Abstract
Background
Meeting increased regulatory requirements for clinical evaluation of medical devices marketed in Europe in accordance with the Medical Device Regulation (EU 2017/745) is challenging, particularly for high-risk devices used in children.
Methods
Within the CORE-MD project, we performed a scoping review on evidence from clinical trials investigating high-risk paediatric medical devices used in paediatric cardiology, diabetology, orthopaedics and surgery, in patients aged 0–21 years. We searched Medline and Embase from 1st January 2017 to 9th November 2022.
Results
From 1692 records screened, 99 trials were included. Most were multicentre studies performed in North America and Europe that mainly had evaluated medical devices from the specialty of diabetology. Most had enrolled adolescents and 39% of trials included both children and adults. Randomized controlled trials accounted for 38% of the sample. Other frequently used designs were before-after studies (21%) and crossover trials (20%). Included trials were mainly small, with a sample size <100 participants in 64% of the studies. Most frequently assessed outcomes were efficacy and effectiveness as well as safety.
Conclusion
Within the assessed sample, clinical trials on high-risk medical devices in children were of various designs, often lacked a concurrent control group, and recruited few infants and young children.
Impact
-
In the assessed sample, clinical trials on high-risk medical devices in children were mainly small, with variable study designs (often without concurrent control), and they mostly enrolled adolescents.
-
We provide a systematic summary of methodologies applied in clinical trials of medical devices in the paediatric population, reflecting obstacles in this research area that make it challenging to conduct adequately powered randomized controlled trials.
-
In view of changing European regulations and related concerns about shortages of high-risk medical devices for children, our findings may assist competent authorities in setting realistic requirements for the evidence level to support device conformity certification.
Similar content being viewed by others
Introduction
Medical devices play a key role in the diagnosis and treatment of many diseases in children. The spectrum ranges from low-risk devices like dressing materials and wheelchairs to those of high-risk like catheters, ventilators, implants or pacemakers. While overall about 500,000 devices are available on the EU market,1,2 the number of those approved for the paediatric age group is not specified as there is no central database in Europe.3 Despite the advances in medical device technology, globally the market of medical devices is clearly dominated by devices for adults, while products specifically approved for paediatric use are in substantially lower number and often unavailable.4 Consequently, off-label use of adult versions of medical devices is often best practice, despite little to no evidence about suitability and safety of their use in children.5,6
In Europe, the regulation for approval of medical devices changed to improve the safety for patients by enhancing the regulatory requirements for evidence-based clinical evaluation of medical devices. Products marketed in the EU that were approved under the prior directives, as well as newly developed devices, will need to comply with the new Medical Device Regulation (MDR; EU 2017/745; https://eur-lex.europa.eu/eli/reg/2017/745/oj) by 26 May 2024, and fully so after the transition period which has been extended to December 2027 for high-risk medical devices.7,8
Meeting these new regulatory requirements can be challenging for manufacturers, especially with regard to clinical investigation of devices intended for patients in the paediatric age group. For many clinical conditions and diseases for which high-risk medical devices are intended, the numbers of patients are limited, events are rare, and the population under study is likely to be heterogeneous ranging from very small preterm infants to adolescents.9 In addition, both ethical considerations and parental concerns can make it complicated to recruit and enrol infants, children and adolescents, who constitute a vulnerable population, to trials.10 An additional barrier faced by manufacturers is high financial regulatory costs in Europe,3,11 with a low likelihood for achieving a return on investment due to the relatively small market for high-risk medical devices in the paediatric age group.
Together, these factors may result in market withdrawal of innovative medical devices for children, and lack of investment in further development and market introduction of new paediatric medical devices.3 While achieving and documenting the highest possible level of safety and efficacy for medical devices used in children is a laudable goal, at the same time providing access to innovative medical devices for the youngest patients and secure access to related state-of-the-art and potentially life-saving interventions remain equally important.
The project “Coordinating Research and Evidence for Medical Devices” (CORE–MD; https://www.core-md.eu/) is an EU Horizon 2020 project that reviews methodologies for the clinical investigation of high-risk medical devices, including those applied specifically in children, in order to recommend an appropriate balance between efficacy, safety and innovation.11 As part of this project, we systematically summarize published clinical evidence on high-risk medical devices, namely available evidence from clinical trials in children, in order to identify and describe methodologies applied in this research area. Given our broad review questions and thus the exploratory character of the review, we conducted a scoping review. This approach is recommended when the purpose of the review is to “scope a body of literature, clarify concepts or to investigate research conduct”,12 and it is typically used to provide an overview and map of the available evidence in a given field and to identify knowledge gaps.12
Methods
This scoping review was performed in accordance with the previously developed protocol, which was registered and published at the Open Science Framework (https://osf.io/uzekt).13
We conducted this review in accordance with the methodology of the Joanna Briggs Institute’s (JBI) Reviewers’ Manual14 and report the results following the PRISMA-ScR guidelines15 (Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews).
