev. Sci. Tech. Int. Epizoot. 31, 829–844 (2012).
Farooq, Z. et al. European projections of West Nile virus transmission under climate change scenarios. One Health 16, 100509 (2023).
Hu, W. et al. Development of a predictive model for Ross River Virus Disease in Brisbane, Australia. Am. J. Trop. Med. Hyg. 71, 129–137 (2004).
Tong, S., Hu, W. & McMichael, A. J. Climate variability and Ross River virus transmission in Townsville Region, Australia, 1985–1996. Trop. Med. Int. Health 9, 298–304 (2004).
Werner, A. K. et al. Environmental drivers of Ross River virus in southeastern Tasmania, Australia: towards strengthening public health interventions. Epidemiol. Infect. 140, 359–371 (2012).
Naish, S. et al. Socio-environmental predictors of Barmah forest virus transmission in coastal areas, Queensland, Australia. Trop. Med. Int. Health 14, 247–256 (2009).
Naish, S., Mengersen, K., Hu, W. & Tong, S. Forecasting the future risk of Barmah Forest Virus disease under climate change scenarios in Queensland, Australia. PloS One 8, e62843–e62843 (2013).
Sakkas, H., Bozidis, P., Franks, A., Papadopoulou, C. & Oropouche Fever: A review. Viruses 10, 175 (2018).
Files, M. A. et al. Baseline mapping of Oropouche virology, epidemiology, therapeutics, and vaccine research and development. Npj Vaccines 7, 1–10 (2022).
Diagne, C. T. et al. Mayaro Virus Pathogenesis and transmission mechanisms. Pathogens 9, 738 (2020).
Alomar, A. A. & Alto, B. W. Temperature-mediated effects on Mayaro Virus Vector competency of Florida Aedes aegypti Mosquito Vectors. Viruses 14, 880 (2022).
Semret, M., Ndao, M., Jacobs, J. & Yansouni, C. P. Point-of-care and point-of-‘can’: leveraging reference-laboratory capacity for integrated diagnosis of fever syndromes in the tropics. Clin. Microbiol. Infect. 24, 836–844 (2018).
Romero-Alvarez, D. & Escobar, L. E. Oropouche fever, an emergent disease from the Americas. Microbes Infect. 20, 135–146 (2018).
Caicedo, E.-Y. et al. The epidemiology of Mayaro virus in the Americas: A systematic review and key parameter estimates for outbreak modelling. PLoS Negl. Trop. Dis. 15, e0009418 (2021).
Romandini, A. et al. Antibiotic resistance in pediatric infections: global emerging threats, predicting the near future. Antibiotics 10, 393 (2021).
Jaenson, T. G., Hjertqvist, M., Bergström, T. & Lundkvist, Å. Why is tick-borne encephalitis increasing? A review of the key factors causing the increasing incidence of human TBE in Sweden. Parasit. Vectors 5, 184 (2012).
Blair, P. W. et al. An emerging biothreat: Crimean-Congo Hemorrhagic Fever Virus in Southern and Western Asia. Am. J. Trop. Med. Hyg. 100, 16–23 (2019).
Gilbert, L. The impacts of climate change on ticks and tick-borne disease risk. Annu. Rev. Entomol. 66, 373–388 (2021).
Danielová, V., Kliegrová, S., Daniel, M. & Benes, C. Influence of climate warming on tickborne encephalitis expansion to higher altitudes over the last decade (1997-2006) in the Highland Region (Czech Republic). Cent. Eur. J. Public Health 16, 4–11 (2008).
Lukan, M., Bullova, E. & Petko, B. Climate warming and Tick-borne Encephalitis, Slovakia. Emerg. Infect. Dis. 16, 524–526 (2010).
Nuttall, P. A. Climate change impacts on ticks and tick-borne infections. Biologia 77, 1503–1512 (2022).
Ogden, N. H., Ben Beard, C., Ginsberg, H. S. & Tsao, J. I. Possible effects of climate change on ixodid ticks and the pathogens they transmit. Predictions Observations. J. Med. Entomol. 58, 1536–1545 (2021).
