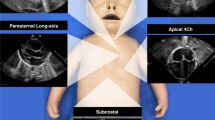
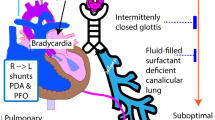
Abstract
The highest incidence of sepsis across all age groups occurs in neonates leading to substantial mortality and morbidity. Cardiovascular dysfunction frequently complicates neonatal sepsis including biventricular systolic and/or diastolic dysfunction, vasoregulatory failure, and pulmonary arterial hypertension. The haemodynamic response in neonatal sepsis can be hyperdynamic or hypodynamic and the underlying pathophysiological mechanisms are heterogeneous. The diagnosis and definition of both neonatal sepsis and cardiovascular dysfunction complicating neonatal sepsis are challenging and not consensus-based. Future developments in neonatal sepsis management will be facilitated by common definitions and datasets especially in neonatal cardiovascular optimisation.
Impact
-
Cardiovascular dysfunction is common in neonatal sepsis but there is no consensus-based definition, making calculating the incidence and designing clinical trials challenging.
-
Neonatal cardiovascular dysfunction is related to the inflammatory response, which can directly target myocyte function and systemic haemodynamics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Reinhart, K. et al. Recognizing sepsis as a global health priority — a WHO resolution. N. Engl. J. Med. 377, 414–417 (2017).
Fleischmann-Struzek, C. et al. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir. Med. 6, 223–230 (2018).
Schlapbach, L. J. et al. Impact of sepsis on neurodevelopmental outcome in a Swiss national cohort of extremely premature infants. Pediatrics 128, e348–e357 (2011).
Hayes, R. et al. Neonatal sepsis definitions from randomised clinical trials. Pediatr. Res. 93, 1141–1148 (2023).
Deshpande, S. et al. Pulmonary hypertension in late onset neonatal sepsis using functional echocardiography: a prospective study. J. Ultrasound 25, 233–239 (2022).
de Waal, K. & Evans, N. Hemodynamics in preterm infants with late-onset sepsis. J. Pediatr. 156, 918–922.e1 (2010).
Habimana, R. et al. Sepsis-induced cardiac dysfunction: a review of pathophysiology. Acute Crit. Care 35, 57–66 (2020).
Prusakowski, M. K. & Chen, A. P. Pediatric sepsis. Emerg. Med. Clin. North Am. 35, 123–138 (2017).
Gonçalves, L. F., Chaiworapongsa, T. & Romero, R. Intrauterine infection and prematurity. Ment. Retard. Dev. Disabil. Res. Rev. 8, 3–13 (2002).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315, 801 (2016).
Morin, L. et al. The current and future state of pediatric sepsis definitions: an international survey. Pediatrics 149, e2021052565 (2022).
Matics, T. J. & Sanchez-Pinto, L. N. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the Sepsis-3 definitions in critically ill children. JAMA Pediatr. 171, e172352 (2017).
Schlapbach, L. J., Straney, L., Bellomo, R., MacLaren, G. & Pilcher, D. Prognostic accuracy of age-adapted SOFA, SIRS, PELOD-2, and qSOFA for in-hospital mortality among children with suspected infection admitted to the intensive care unit. Intensive Care Med. 44, 179–188 (2018).
Molloy, E. J. & Bearer, C. F. Paediatric and neonatal sepsis and inflammation. Pediatr. Res. 91, 267–269 (2022).
Molloy, E. J. et al. Neonatal sepsis: need for consensus definition, collaboration and core outcomes. Pediatr. Res. 88, 2–4 (2020).
Henry, C. J. et al. Neonatal sepsis: a systematic review of core outcomes from randomised clinical trials. Pediatr. Res. 91, 735–742 (2022).
McGovern, M., Giannoni, E., Kuester, H., Turner, M. A., van den Hoogen, A. & Bliss, J. M. et al. Challenges in developing a consensus definition of neonatal sepsis. Pediatr. Res. 88, 14–26 (2020).
ELFIN Trial Investigators Group. Enteral lactoferrin supplementation for very preterm infants: a randomised placebo-controlled trial. Lancet 393, 423–433 (2019).