Inclusion and exclusion criteria
Participants
The study population of interest was children and young people from different age-groups covering the range from 0 to <21 years, including preterms, neonates, infants, toddlers, children and adolescents, with any medical condition as an indication for the use of a specific medical device. Mixed population studies that involved both children and adults were also eligible for inclusion.
Concept
Medical devices, including paediatric medical devices, are categorized in different risk classes according to the EU Medical Device Regulation (MDR), as well as to the U.S. Food and Drug Administration (FDA) regulations. However, the classification rules are different and some products may fall into different risk categories. The focus of this review was on high-risk medical devices. According to the MDR, high-risk medical devices are “class III implantable devices and class IIb active devices that are intended to administer or remove medicinal products from the body”.16 According to the FDA, high-risk medical devices are class III devices that “usually sustain or support life, are implanted, or present potential unreasonable risk of illness or injury”.17 Examples of high-risk medical devices are prosthetic heart valves, closed-loop insulin delivery systems, defibrillators or deep-brain stimulation. In our review, studies on class IIb and III medical devices according to the MDR, and on class III medical devices according to the FDA were considered for inclusion.
For feasibility reasons, we focused on selected medical devices, based on a pre-defined list of high-risk paediatric medical devices from cardiology, diabetology, orthopaedics and surgery. This selection is in line with the similar reviews done by the CORE-MD consortium for adult populations18,19,20 and it covers clinical specialties (cardiology; clinical chemistry that includes insulin pumps and glucose sensors) that are frequently represented among approved devices in children.21 In Europe, medical devices are not listed centrally. The European Database on Medical Devices (EUDAMED) will be mandatory in the future to track all devices placed on the EU market under the MDR, but is still under development. Therefore, we developed the list of devices of interest (Supplementary Table 1) using sources based on FDA records.
We used the device list provided by ref. 21, who identified all high-risk medical devices with paediatric age indications listed in the FDA Premarket Approval (PMA) database from inception to February 2020 in their study. Additionally, we supplemented this list by searching the following FDA resources:
-
Premarket Approvals (PMA) database: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pmasimplesearch.cfm (from March 2020 to June 2022)
-
Annual Reports to Congress on Premarket Approval of Paediatric Uses of Devices, covering approved PMA and Humanitarian Device Exemption (HDE) applications (available from 2008 to 2017)
-
Humanitarian Device Exemption (HDE) database (from 2018 to June 2022)
In this scoping review, we investigated designs and methods applied in clinical trials with the use of a high-risk medical device in children as an intervention. According to the International Committee of Medical Journal Editors (ICMJE) a clinical trial is “any research project that prospectively assigns people or a group of people to an intervention, with or without concurrent comparison or control groups, to study the relationship between a health-related intervention and a health outcome”.22 While there are many other definitions of a “clinical trial”,23,24 in any case a clinical trial is an interventional study and thus differentiates itself from studies of observational design.
Due to the nature of this review, the list of outcomes of interest remained open but included the following:
-
Country; single- or multicentre, national or international study
-
Study design (e.g., controlled clinical trial, crossover trial, single-arm interventional study)
-
Sample size and proportion of paediatric participants
-
Target population characteristics (age, sex)
-
Type of device and indication for its use
-
Assessed study outcomes (e.g., safety, performance, efficacy, patient reported outcomes)
-
Approving body
-
Funding (e.g., industry sponsorship)
Context
We included any clinical trials’ reports on high-risk medical devices in children, including those on pre- and post-market clinical investigation. No restrictions were applied in terms of study setting or device indications for use, with areas of application including cardiology, diabetology, orthopaedics and surgery.
Types of sources
Clinical trials of any design (e.g., randomized and non-randomized controlled clinical trials, interventional studies without concurrent controls, before–after studies, crossover trials) and qualitative studies focused on the intervention being trialled, were eligible for inclusion. Evidence from systematic reviews and observational studies (prospective, retrospective) was not of interest. Conference abstracts, commentaries, editorials, letters and book chapters were excluded.