Daniel, M. et al. Increased relative risk of tick-borne Encephalitis in warmer weather. Front. Cell. Infect. Microbiol. 8, 1012–1025 (2018).
Nah, K., Bede-Fazekas, Á., Trájer, A. J. & Wu, J. The potential impact of climate change on the transmission risk of tick-borne encephalitis in Hungary. BMC Infect. Dis. 20, 34 (2020).
Tokarevich, N. K. et al. The impact of climate change on the expansion of Ixodes persulcatus habitat and the incidence of tick-borne encephalitis in the north of European Russia. Glob. Health Action 4, 8448 (2011).
Nili, S., Khanjani, N., Jahani, Y. & Bakhtiari, B. The effect of climate variables on the incidence of Crimean Congo Hemorrhagic Fever (CCHF) in Zahedan, Iran. BMC Public Health 20, 1893 (2020).
Ansari, H. et al. Crimean-Congo hemorrhagic fever and its relationship with climate factors in southeast Iran: A 13-year experience. J. Infect. Dev. Ctries. 8, 749–757 (2014).
Mostafavi, E., Chinikar, S., Bokaei, S. & Haghdoost, A. Temporal modeling of Crimean-Congo hemorrhagic fever in eastern Iran. Int. J. Infect. Dis. 17, e524–e528 (2013).
Papa, A. et al. Crimean-Congo Hemorrhagic Fever Virus, Greece. Emerg. Infect. Dis. 20, 288–290 (2014).
Stavropoulou, E. & Troillet, N. Crimean-Congo hemorrhagic fever: an emerging viral hemorrhagic fever in Europe. Rev. Médicale Suisse 14, 1786–1789 (2018).
Bernard, C. et al. Systematic Review on Crimean–Congo Hemorrhagic Fever Enzootic Cycle and Factors Favoring Virus Transmission: Special Focus on France, an Apparently Free-Disease Area in Europe. Front. Vet. Sci. 9, 932304 (2022).
Kuehnert, P. A., Stefan, C. P., Badger, C. V. & Ricks, K. M. Crimean-Congo Hemorrhagic Fever Virus (CCHFV): A silent but widespread threat. Curr. Trop. Med. Rep. 8, 141–147 (2021).
Dreshaj, S. et al. Current situation of Crimean-Congo hemorrhagic fever in Southeastern Europe and neighboring countries: a public health risk for the European Union? Travel Med. Infect. Dis. 14, 81–91 (2016).
Kuehn, L. & McCormick, S. Heat exposure and maternal health in the face of climate change. Int. J. Environ. Res. Public. Health 14, 853 (2017).
Lawn, J., Kerber, K., Enweronu-Laryea, C. & Massee Bateman, O. Newborn survival in low resource settings—are we delivering? BJOG Int. J. Obstet. Gynaecol. 116, 49–59 (2009).
Cappelletti, M., Della Bella, S., Ferrazzi, E., Mavilio, D. & Divanovic, S. Inflammation and preterm birth. J. Leukoc. Biol. 99, 67–78 (2016).
Nadeau, H. C. G., Subramaniam, A. & Andrews, W. W. Infection and preterm birth. Semin. Fetal Neonatal Med. 21, 100–105 (2016).
Olsen, S. J. et al. The effect of influenza vaccination on birth outcomes in a cohort of pregnant women in Lao PDR, 2014–2015. Clin. Infect. Dis. 63, 487–494 (2016).
Bobei, T.-I. et al. The impact of SARS-CoV-2 infection on premature birth—our experience as COVID Center. Medicina 58, 587 (2022).
Kayem, N. D. et al. Lassa fever in pregnancy: a systematic review and meta-analysis. Trans. R. Soc. Trop. Med. Hyg. 114, 385–396 (2020).
Coler, B. et al. Common pathways targeted by viral hemorrhagic fever viruses to infect the placenta and increase the risk of stillbirth. Placenta https://doi.org/10.1016/j.placenta.2022.10.002 (2022).
Moucheraud, C. et al. Consequences of maternal mortality on infant and child survival: a 25-year longitudinal analysis in Butajira Ethiopia (1987-2011). Reprod. Health 12, S4 (2015).