The International Neonatal Immunotherapy Study (INIS) Collaborative Group. Treatment of neonatal sepsis with intravenous immune globulin. N. Engl. J. Med. 365, 1201–1211 (2011).
Wynn, J. L. & Polin, R. A. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr. Res. 88, 85–90 (2020).
Fleiss, N. et al. Evaluation of the neonatal sequential organ failure assessment and mortality risk in preterm infants with late-onset infection. JAMA Netw. Open 4, e2036518 (2021).
Aziz, K. B. et al. Maximum vasoactive-inotropic score and mortality in extremely premature, extremely low birth weight infants. J. Perinatol. 41, 2337–2344 (2021).
Kharrat, A. et al. Validity of the vasoactive-inotropic score in preterm neonates receiving cardioactive therapies. Early Hum. Dev. 173, 105657 (2022).
Demirhan, S., Topcuoglu, S., Karadag, N., Ozalkaya, E. & Karatekin, G. Vasoactive inotropic score as a predictor of mortality in neonatal septic shock. J. Trop. Pediatr. 68, fmac100 (2022).
Wynn, J. L. et al. Timing of multiorgan dysfunction among hospitalized infants with fatal fulminant sepsis. Am. J. Perinatol. 34, 633–639 (2017).
Giannoni, E. et al. Neonatal sepsis of early onset, and hospital-acquired and community-acquired late onset: a prospective population-based cohort study. J. Pediatr. 201, 106–114.e4 (2018).
Kermorvant-Duchemin, E., Laborie, S., Rabilloud, M., Lapillonne, A. & Claris, O. Outcome and prognostic factors in neonates with septic shock. Pediatr. Crit. Care Med. 9, 186–191 (2008).
Groeneveld, A. B., Nauta, J. J. & Thijs, L. G. Peripheral vascular resistance in septic shock: its relation to outcome. Intensive Care Med. 14, 141–147 (1988).
Ceneviva, G., Paschall, J. A., Maffei, F. & Carcillo, J. A. Hemodynamic support in fluid-refractory pediatric septic shock. Pediatrics 102, e19 (1998).
Pollack, M. M., Fields, A. I. & Ruttimann, U. E. Distributions of cardiopulmonary variables in pediatric survivors and nonsurvivors of septic shock. Crit. Care Med. 13, 454–459 (1985).
Mercier, J.-C., Beaufils, F., Hartmann, J.-F. & Azema, D. Hemodynamic patterns of meningococcal shock in children. Crit. Care Med. 16, 27–33 (1988).
El-Khuffash, A. F. & McNamara, P. J. Neonatologist-performed functional echocardiography in the neonatal intensive care unit. Semin. Fetal Neonatal Med. 16, 50–60 (2011).
Cerritelli, F. et al. A review on the vagus nerve and autonomic nervous system during fetal development: searching for critical windows. Front. Neurosci. 15, 721605 (2021).
Galland, B. C., Taylor, B. J., Bolton, D. P. G. & Sayers, R. M. Heart rate variability and cardiac reflexes in small for gestational age infants. J. Appl. Physiol. (1985) 100, 933–939 (2006).
Saini, S. S., Kumar, P. & Kumar, R. M. Hemodynamic changes in preterm neonates with septic shock: a prospective observational study. Pediatr. Crit. Care Med. 15, 443–450 (2014).
Deshpande, S., Suryawanshi, P., Chaudhary, N. & Maheshwari, R. Cardiac output in late onset neonatal sepsis. J. Clin. Diagn. Res. https://doi.org/10.7860/JCDR/2017/30312.10871 (2017).
Yengkhom, R. et al. Point of care neonatal ultrasound in late-onset neonatal sepsis. J. Neonatol. 35, 59–63 (2021).
Briegel, J., Jochum, M., Gippner-Steppert, C. & Thiel, M. Immunomodulation in septic shock: hydrocortisone differentially regulates cytokine responses. J. Am. Soc. Nephrol. 12, S70–S74 (2001).