Search strategy
We searched the following electronic medical databases: MEDLINE (PubMed) and EMBASE (Ovid). Database-specific search strategies were developed based on the predefined list of high-risk medical devices, with the use of trade and generic devices’ names and clinical trials search filters. The timeframe for our search was from 1st January 2017 to 9th November 2022. We restricted our search to sources and papers published in English language only. The detailed search strategy is provided in Supplementary Table 2.
Literature selection
Records identified after applying our search strategy were uploaded into reference manager EndNote (Version X8 and 20) and duplicates were removed. Titles and abstracts were screened against the inclusion criteria by one reviewer (PD, KG, MK).25 This process was pilot-tested on a selected subgroup of references with the involvement of all reviewers. Full text articles were obtained for abstracts that needed to be included or that appeared unclear. They were independently evaluated by two reviewers (PD, KG, MK). Any disagreements or uncertainties regarding inclusion were resolved through discussion and record assessment by the third independent reviewer (BPG).
Data extraction
Data extraction was performed manually by two independent reviewers for each included article using a pre-specified data extraction form, and it was later cross-checked for any discrepancies. We extracted information on authors, year of publication, study setting and design, sample size, participant demographic characteristics (age, sex), study aim, medical device characteristics (trade and generic name, medical condition the device is intended for), assessed study outcomes, funding, if the device is on the market and if the study serves for clinical evaluation purposes.
Data analysis
We used basic descriptive statistics (e.g., frequencies, proportions) to summarize the study designs, sample sizes and proportion of paediatric participants within the study sample, the study setting and population characteristics (with main emphasis on the age groups), the type of devices and their distribution across studied clinical specialties, assessed study outcomes and sources of funding.
Results
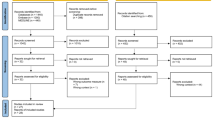
Of 1692 records identified, 104 reports26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129 on 99 trials were included in this scoping review (Fig. 1). We considered multiple reports as one trial if the population enrolled was fully the same. Excluded studies together with reasons for exclusion are presented in Supplementary Table 3 and details on characteristics of each included study in Supplementary Tables 4 and 5.
Study setting
90% of the included studies were conducted in countries of very high Human Development Index (HDI), mainly in North America (38%) and Europe (35%). 65% of the studies were multicentre. Of those, 73% trials were conducted within one country and 27% enrolled participants from different countries. Distributions of the trials across continents and by centre are shown in Fig. 2a, b.
Evaluated medical devices
Most of the included trials (n = 87, 88%) evaluated the use of medical devices from the clinical specialty of diabetology, followed by cardiology (n = 12, 12%). These included closed loop systems, glucose monitoring devices and insulin pumps. We identified no trials that had evaluated the use of medical devices from the clinical specialities of paediatric orthopaedics or paediatric surgery. The list of evaluated medical devices (generic names) by clinical specialty is provided in Table 1. Eight trials did not report the trade name of the evaluated device or the exact model that had been evaluated. 53% of the trials studied a medical device already available on the market. The other trials studied a medical device that was not on the market, or else information about the status of the device was unclear.
Population
60 trials (61%) enrolled only paediatric populations (participants <21 years of age). The remaining 39 trials (39%) evaluated the device of interest in a mixed population, consisting of both children and adult study participants. Within the studies with mixed populations, 64% (n = 25) reported the exact number of children with a proportion from 10% to 89% (median 52%, interquartile range, IQR 45–65%).
We categorized the age groups of interest as followed: neonates from birth through the first 28 days, infants from 29 days to 2 years of age, children from 2 years to 12 years of age, and adolescents from 12 to 21 years, according to ref. 21 and the FDA classification.130
Most studies included children and adolescents (49%), followed by adolescents (33%) or children only (14%) (Fig. 3). Of the remaining reports, two studies (2%) enrolled participants of a broader age range including infants, children and adolescents, one report (1%) included infants and children, and one report (1%) enrolled neonates.
Overall, 51 studies (51.5%) included multiple paediatric age groups, and 48 studies only a single paediatric age group (48.5%). Among the studies with mixed populations (n = 39), 36 studies (92%) included adolescents over 18 years of age.
Study designs
The largest single category of included studies were randomized controlled trials (RCTs) (38%), followed by baseline-controlled trials (before-after studies) (21%) and trials of crossover design (20%) (Table 2). Of all controlled clinical trials and crossover trials, 90% were randomized. All crossover trials and most of the RCTs were open-label studies, with blinding (single or double) applied in only 13% of RCTs.