Paixão, E. S., Teixeira, M. G., Costa, M., da, C. N., Barreto, M. L. & Rodrigues, L. C. Symptomatic Dengue during Pregnancy and Congenital Neurologic Malformations. Emerg. Infect. Dis. 24, 1748–1750 (2018).
Satterfield-Nash, A. Health and Development at Age 19–24 months of 19 children who were born with microcephaly and laboratory evidence of congenital Zika Virus Infection During the 2015 Zika Virus Outbreak—Brazil, 2017. MMWR Morb. Mortal. Wkly. Rep. 66, 1347–1351 (2017).
Contopoulos-Ioannidis, D., Newman-Lindsay, S., Chow, C. & LaBeaud, A. D. Mother-to-child transmission of Chikungunya virus: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 12, e0006510 (2018).
Neu, N., Duchon, J. & Zachariah, P. TORCH Infections. Clin. Perinatol. 42, 77–103 (2015).
Pesch, M. H., Kuboushek, K., McKee, M. M., Thorne, M. C. & Weinberg, J. B. Congenital cytomegalovirus infection. BMJ 373, n1212 (2021).
Beigi, R. H. et al. The need for inclusion of pregnant women in COVID-19 vaccine trials. Vaccine 39, 868–870 (2021).
Dad, N., Buhmaid, S. & Mulik, V. Vaccination in pregnancy – The when, what and how? Eur. J. Obstet. Gynecol. Reprod. Biol. 265, 1–6 (2021).
Burrell, A. L., Evans, J. P. & De Kauwe, M. G. Anthropogenic climate change has driven over 5 million km2 of drylands towards desertification. Nat. Commun. 11, 3853 (2020).
Carlton, E. J., Woster, A. P., DeWitt, P., Goldstein, R. S. & Levy, K. A systematic review and meta-analysis of ambient temperature and diarrhoeal diseases. Int. J. Epidemiol. 45, 117–130 (2016).
Levy, K., Smith, S. M. & Carlton, E. J. Climate change impacts on waterborne diseases: Moving toward designing interventions. Curr. Environ. Health Rep. 5, 272–282 (2018).
Thiam, S. et al. Association between childhood diarrhoeal incidence and climatic factors in urban and rural settings in the Health District of Mbour, Senegal. Int. J. Environ. Res. Public. Health 14, 1049 (2017).
Dimitrova, A., Gershunov, A., Levy, M. C. & Benmarhnia, T. Uncovering social and environmental factors that increase the burden of climate-sensitive diarrheal infections on children. Proc. Natl Acad. Sci. 120, e2119409120 (2023).
Gall, A. M., Mariñas, B. J., Lu, Y., Shisler, J. L. & Waterborne Viruses: A barrier to safe drinking water. PLoS Pathog. 11, e1004867 (2015).
Herrador, B. G. et al. Association between heavy precipitation events and waterborne outbreaks in four Nordic countries, 1992-2012. J. Water Health 14, 1019–1027 (2016).
Levy, K., Woster, A. P., Goldstein, R. S. & Carlton, E. J. Untangling the impacts of climate change on waterborne diseases: a systematic review of relationships between diarrheal diseases and temperature, rainfall, flooding, and drought. Environ. Sci. Technol. 50, 4905–4922 (2016).
Semenza, J. C. & Paz, S. Climate change and infectious disease in Europe: Impact, projection and adaptation. Lancet Reg. Health Eur. 9, 100230–100230 (2021).
Tavakol-Davani, H. et al. How does climate change affect combined sewer overflow in a system benefiting from rainwater harvesting systems? Sustain. Cities Soc. 27, 430–438 (2016).
Azage, M., Kumie, A., Worku, A. & Bagtzoglou, A. C. Childhood diarrhea exhibits spatiotemporal variation in Northwest Ethiopia: A SaTScan spatial statistical analysis. PloS One 10, e0144690–e0144690 (2015).
McIver, L. J. et al. Review of climate change and water-related diseases in Cambodia and findings from stakeholder knowledge assessments. Asia. Pac. J. Public Health 28, 49S–58S (2016).