Soliman, A. T. et al. Circulating adrenocorticotropic hormone (ACTH) and cortisol concentrations in normal, appropriate-for-gestational-age newborns versus those with sepsis and respiratory distress: cortisol response to low-dose and standard-dose ACTH tests. Metabolism 53, 209–214 (2004).
Ng, P. C. Adrenocortical insufficiency and refractory hypotension in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 101, F571–F576 (2016).
Scott, S. M. & Watterberg, K. L. Effect of gestational age, postnatal age, and illness on plasma cortisol concentrations in premature infants. Pediatr. Res. 37, 112–116 (1995).
Huysman, M. W. A., Hokken-Koelega, A. C. S., De Ridder, M. A. J. & Sauer, P. J. J. Adrenal function in sick very preterm infants. Pediatr. Res. 48, 629–633 (2000).
Seri, I., Tan, R. & Evans, J. Cardiovascular effects of hydrocortisone in preterm infants with pressor-resistant hypotension. Pediatrics 107, 1070–1074 (2001).
Ng, P. C. et al. A double-blind, randomized, controlled study of a “stress dose” of hydrocortisone for rescue treatment of refractory hypotension in preterm infants. Pediatrics 117, 367–375 (2006).
Higgins, S., Friedlich, P. & Seri, I. Hydrocortisone for hypotension and vasopressor dependence in preterm neonates: a meta-analysis. J. Perinatol. 30, 373–378 (2010).
Kharrat, A. & Jain, A. Hemodynamic dysfunction in neonatal sepsis. Pediatr. Res. 91, 413–424 (2022).
Meadow, W. L. & Meus, P. J. Unsuspected mesenteric hypoperfusion despite apparent hemodynamic recovery in the early phase of septic shock in piglets. Circ. Shock 15, 123–129 (1985).
Meadow, W. L. & Meus, P. J. Early and late hemodynamic consequences of group B beta streptococcal sepsis in piglets: effects on systemic, pulmonary, and mesenteric circulations. Circ. Shock 19, 347–356 (1986).
Davis, A. L. et al. American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit. Care Med. 45, 1061–1093 (2017).
Truog, W. E., Gibson, R. L., Henderson, W. R. & Redding, G. J. Tumor necrosis factor-induced neonatal pulmonary hypertension: effects of dazmegrel pretreatment. Pediatr. Res. 27, 466–471 (1990).
Anderson, P. A. W. The heart and development. Semin. Perinatol. 20, 482–509 (1996).
Marijianowski, M. M. H., van der Loos, C. M., Mohrschladt, M. F. & Becker, A. E. The neonatal heart has a relatively high content of total collagen and type I collagen, a condition that may explain the less compliant state. J. Am. Coll. Cardiol. 23, 1204–1208 (1994).
Crepaz, R., Pitscheider, W., Radetti, G. & Gentili, L. Age-related variation in left ventricular myocardial contractile state expressed by the stress velocity relation. Pediatr. Cardiol. 19, 463–467 (1998).
Rowland, D. G. & Gutgesell, H. P. Noninvasive assessment of myocardial contractility, preload, and afterload in healthy newborn infants. Am. J. Cardiol. 75, 818–821 (1995).
Vrancken, S. L., van Heijst, A. F. & de Boode, W. P. Neonatal hemodynamics: from developmental physiology to comprehensive monitoring. Front. Pediatr. 6, 87 (2018).
Rudolph, A. Myocardial growth before and after birth: clinical implications. Acta Paediatr. 89, 129–133 (2000).
Smith, A. et al. Comparison of left ventricular rotational mechanics between term and extremely premature infants over the first week of age. Open Heart 8, e001458 (2021).
Singh, Y., Katheria, A. C. & Vora, F. Advances in diagnosis and management of hemodynamic instability in neonatal shock. Front. Pediatr. 6, 2 (2018).
Drosatos, K. et al. Pathophysiology of sepsis-related cardiac dysfunction: driven by inflammation, energy mismanagement, or both? Curr. Heart Fail. Rep. 12, 130–140 (2015).
Poelaert, J., Declerck, C., Vogelaers, D., Colardyn, F. & Visser, C. A. Left ventricular systolic and diastolic function in septic shock. Intensive Care Med. 23, 553–560 (1997).