Sample size
The sample size varied across the studies and ranged from 10 to 1000 participants. Most of the included trials were small, with the median number of participants 59 (IQR 30–124.5) and with a sample size <100 participants in 64% of the studies. Figure 4a, b shows the distribution of the sample sizes of the included studies (continuously, per category: 0–29, 30–99, 100–199, ≥200).
In the subgroup of studies (n = 60) that enrolled only a paediatric population (participants <21 years of age), the median sample size was 48 (IQR 24–102). The median number of study participants was similar in the paediatric studies from the field of diabetology only (57 trials, median sample size of 50, IQR 24–105), but was significantly lower in studies from the field of cardiology (3 trials, median sample size 17, IQR 14.5–30.5).
Assessed outcomes
Most studies assessed the efficacy or effectiveness of the device used (79%), and the safety of the intervention (73%). Patient-reported outcomes were assessed in 24% of the trials. 23% of trials focused on the performance of the device. Usability was examined only in studies from the field of diabetology. Table 3 shows all different types of outcomes assessed in the included studies.
Funding
32 trials (32%) were industry funded, and 42 trials (43%) were partly industry funded meaning that devices were provided by industry for free or at a discounted price. Of all studies in which industry was involved in the funding, 10 trials specifically mentioned that they were investigator-initiated. 13 trials (13%) were non-industry funded. No funding had been received, or funding was not reported in 12 trials (12%).
Discussion
Summary of findings
This study provides an overview on designs and methodologies applied in a systematically selected sample of recent clinical trials evaluating high-risk medical devices in infants, children and adolescents. Our sample was dominated by devices from the clinical specialty of diabetology, while we identified only few studies of cardiology devices and none of orthopaedic or surgery devices in children. Of all identified clinical trials, 38% were RCTs. Remaining trials were of various study designs, often without a concurrent control group, and included crossover trials and before-after studies. Other study characteristics such as small sample sizes and multicentricity were common. Identified studies were mostly conducted in adolescents and older children, with a very low number in neonates, infants and young children.
We based our search on devices with an approved indication from the FDA for use in paediatric patients, although mostly, they had not been approved for the youngest children.21 This may to some extent explain the fact that we found nearly no studies performed in infants and young children. Overall, both low number of devices approved for this young age group and low number of clinical trials identified by us that targeted this population may primarily indicate greater barriers in obtaining clinical evidence in this group. Both ethical aspects and parental concerns can hamper participant recruitment and enrolment and make it more difficult to perform clinical studies in children, particularly in younger age groups, also because of limited number of patients available and rarity of events.9,10 These barriers likely influence not only the number of studies, but also their design.
Only 38% of the clinical trials within our sample were RCTs, which is similar to the recent report on clinical evidence for FDA first-time approved high-risk therapeutic devices, showing that trials with randomization accounted for 49% of pivotal studies for paediatric devices.131 Unsurprisingly, nearly all of the identified RCTs had been conducted among patients with type 1 diabetes, one of the most common chronic diseases in children.132 It is also not unexpected that all crossover trials in our sample were from the field of diabetology, as this study design provides a high level of evidence in patients with chronic diseases, such as diabetes, with a temporary/reversible effect induced by a device (e.g., glycemic control), and at the same time allows for significant sample size reduction because study participants serve as their own controls.133 In contrast, within our small sample of studies from the specialty of cardiology, the leading study designs were uncontrolled studies and before-after studies. Considering the type of indications for high-risk medical device use in paediatric cardiology, such as rare congenital heart defects often requiring urgent and life-saving interventions at a very young age, these findings appear understandable. Other trial design features, such as mixed population under study, multicentricity or small sample sizes that characterized trials within our sample are also likely to be derived from the above-mentioned barriers in study participant recruitment.
We identified a substantially higher proportion of studies conducted in type 1 diabetes patients, than in the other specialities that we included, which again is likely to be at least partly explained by the relatively high prevalence of this disease. Moreover, evaluated devices from this clinical field were basically identical to those used in adults. For manufacturers, it seems to be financially more attractive to conduct studies on devices with large sales volumes, and with long-term use of the devices, which can provide a significant profit margin. We speculate that this is an additional reason why most included studies were performed on commercially attractive devices such as diabetic devices. Additionally, diabetic devices tend to be subject to health technology assessments for reimbursement purposes and, therefore, need more studies of high quality confirming their effectiveness to meet the reimbursement standards set by national authorities. Interestingly, we did not identify any studies of orthopaedic devices in children. However, orthopaedics, with a low number of devices indicated for children within this subspecialty, is not one of the leading fields for devices in paediatrics compared to cardiology, clinical chemistry, ophthalmology or otolaryngology21 and in contrast to devices in adults.