Horn, L. M. et al. Association between precipitation and diarrheal disease in Mozambique. Int. J. Environ. Res. Public. Health 15, 709 (2018).
Liu, Z. et al. Association between floods and infectious diarrhea and their effect modifiers in Hunan province, China: A two-stage model. Sci. Total Environ. 626, 630–637 (2018).
Sharma, S., Sachdeva, P. & Virdi, J. S. Emerging water-borne pathogens. Appl. Microbiol. Biotechnol. 61, 424–428 (2003).
Zhao, H. et al. Detection of a Bocavirus circular genome in fecal specimens from children with acute Diarrhea in Beijing, China. PLoS ONE 7, e48980 (2012).
Lee, C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 12, 193 (2015).
Ahmed, K. et al. An outbreak of gastroenteritis by emerging norovirus GII.2[P16] in a kindergarten in Kota Kinabalu, Malaysian Borneo. Sci. Rep. 10, 7137 (2020).
Becker-Dreps, S., González, F. & Bucardo, F. Sapovirus: an emerging cause of childhood diarrhea. Curr. Opin. Infect. Dis. 33, 388–397 (2020).
Kumazaki, M. & Usuku, S. Influence of herd immunity on norovirus: a long-term field study of repeated viral gastroenteritis outbreaks at the same facilities. BMC Infect. Dis. 23, 265 (2023).
WHO. Statement of the thirty-fourth Polio IHR Emergency Committee. World Health Organization https://www.who.int/news/item/02-02-2023-statement-of-the-thirty-fourth-polio-ihr-emergency-committee (2023).
WHO. Poliomyelitis. World Health Organization https://www.who.int/news-room/fact-sheets/detail/poliomyelitis (2022).
Nagata, J. M., Epstein, A., Ganson, K. T., Benmarhnia, T. & Weiser, S. D. Drought and child vaccination coverage in 22 countries in sub-Saharan Africa: A retrospective analysis of national survey data from 2011 to 2019. PLoS Med 18, e1003678 (2021).
Mahmud, A. S., Martinez, P. P., He, J. & Baker, R. E. The impact of climate change on vaccine-preventable diseases: insights from current research and new directions. Curr. Environ. Health Rep. 7, 384–391 (2020).
Coates, S. J., Davis, M. D. P. & Andersen, L. K. Temperature and humidity affect the incidence of hand, foot, and mouth disease: a systematic review of the literature–a report from the International Society of Dermatology Climate Change Committee. Int. J. Dermatol. 58, 388–399 (2019).
Yi, L. et al. The impact of climate variability on infectious disease transmission in China: Current knowledge and further directions. Environ. Res. 173, 255–261 (2019).
Qi, H. et al. Impact of meteorological factors on the incidence of childhood hand, foot, and mouth disease (HFMD) analyzed by DLNMs-based time series approach. Infect. Dis. Poverty 7, 7 (2018).
Xu, M. et al. Non-linear association between exposure to ambient temperature and children’s hand-foot-and-mouth disease in Beijing, China. PLOS ONE 10, e0126171 (2015).
Jiang, F. C. et al. Meteorological factors affect the hand, foot, and mouth disease epidemic in Qingdao, China, 2007–2014. Epidemiol. Infect. 144, 2354–2362 (2016).
Du, Z. et al. Weather effects on hand, foot, and mouth disease at individual level: a case-crossover study. BMC Infect. Dis. 19, 1029 (2019).
Wu, H., Wang, H., Wang, Q., Xin, Q. & Lin, H. The effect of meteorological factors on adolescent hand, foot, and mouth disease and associated effect modifiers. Glob. Health Action 7, 24664 (2014).
Hao, J. et al. Impact of ambient temperature and relative humidity on the incidence of hand-foot-mouth disease in Wuhan, China. Int. J. Environ. Res. Public. Health 17, 428 (2020).
Onozuka, D. & Hashizume, M. Effect of weather variability on the incidence of mumps in children: a time-series analysis. Epidemiol. Infect. 139, 1692–1700 (2011).