Kimchi, A. et al. Right ventricular performance in septic shock: a combined radionuclide and hemodynamic study. J. Am. Coll. Cardiol. 4, 945–951 (1984).
Parker, M. M. Profound but reversible myocardial depression in patients with septic shock. Ann. Intern. Med. 100, 483 (1984).
Bouhemad, B. et al. Isolated and reversible impairment of ventricular relaxation in patients with septic shock. Crit. Care Med. 36, 766–774 (2008).
L’Heureux, M., Sternberg, M., Brath, L., Turlington, J. & Kashiouris, M. G. Sepsis-induced cardiomyopathy: a comprehensive review. Curr. Cardiol. Rep. 22, 35 (2020).
Vieillard-Baron, A. et al. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit. Care Med. 36, 1701–1706 (2008).
Beesley, S. J. et al. Septic cardiomyopathy. Crit. Care Med. 46, 625–634 (2018).
Vieillard-Baron, A., Prin, S., Chergui, K., Dubourg, O. & Jardin, F. Hemodynamic instability in sepsis: bedside assessment by Doppler echocardiography. Am. J. Respir. Crit. Care Med. 168, 1270–1276 (2003).
Torgersen, C. et al. Macroscopic postmortem findings in 235 surgical intensive care patients with sepsis. Anesth. Analg. 108, 1841–1847 (2009).
Parrillo, J. E. Septic shock in humans: advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann. Intern. Med. 113, 227 (1990).
Merx, M. W. & Weber, C. Sepsis and the heart. Circulation 116, 793–802 (2007).
Jain, A., Sankar, J., Anubhuti, A., Yadav, D. K. & Sankar, M. J. Prevalence and outcome of sepsis-induced myocardial dysfunction in children with ‘sepsis’ ‘with’ and ‘without shock’—a prospective observational study. J. Trop. Pediatr. 64, 501–509 (2018).
Yang, C. et al. NT-Pro-BNP and echocardiography for the early assessment of cardiovascular dysfunction in neonates with sepsis. Medicine (Baltimore) 101, e30439 (2022).
Alzahrani, A. K. Cardiac function affection in infants with neonatal sepsis. J. Clin. Trials 7, 329 (2017).
Abdel-Hady, H. E., Matter, M. K. & El-Arman, M. M. Myocardial dysfunction in neonatal sepsis: a tissue Doppler imaging study. Pediatr. Crit. Care Med. 13, 318–323 (2012).
Fahmey, S. S., Hodeib, M., Refaat, K. & Mohammed, W. Evaluation of myocardial function in neonatal sepsis using tissue Doppler imaging. J. Matern. Fetal Neonatal Med. 33, 3752–3756 (2020).
Tomerak, R. H., El-Badawy, A. A., Hussein, G., Kamel, N. R. M. & Razak, A. R. A. Echocardiogram done early in neonatal sepsis: what does it add? J. Investig. Med. 60, 680–684 (2012).
Awny MM, Abd-Rab-Alrasol OT, Al Biltagi MA, Al-Asy HM, El-Mahdy HS. Cardiac functions by tissue Doppler and Speckle tracking echocardiography in neonatal sepsis and its correlation with sepsis markers and cardiac troponin-T. J. Pediatr. Neonatal Care 5, 11–12 (2016).
Kumar, A. et al. Experimental human endotoxemia is associated with depression of load-independent contractility indices. Chest 126, 860–867 (2004).
Uchimido, R., Schmidt, E. P. & Shapiro, N. I. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit. Care 23, 16 (2019).
De Backer, D., Orbegozo Cortes, D., Donadello, K. & Vincent, J.-L. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence 5, 73–79 (2014).
Kumar, A., Kumar, A., Paladugu, B., Mensing, J. & Parrillo, J. E. Transforming growth factor-β1 blocks in vitro cardiac myocyte depression induced by tumor necrosis factor-α, interleukin-1β, and human septic shock serum. Crit. Care Med. 35, 358–364 (2007).