As anticipated, most often reported study outcomes in our sample were efficacy or effectiveness of the device used, and safety of the intervention. Nearly one quarter of studies assessed patient-reported outcomes, which provide valuable information about the impact of a treatment from the perspective of a patient that often cannot be captured by clinical measures.134 Our results indicate that there is room left for improvement and inclusion of patient-reported outcomes. As the MDR also mentions “meaningful, measurable patient-relevant clinical outcome(s),6 under clinical benefit to be assessed, this can increase the inclusion of these types of outcomes in future studies.
Finally, our findings show a substantial contribution of commercial manufacturers in the identified clinical trials, which comes with clear benefits but also with concerns about potential bias introduced in company-sponsored studies. A physician-initiated industry-sponsored study model is among the solutions to consider in order to reduce bias associated with medical device companies involvement into device research.135 Further, ensuring good clinical practice by applying the ISO 14155 standard and requiring sponsors to publish all clinical investigation reports as newly required by the MDR, are means of defense against possible bias.
Given the concerns about limited availability of some high-risk paediatric medical devices in Europe,3,136 European clinical experts have recently developed recommendations on clinical investigation and evaluation of high-risk medical devices for children.137 Findings obtained from this review assisted in the development of these recommendations.
Strengths and limitations
To our knowledge this is the first systematic summary of the methodologies applied in clinical trials on high-risk medical devices in children, not limited to studies identified through FDA sources and exclusively supporting FDA approval of medical devices, but exploring published evidence from various settings and regulatory systems. In addition, a wide range of devices from different clinical specialties was covered in our search. We applied rigorous methods for the review conduct, as proposed in JBI Reviewers’ Manual, with respect to different review stages, including study identification and selection process, data extraction and synthesis.
Although due to feasibility reasons we did not apply free text words and standardized keywords to all concepts covered in our search strategy, both generic and trades names of devices of interest were covered in attempt to identify relevant trials. While we applied validated search filters for clinical trials, we acknowledge that their sensitivity to identify non-randomized trials may be lower as compared to RCTs.138 Therefore, we cannot exclude that some potentially eligible studies might have been omitted by us. Finally, our review was limited to studies published in English in the last 5 years, and did not include unpublished or grey literature or potentially existing confidential data used for device evaluation purposes. However, it should be noted that the aim of this scoping review was to obtain a representative sample of recent clinical trials on high-risk medical devices in children, rather than to identify the totality of evidence. Our selection of devices of interest was based on the U.S. FDA sources as there is no central European database of (paediatric) medical devices,3 which can be considered as a limitation of this review. Other challenges in the review conduct concerned determination of device class, complicated by the different device classification systems (e.g., FDA vs. EU criteria for high-risk medical devices), changes in classification over time, or no central source of information regarding device class in Europe.
Conclusion
While RCTs are considered the gold standard for the effectiveness and safety assessment of a medical intervention, they may not always be feasible in clinical investigation of medical devices in children. Clinical trials of other designs, as those identified in our review, offer a compromise between the highest level of evidence and the lack of evidence. Paediatric devices require specific considerations and have unique barriers to their development. The findings from this scoping review may assist regulators and competent authorities in setting achievable and context-tailored requirements for clinical evidence supporting device conformity. Urgent actions are needed in Europe to ensure both the safety and the continued availability of devices that are essential to treat sick children. Capitalizing on respective evidence-based summaries supports regulatory decision-making processes.
Data availability
In this study, we used only publically available, published data.
References
Melvin, T. & Torre, M. New medical device regulations: the regulator’s view. EFORT Open Rev. 4, 351–356 (2019).
European Commission. Medical Devices—Sector—Overview, https://health.ec.europa.eu/medical-devices-sector/overview_en (2023).
Melvin, T., Kenny, D., Gewillig, M. & Fraser, A. G. Orphan medical devices and pediatric cardiology—what interventionists in Europe need to know, and what needs to be done. Pediatr. Cardiol. 44, 271–279 (2023).