Nhat, P. D., Phu, V. L., Chính, Đ. V., Tam, D. T. M. & Thanh, M. T. Impact of weather factors on hospital admission of hand, foot and mouth disease under climate change scenarios in Ho Chi Minh City. IOP Conf. Ser. Earth Environ. Sci. 964, 012016 (2022).
Hanna, J., Brauer, P. R., Morse, E., Berson, E. & Mehra, S. Epidemiological analysis of croup in the emergency department using two national datasets. Int. J. Pediatr. Otorhinolaryngol. 126, 109641 (2019).
Dalziel, S. R. et al. Bronchiolitis. Lancet 400, 392–406 (2022).
Marangu, D. & Zar, H. J. Childhood pneumonia in low-and-middle-income countries: An update. Paediatr. Respir. Rev. 32, 3–9 (2019).
Simoes, E. A. F. et al. Acute respiratory infections in children. in Disease Control Priorities in Developing Countries (eds. Jamison, D. T. et al.) (The International Bank for Reconstruction and Development/The World Bank, 2006).
Caballero, M. T. & Polack, F. P. Respiratory syncytial virus is an “opportunistic” killer. Pediatr. Pulmonol. 53, 664–667 (2018).
Al-Toum, R., Bdour, S. & Ayyash, H. Epidemiology and clinical characteristics of respiratory syncytial virus infections in Jordan. J. Trop. Pediatr. 52, 282–287 (2006).
Viegas, M., Barrero, P. R., Maffey, A. F. & Mistchenko, A. S. Respiratory viruses seasonality in children under five years of age in Buenos Aires, ArgentinaA five-year analysis. J. Infect. 49, 222–228 (2004).
Nenna, R. et al. Respiratory syncytial virus bronchiolitis, weather conditions and air pollution in an Italian urban area: An observational study. Environ. Res. 158, 188–193 (2017).
Tchidjou, H. K. et al. Seasonal pattern of hospitalization from acute respiratory infections in Yaoundé, Cameroon. J. Trop. Pediatr. 56, 317–320 (2010).
Nascimento-Carvalho, C. M. et al. Seasonal patterns of viral and bacterial infections among children hospitalized with community-acquired pneumonia in a tropical region. Scand. J. Infect. Dis. 42, 839–844 (2010).
Dowell, S. F. & Ho, M. S. Seasonality of infectious diseases and severe acute respiratory syndrome–what we don’t know can hurt us. Lancet Infect. Dis. 4, 704–708 (2004).
He, D., Chiu, A. P. Y., Lin, Q. & Cowling, B. J. Differences in the seasonality of Middle East respiratory syndrome coronavirus and influenza in the Middle East. Int. J. Infect. Dis. 40, 15–16 (2015).
Darling, N. D. et al. Retrospective, epidemiological cluster analysis of the Middle East respiratory syndrome coronavirus (MERS-CoV) epidemic using open source data. Epidemiol. Infect. 145, 3106–3114 (2017).
Tuncer, N. & Martcheva, M. Modeling seasonality in Avian Influenza H5N1. J. Biol. Syst. 21, 1340004 (2013).
Davis, R. E., Dougherty, E., McArthur, C., Huang, Q. S. & Baker, M. G. Cold, dry air is associated with influenza and pneumonia mortality in Auckland, New Zealand. Influenza Other Respir. Viruses 10, 310–313 (2016).
Marr, L. C., Tang, J. W., Van Mullekom, J. & Lakdawala, S. S. Mechanistic insights into the effect of humidity on airborne influenza virus survival, transmission and incidence. J. R. Soc. Interface 16, 20180298 (2019).
Lam, E. K. S., Morris, D. H., Hurt, A. C., Barr, I. G. & Russell, C. A. The impact of climate and antigenic evolution on seasonal influenza virus epidemics in Australia. Nat. Commun. 11, 2741 (2020).
Leung, N. H. L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 19, 528–545 (2021).
Miyayo, S. F., Owili, P. O., Muga, M. A. & Lin, T.-H. Analysis of Pneumonia occurrence in relation to climate change in Tanga, Tanzania. Int. J. Environ. Res. Public. Health 18, 4731 (2021).