Bernardin, G., Strosberg, A. D., Bernard, A., Mattei, M. & Marullo, S. Beta-adrenergic receptor-dependent and -independent stimulation of adenylate cyclase is impaired during severe sepsis in humans. Intensive Care Med. 24, 1315–1322 (1998).
Jarkovska, D. et al. Cellular mechanisms of myocardial depression in porcine septic shock. Front. Physiol. 9, 726 (2018).
Yu, P. et al. Myocardial collagen changes and edema in rats with hyperdynamic sepsis. Crit. Care Med. 25, 657–662 (1997).
Chagnon, F., Bentourkia, M., Lecomte, R., Lessard, M. & Lesur, O. Endotoxin-induced heart dysfunction in rats: assessment of myocardial perfusion and permeability and the role of fluid resuscitation. Crit. Care Med. 34, 127–133 (2006).
Vasques-Nóvoa, F. et al. Myocardial edema: an overlooked mechanism of septic cardiomyopathy? Shock 53, 616–619 (2020).
Comstock, K. L. et al. LPS-induced TNF-alpha release from and apoptosis in rat cardiomyocytes: obligatory role for CD14 in mediating the LPS response. J. Mol. Cell. Cardiol. 30, 2761–2775 (1998).
Carlson, D. L., Willis, M. S., White, D. J., Horton, J. W. & Giroir, B. P. Tumor necrosis factor-α-induced caspase activation mediates endotoxin-related cardiac dysfunction. Crit. Care Med. 33, 1021–1028 (2005).
Landry, D. W. & Oliver, J. A. The pathogenesis of vasodilatory shock. N. Engl. J. Med. 345, 588–595 (2001).
Kakihana, Y., Ito, T., Nakahara, M., Yamaguchi, K. & Yasuda, T. Sepsis-induced myocardial dysfunction: pathophysiology and management. J. Intensive Care 4, 22 (2016).
O’Hare, F. M., William Watson, R. & Molloy, E. J. Toll-like receptors in neonatal sepsis. Acta Paediatr. 102, 572–578 (2013).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007).
Adib-Conquy, M. & Cavaillon, J.-M. Réponse inflammatoire et anti-inflammatoire de l’hôte au cours du sepsis. Pathol. Biol. 60, 306–313 (2012).
Soriano, F. G., Lorigados, C. B., Pacher, P. & Szabó, C. Effects of a potent peroxynitrite decomposition catalyst in murine models of endotoxemia and sepsis. Shock 35, 560–566 (2011).
Greer, J. Pathophysiology of cardiovascular dysfunction in sepsis. BJA Educ. 15, 316–321 (2015).
Schultz, C. et al. Immature anti-inflammatory response in neonates. Clin. Exp. Immunol. 135, 130–136 (2003).
Schultz, C. et al. Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatr. Res. 51, 317–322 (2002).
Kollmann, T. R., Kampmann, B., Mazmanian, S. K., Marchant, A. & Levy, O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity 46, 350–363 (2017).
Kollmann, T. R., Levy, O., Montgomery, R. R. & Goriely, S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 37, 771–783 (2012).
Khadour, F. H. et al. Enhanced NO and superoxide generation in dysfunctional hearts from endotoxemic rats. Am. J. Physiol. Heart Circ. Physiol. 283, H1108–H1115 (2002).
Hickson-Bick, D. L. M., Jones, C. & Buja, L. M. The response of neonatal rat ventricular myocytes to lipopolysaccharide-induced stress. Shock 25, 546–552 (2006).
Hobai, I. A., Edgecomb, J., LaBarge, K. & Colucci, W. S. Dysregulation of intracellular calcium transporters in animal models of sepsis-induced cardiomyopathy. Shock 43, 3–15 (2015).
Zhang, M. et al. Clinical characteristics of severe neonatal enterovirus infection: a systematic review. BMC Pediatr. 21, 127 (2021).
Ronchi, A., Doern, C., Brock, E., Pugni, L. & Sánchez, P. J. Neonatal adenoviral infection: a seventeen year experience and review of the literature. J. Pediatr. 164, 529–535.e4 (2014).