Hwang, T. J., Kesselheim, A. S. & Bourgeois, F. T. Postmarketing trials and pediatric device approvals. Pediatrics 133, e1197–e1202 (2014).
American Academy of Pediatrics Policy Statement, Section on cardiology and cardiac surgery & section on orthopedics. Off-Label Use of Medical Devices in Children. Pediatrics 139, e20163439 (2017).
Espinoza, J. C. The scarcity of approved pediatric high-risk medical devices. JAMA Netw. Open 4, e2112760 (2021).
European Commission. Regulation (Eu) 2017/745 of the European Parliament and of the Council of 5 April 2017 on Medical Devices, Amending Directive 2001/83/Ec, Regulation (Ec) No 178/2002 and Regulation (Ec) No 1223/2009 and Repealing Council Directives 90/385/Eec and 93/42/Eec (Text with EEA Relevance). Off. J. Eur. Union 60, 1–175 (2017).
European Commission. Regulation (Eu) 2023/607 of the European Parliament and of the Council of 15 March 2023 Amending Regulations (Eu) 2017/745 and (Eu) 2017/746 as Regards the Transitional Provisions for Certain Medical Devices and in Vitro Diagnostic Medical Devices (Text with EEA Relevance). Off. J. Eur. Union 66, 24–29 (2023).
Fleurence, R. L., Forrest, C. B. & Shuren, J. Strengthening the evidence base for pediatric medical devices using real-world data. J. Pediatr. 214, 209–211 (2019).
Bates, K. E. et al. Pediatric cardiovascular safety: challenges in drug and device development and clinical application. Am. Heart J. 164, 481–492 (2012).
Fraser, A. G. et al. Improved clinical investigation and evaluation of high-risk medical devices: the rationale and objectives of CORE-MD (Coordinating Research and Evidence for Medical Devices). Eur. Heart J. Qual. Care Clin. Outcomes 8, 249–258 (2022).
Munn, Z. et al. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 18, 143 (2018).
Guerlich, K., Patro-Golab, B., Kammermeier, M., Dworakowski, P. & Koletzko, B. Clinical Evidence for High-Risk Medical Devices in Children: A Protocol for a Scoping Review, https://osf.io/uzekt (2023).
Peters, M. D. J. et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Implement 19, 3–10 (2021).
Tricco, A. C. et al. Prisma extension for scoping reviews (Prisma-Scr): checklist and explanation. Ann. Intern Med. 169, 467–473 (2018).
European Medicines Agency (EMA). Medical Devices, https://www.ema.europa.eu/en/human-regulatory/overview/medical-devices (2023).
US Food and Drug Administration (FDA). Learn If a Medical Device Has Been Cleared by FDA for Marketing, https://www.fda.gov/medical-devices/consumers-medical-devices/learn-if-medical-device-has-been-cleared-fda-marketing (2023).
Siontis, G. et al. Clinical Evidence for High-Risk Medical Devices in Cardiology: A Protocol for a Systematic Review and Meta-Epidemiological Investigation, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022308593 (2022).
Bano, A. et al. Clinical Evidence of High-Risk Medical Devices for Diabetes Management: A Systematic Review and Meta-Analysis, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022366871 (2022).
Smith, J., Combescure, C., Barea, C. & Lübbeke, A. Clinical Investigations to Evaluate High-Risk Orthopaedic Devices: Systematic Review and Meta-Analysis, https://osf.io/6gmyx (2021).
Lee, S. J. et al. Quantification of US Food And Drug Administration premarket approval statements for high-risk medical devices with pediatric age indications. JAMA Netw. Open 4, e2112562 (2021).
International Committee of Medical Journal Editors. Clinical Trials, https://www.icmje.org/recommendations/browse/publishing-and-editorial-issues/clinical-trial-registration.html (2023).
World Health Organization (WHO). Glossary: Clinical Trial Definition, https://www.who.int/clinical-trials-registry-platform/about/glossary (2023).
National Institutes of Health (NIH). Nih’s Definition of a Clinical Trial, https://grants.nih.gov/policy/clinical-trials/definition.htm (2023).
Lefebvre, C. et al. Chapter 4: Searching for and selecting studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 63 (Updated February 2022) (Higgins, J. P. T. et al. eds.) (Cochrane, 2022).
Abraham, M. B. et al. Reduction in hypoglycemia with the predictive low-glucose management system: a long-term randomized controlled trial in adolescents with type 1 diabetes. Diabetes Care 41, 303–310 (2018).