Sajadi, M. M. et al. Temperature, humidity, and latitude analysis to estimate potential spread and seasonality of Coronavirus Disease 2019 (COVID-19). JAMA Netw. Open 3, e2011834 (2020).
Liu, X. et al. The role of seasonality in the spread of COVID-19 pandemic. Environ. Res. 195, 110874 (2021).
D’Amico, F. et al. COVID-19 seasonality in temperate countries. Environ. Res. 206, 112614 (2022).
Towers, S. et al. Climate change and influenza: the likelihood of early and severe influenza seasons following warmer than average winters. PLoS Curr. 5, ecurrents.flu.3679b56a3a5313dc7c043fb944c6f138 (2013).
Mirsaeidi, M. et al. Climate change and respiratory infections. Ann. Am. Thorac. Soc. 13, 1223–1230 (2016).
Irwin, C. K. et al. Using the systematic review methodology to evaluate factors that influence the persistence of influenza virus in environmental matrices. Appl. Environ. Microbiol. 77, 1049–1060 (2011).
Dombrovsky, L. et al. Modeling evaporation of water droplets as applied to survival of airborne viruses. Atmosphere 11, 965 (2020).
Takaro, T. K., Knowlton, K. & Balmes, J. R. Climate change and respiratory health: current evidence and knowledge gaps. Expert Rev. Respir. Med. 7, 349–361 (2013).
Evans, G. W. Projected behavioral impacts of global climate change. Annu. Rev. Psychol. 70, 449–474 (2019).
Nardell, E., Lederer, P., Mishra, H., Nathavitharana, R. & Theron, G. Cool but dangerous: How climate change is increasing the risk of airborne infections. Indoor Air 30, 195–197 (2020).
Zhao, Q. et al. Global climate change and human health: Pathways and possible solutions. Eco-Environ. Health 1, 53–62 (2022).
Staddon, C. et al. Water insecurity compounds the global coronavirus crisis. Water Int 45, 416–422 (2020).
Haddout, S., Priya, K. L., Hoguane, A. M. & Ljubenkov, I. Water scarcity: A big challenge to slums in Africa to fight against COVID-19. Sci. Technol. Libr. 39, 281–288 (2020).
Xu, R. et al. Wildfires, global climate change, and human health. N. Engl. J. Med. 383, 2173–2181 (2020).
Romanello, M. et al. The 2022 report of the Lancet Countdown on health and climate change: health at the mercy of fossil fuels. Lancet 400, 1619–1654 (2022).
Neuzil, K. M., Wright, P. F., Mitchel, E. F. & Griffin, M. R. The burden of influenza illness in children with asthma and other chronic medical conditions. J. Pediatr. 137, 856–864 (2000).
Wu, P. & Hartert, T. V. Evidence for a causal relationship between respiratory syncytial virus infection and asthma. Expert Rev. Anti Infect. Ther. 9, 731–745 (2011).
Guarnieri, M. & Balmes, J. R. Outdoor air pollution and asthma. Lancet 383, 1581–1592 (2014).
Victora, C. G. et al. Countdown to 2015: a decade of tracking progress for maternal, newborn, and child survival. Lancet Lond. Engl. 387, 2049–2059 (2016).
Odo, D. B. et al. Ambient air pollution and acute respiratory infection in children aged under 5 years living in 35 developing countries. Environ. Int. 159, 107019 (2022).
Kodgule, R. & Salvi, S. Exposure to biomass smoke as a cause for airway disease in women and children. Curr. Opin. Allergy Clin. Immunol. 12, 82–90 (2012).
World Health Organization. Air pollution and child health: prescribing clean air. Summary. https://www.who.int/publications/i/item/WHO-CED-PHE-18-01 (2018).
Larson, P. S. et al. Long-term PM2.5 exposure is associated with symptoms of acute respiratory infections among children under five years of age in Kenya, 2014. Int. J. Environ. Res. Public. Health 19, 2525 (2022).
Werz, M. & Hoffman, M. Europe’s twenty-first century challenge: climate change, migration and security. Eur. View 15, 145–154 (2016).