Pawar, R. et al. Neonatal Multisystem Inflammatory Syndrome (MIS-N) associated with prenatal maternal SARS-CoV-2: a case series. Children (Basel). 8, 572 (2021).
DiGiulio, D. B. et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am. J. Reprod. Immunol. 64, 38–57 (2010).
Gotsch, F. et al. The fetal inflammatory response syndrome. Clin. Obstet. Gynecol. 50, 652–683 (2007).
Kim, C. J. et al. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol. 213, S29–S52 (2015).
Kalikkot Thekkeveedu, R., Guaman, M. C. & Shivanna, B. Bronchopulmonary dysplasia: a review of pathogenesis and pathophysiology. Respir. Med. 132, 170–177 (2017).
Perniciaro, S. et al. Early- and late-respiratory outcome in very low birth weight with or without intrauterine inflammation. Am. J. Perinatol. 37, S76–S83 (2020).
Resch, B. et al. Risk factors and determinants of neurodevelopmental outcome in cystic periventricular leucomalacia. Eur. J. Pediatr. 159, 663–670 (2000).
Grether, J. K. & Nelson, K. B. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA 278, 207–211 (1997).
Yoon, B. H. et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1β, and tumor necrosis factor-α), neonatal brain white matter lesions, and cerebral palsy. Am. J. Obstet. Gynecol. 177, 19–26 (1997).
Yanowitz, T. D. et al. Hemodynamic disturbances in premature infants born after chorioamnionitis: association with cord blood cytokine concentrations. Pediatr. Res. 51, 310–316 (2002).
Rounioja, S. Intra-amniotic lipopolysaccharide leads to fetal cardiac dysfunction: a mouse model for fetal inflammatory response. Cardiovasc. Res. 60, 156–164 (2003).
Durosier, L. D. et al. Does heart rate variability reflect the systemic inflammatory response in a fetal sheep model of lipopolysaccharide-induced sepsis? Physiol. Meas. 36, 2089–2102 (2015).
Stone, M. L. et al. Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. J. Perinatol. 33, 847–850 (2013).
Griffin, M. P. & Moorman, J. R. Toward the early diagnosis of neonatal sepsis and sepsis-like illness using novel heart rate analysis. Pediatrics 107, 97–104 (2001).
Griffin, M. P. et al. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics 116, 1070–1074 (2005).
Weitkamp, J.-H. et al. Meningitis, urinary tract, and bloodstream infections in very low birth weight infants enrolled in a heart rate characteristics monitoring trial. Pediatr. Res. 87, 1226–1230 (2020).
Kyozuka, H., Yasuda, S., Hiraiwa, T., Nomura, Y. & Fujimori, K. The change of fetal heart rate short-term variability during the course of histological chorioamnionitis in fetal sheep. Eur. J. Obstet. Gynecol. Reprod. Biol. 228, 32–37 (2018).
Garzoni, L., Faure, C. & Frasch, M. G. Fetal cholinergic anti-inflammatory pathway and necrotizing enterocolitis: the brain-gut connection begins in utero. Front. Integr. Neurosci. 7, 57 (2013).
Romero, R. et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J. Matern. Fetal Neonatal Med. 16, 146–157 (2004).
Letti Müller, A. L. et al. Tei index to assess fetal cardiac performance in fetuses at risk for fetal inflammatory response syndrome. Ultrasound Obstet. Gynecol. 36, 26–31 (2010).
Kelleher, M. A. et al. Maternal azithromycin therapy for Ureaplasma parvum intraamniotic infection improves fetal hemodynamics in a nonhuman primate model. Am. J. Obstet. Gynecol. 223, 578.e1–578.e11 (2020).
Author information
Authors and Affiliations
Consortia
Contributions
S.M.D and E.J.M: manuscript design, manuscript draft. All authors contributed to editing, including revising the paper for important intellectual content and approved the final draft. S.L: created figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Duignan, S.M., Lakshminrusimha, S., Armstrong, K. et al. Neonatal sepsis and cardiovascular dysfunction I: mechanisms and pathophysiology. Pediatr Res 95, 1207–1216 (2024). https://doi.org/10.1038/s41390-023-02926-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02926-2