Abraham, M. B. et al. Characteristics of automated insulin suspension and glucose responses with the predictive low-glucose management system. Diabetes Technol. Ther. 21, 28–34 (2019).
Abraham, M. B. et al. Effect of a hybrid closed-loop system on glycemic and psychosocial outcomes in children and adolescents with type 1 diabetes: a randomized clinical trial. JAMA Pediatr. 175, 1227–1235 (2021).
Adams, R. N. et al. Psychosocial and human factors during a trial of a hybrid closed loop system for type 1 diabetes management. Diabetes Technol. Ther. 20, 648–653 (2018).
Al Hayek, A. A., Robert, A. A. & Al Dawish, M. A. Evaluation of freestyle libre flash glucose monitoring system on glycemic control, health-related quality of life, and fear of hypoglycemia in patients with type 1 diabetes. Clin. Med. Insights Endocrinol. Diabetes 10, 1–6 (2017).
Al Hayek, A. A. & Al Dawish, M. A. The potential impact of the freestyle libre flash glucose monitoring system on mental well-being and treatment satisfaction in patients with type 1 diabetes: a prospective study. Diabetes Ther. 10, 1239–1248 (2019).
Al Hayek, A. A., Robert, A. A. & Al Dawish, M. A. Differences of FreeStyle Libre flash glucose monitoring system and finger pricks on clinical characteristics and glucose monitoring satisfactions in type 1 diabetes using insulin pump. Clin. Med. Insights: Endocrinol. Diabetes 12, 1179551419861102 (2019).
Al-Musa, H. M. & Aftab, R. Effectiveness of continuous glucose monitoring for managing type-1 diabetic patients and barrier to its use: a quasi interventional trial. J. Krishna Inst. Med. Sci. Univ. 7, 68–79 (2018).
Anderson, S. M. et al. The international diabetes closed-loop study: testing artificial pancreas component interoperability. Diabetes Technol. Ther. 21, 73–80 (2019).
Aronson, R., Abitbol, A. & Tweden, K. S. First assessment of the performance of an implantable continuous glucose monitoring system through 180 days in a primarily adolescent population with type 1 diabetes. Diabetes Obes. Metab. 21, 1689–1694 (2019).
Barnard, K. D. et al. Closing the loop in adults, children and adolescents with suboptimally controlled type 1 diabetes under free living conditions: a psychosocial substudy. J. Diabetes Sci. Technol. 11, 1080–1088 (2017).
Battelino, T., Nimri, R., Dovc, K., Phillip, M. & Bratina, N. Prevention of hypoglycemia with predictive low glucose insulin suspension in children with type 1 diabetes: a randomized controlled trial. Diabetes Care 40, 764–770 (2017).
Beardsall, K., Thomson, L., Elleri, D., Dunger, D. B. & Hovorka, R. Feasibility of automated insulin delivery guided by continuous glucose monitoring in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 105, F279–F284 (2020).
Beato-Vibora, P. I. et al. Rapid improvement in time in range after the implementation of an advanced hybrid closed-loop system in adolescents and adults with type 1 diabetes. Diabetes Technol. Ther. 23, 609–615 (2021).
Bergenstal, R. M. et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet 397, 208–219 (2021).
Biester, T. et al. “Let the algorithm do the work”: reduction of hypoglycemia using sensor-augmented pump therapy with predictive insulin suspension (SmartGuard) in pediatric type 1 diabetes patients. Diabetes Technol. Ther. 19, 173–182 (2017).
Biester, T. et al. Dream5: an open-label, randomized, cross-over study to evaluate the safety and efficacy of day and night closed-loop control by comparing the MD-Logic automated insulin delivery system to sensor augmented pump therapy in patients with type 1 diabetes at home. Diabetes Obes. Metab. 21, 822–828 (2019).
Bisio, A. et al. Sleep and diabetes-specific psycho-behavioral outcomes of a new automated insulin delivery system in young children with type 1 diabetes and their parents. Pediatr. Diabetes 22, 495–502 (2021).
Blair, J. C. et al. Continuous subcutaneous insulin infusion versus multiple daily injection regimens in children and young people at diagnosis of type 1 diabetes: pragmatic randomised controlled trial and economic evaluation. BMJ (Online) 365, 1226 (2019). (no pagination).