Kaczan, D. J. & Orgill-Meyer, J. The impact of climate change on migration: a synthesis of recent empirical insights. Clim. Change 158, 281–300 (2020).
Hoffmann, R., Dimitrova, A., Muttarak, R., Crespo Cuaresma, J. & Peisker, J. A meta-analysis of country-level studies on environmental change and migration. Nat. Clim. Change 10, 904–912 (2020).
UNICEF. New guidelines provide first global policy framework on protecting children on the move in face of climate change. https://www.unicef.org/press-releases/new-guidelines-provide-first-global-policy-framework-protect-children-move-displaced-face (2022).
De Bruijn, B. The living conditions and well-being of refugees. Hum. Dev. Res. Pap. 25, (2009).
Sverdlik, A. Ill-health and poverty: a literature review on health in informal settlements. Environ. Urban. 23, 123–155 (2011).
Hunter, L. M. et al. Scales and sensitivities in climate vulnerability, displacement, and health. Popul. Environ. 43, 61–81 (2021).
Altare, C. et al. Infectious disease epidemics in refugee camps: a retrospective analysis of UNHCR data (2009-2017). J. Glob. Health Rep. 3, e2019064 (2019).
Shimakawa, Y., Camélique, O. & Ariyoshi, K. Outbreak of chickenpox in a refugee camp of northern Thailand. Confl. Health 4, 4 (2010).
Hsan, K., Naher, S., Gozal, D., Griffiths, M. D. & Furkan Siddique, Md. R. Varicella outbreak among the Rohingya refugees in Bangladesh: Lessons learned and potential prevention strategies. Travel Med. Infect. Dis. 31, 101465 (2019).
Ahmed, J. A. et al. Epidemiology of respiratory viral infections in two long-term refugee camps in Kenya, 2007-2010. BMC Infect. Dis. 12, 7 (2012).
Mohamed, G. A. et al. Etiology and incidence of viral acute respiratory infections among refugees aged 5 years and older in Hagadera Camp, Dadaab, Kenya. Am. J. Trop. Med. Hyg. 93, 1371–1376 (2015).
WHO & CDC. Outbreak of Poliomyelitis—Somalia and Kenya, May 2013. Morb. Mortal. Wkly. Rep. 62, 484 (2013).
Harvey, B. et al. Planning and implementing a targeted polio vaccination campaign for Somali mobile populations in Northeastern Kenya based on migration and settlement patterns. Ethn. Health 27, 817–832 (2022).
Kaic, B., Borcic, B., Ljubicic, M., Brkic, I. & Mihaljevic, I. Hepatitis A control in a refugee camp by active immunization. Vaccine 19, 3615–3619 (2001).
Hakim, M. S. et al. The global burden of hepatitis E outbreaks: a systematic review. Liver Int 37, 19–31 (2017).
Bagulo, H., Majekodunmi, A. O. & Welburn, S. C. Hepatitis E in Sub Saharan Africa – A significant emerging disease. One Health 11, 100186 (2020).
Ahmed, A., Elduma, A., Magboul, B., Higazi, T. & Ali, Y. The first outbreak of dengue fever in Greater Darfur, Western Sudan. Trop. Med. Infect. Dis 4, 43 (2019).
Bonner, P. C. et al. Poor housing quality increases risk of rodent infestation and Lassa Fever in Refugee Camps of Sierra Leone. Am. J. Trop. Med. Hyg. 77, 169–175 (2007).
Kouadio, I. K., Aljunid, S., Kamigaki, T., Hammad, K. & Oshitani, H. Infectious diseases following natural disasters: prevention and control measures. Expert Rev. Anti Infect. Ther. 10, 95–104 (2012).
Mipatrini, D., Stefanelli, P., Severoni, S. & Rezza, G. Vaccinations in migrants and refugees: a challenge for European health systems. A systematic review of current scientific evidence. Pathog. Glob. Health 111, 59–68 (2017).
Kusuma, Y. S., Kumari, R., Pandav, C. S. & Gupta, S. K. Migration and immunization: determinants of childhood immunization uptake among socioeconomically disadvantaged migrants in Delhi, India. Trop. Med. Int. Health 15, 1326–1332 (2010).
McMichael, C. Climate change-related migration and infectious disease. Virulence 6, 548–553 (2015).
Wasserman, S., Tambyah, P. A. & Lim, P. L. Yellow fever cases in Asia: primed for an epidemic - ClinicalKey. Int. J. Infect. Dis. 48, 98–103 (2016).
Sadarangani, S. P., Lim, P. L. & Vasoo, S. Infectious diseases and migrant worker health in Singapore: a receiving country’s perspective. J. Travel Med. 24, 1–9 (2017).
Atif, M., Saqib, A., Ikram, R., Sarwar, M. R. & Scahill, S. The reasons why Pakistan might be at high risk of Crimean Congo haemorrhagic fever epidemic; a scoping review of the literature. Virol. J. 14, 63 (2017).
Zimmerman, C., Kiss, L. & Hossain, M. Migration and Health: a framework for 21st-century policy-making. PLOS Med 8, e1001034 (2011).
Wesolowski, A. et al. Impact of human mobility on the emergence of dengue epidemics in Pakistan. Proc. Natl Acad. Sci. 112, 11887–11892 (2015).
Dhesi, S., Isakjee, A. & Davies, T. Public health in the Calais refugee camp: environment, health and exclusion. Crit. Public Health 28, 140–152 (2018).
Pottie, K. et al. Access to healthcare for the most vulnerable migrants: a humanitarian crisis. Confl. Health 9, 16 (2015).
NOAA. Tropical Cyclone Freddy Breaks Records before Lashing Madagascar. National Environmental Satellite, Data, and Information Service https://www.nesdis.noaa.gov/news/tropical-cyclone-freddy-breaks-records-lashing-madagascar (2023).
WHO Africa. Cyclone Freddy deepens health risks in worst-hit countries. WHO Regional Office for Africa https://www.afro.who.int/news/cyclone-freddy-deepens-health-risks-worst-hit-countries (2023).
Africa Research Bulletin. Cyclone Freddy: Malawi and Mozambique. Afr. Res. Bull. Econ. Financ. Tech. Ser. 60, 24231 (2023).
Chinele, J. Cyclone Freddy Collapses Malawi’s Health System, Washing Away Medicines And Patient Records - Health Policy Watch. Health Policy Watch https://healthpolicy-watch.news/cyclone-freddy-collapses-malawis-health-system-washing-away-medicines-and-patient-records/ (2023).
Wong, J. C. Hospitals face critical shortage of IV bags due to Puerto Rico hurricane. The Guardian https://www.theguardian.com/us-news/2018/jan/10/hurricane-maria-puerto-rico-iv-bag-shortage-hospitals (2018).
Richardson, E. T., Burkett, M. & Farmer, P. Health Effects of Climate Change. In Harrison’s Principles of Internal Medicine (eds. Loscalzo, J. et al.) (McGraw-Hill Education, 2022).
Bendix, A. Surge of viruses leaves children’s hospitals scrambling to free up beds and find room for patients. NBC News https://www.nbcnews.com/health/health-news/virus-surge-childrens-hospitals-scrambling-rcna53891 (2022).
Walter and Patricia Rodney Commission on Reparations. https://www.rodneycommission.org/ (2019).
Ryan, S. J., Carlson, C. J., Mordecai, E. A. & Johnson, L. R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 13, e0007213 (2019).
Funding
Dr. Eugene T. Richardson and Smit Chitre are funded by a Harvard Center for African Studies Motsepe Presidential Research Accelerator grant; Smit Chitre is also funded by the Weatherhead Center for International Affairs Medium Faculty Grant.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article and revising it critically for important intellectual content. Each author has approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
No patient consent was required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chitre, S.D., Crews, C.M., Tessema, M.T. et al. The impact of anthropogenic climate change on pediatric viral diseases. Pediatr Res 95, 496–507 (2024). https://doi.org/10.1038/s41390-023-02929-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02929-z
This article is cited by
-
Trends in prenatal and pediatric viral infections, and the impact of climate change
Pediatric Research (2024)