Boucher, S. E. et al. Effect of 6 months of flash glucose monitoring in youth with type 1 diabetes and high-risk glycemic control: a randomized controlled trial. Diabetes Care 43, 2388–2395 (2020).
Breton, M. D. et al. A randomized trial of closed-loop control in children with type 1 diabetes. N. Engl. J. Med. 383, 836–845 (2020).
Brown, S. A. et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N. Engl. J. Med. 381, 1707–1717 (2019).
Brown, S. A. et al. Glycemic outcomes of use of CLC versus PLGS in type 1 diabetes: a randomized controlled trial. Diabetes Care 43, 1822–1828 (2020).
Burckhardt, M. A. et al. The use of continuous glucose monitoring with remote monitoring improves psychosocial measures in parents of children with type 1 diabetes: a randomized crossover trial. Diabetes Care 41, 2641–2643 (2018).
Burckhardt, M. A. et al. Use of remote monitoring with continuous glucose monitoring in young children with type 1 diabetes: the parents’ perspective. Diabet. Med 36, 1453–1459 (2019).
Burnside, M. J. et al. Open-source automated insulin delivery in type 1 diabetes. N. Engl. J. Med. 387, 869–881 (2022).
Carlson, A. L. et al. Safety and glycemic outcomes during the MiniMedTM advanced hybrid closed-loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol. Ther. 24, 178–189 (2022).
Cobry, E. C. et al. Health-related quality of life and treatment satisfaction in parents and children with type 1 diabetes using closed-loop control. Diabetes Technol. Ther. 23, 401–409 (2021).
Collyns, O. J. et al. Improved glycemic outcomes with Medtronic MiniMed advanced hybrid closed-loop delivery: results from a randomized crossover trial comparing automated insulin delivery with predictive low glucose suspend in people with type1 diabetes. Diabetes Care 44, 969–975 (2021).
De Bock, M. et al. Performance of Medtronic hybrid closed-loop iterations: results from a randomized trial in adolescents with type 1 diabetes. Diabetes Technol. Ther. 20, 693–697 (2018).
Deboer, M. D. et al. Performance of an artificial pancreas system for young children with type 1 diabetes. Diabetes Technol. Ther. 19, 293–298 (2017).
Deja, G. et al. The usefulness of the FlashStyle Libre system in glycemic control in children with type 1 diabetes during summer camp. Pediatr. Endocrinol. Diabetes Metab. 24, 11–19 (2018).
Deshpande, S. et al. Feasibility and preliminary safety of smartphone-based automated insulin delivery in adolescents and children with type 1 diabetes. J. Diabetes Sci. Technol. (2022). https://doi.org/10.1177/19322968221116384. Online ahead of print.
Ekhlaspour, L. et al. Closed loop control in adolescents and children during winter sports: use of the tandem control-IQ AP system. Pediatr. Diabetes 20, 759–768 (2019).
Elbalshy, M. M. et al. The effect of do-it-yourself real-time continuous glucose monitoring on psychological and glycemic variables in children with type 1 diabetes: a randomized crossover trial. Pediatr. Diabetes 23, 480–488 (2022).
Elbarbary, N. S. & Ismail, E. A. R. Glycemic control during Ramadan fasting in adolescents and young adults with type 1 diabetes on MiniMed™ 780g advanced hybrid closed‑loop system: a randomized controlled trial. Diabetes Res. Clin. Pract. 191, 110045 (2022).
Forlenza, G. P. et al. Predictive hyperglycemia and hypoglycemia minimization: in-home double-blind randomized controlled evaluation in children and young adolescents. Pediatr. Diabetes 19, 420–428 (2018).
Forlenza, G. P. et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the prolog trial. Diabetes Care 41, 2155–2161 (2018).
Forlenza, G. P. et al. Safety evaluation of the MiniMed 670g system in children 7-13 years of age with type 1 diabetes. Diabetes Technol. Ther. 21, 11–19 (2019).
Forlenza, G. P. et al. Successful at-home use of the tandem control-IQ artificial pancreas system in young children during a randomized controlled trial. Diabetes Technol. Ther. 21, 159–169 (2019).
Forlenza, G. P. et al. Glycemic outcomes of children 2-6 years of age with type 1 diabetes during the pediatric MiniMed™ 670g system trial. Pediatr. Diabetes 23, 324–329 (2022).
Fox, L. A., Balkman, E., Englert, K., Hossain, J. & Mauras, N. Safety of using real-time sensor glucose values for treatment decisions in adolescents with poorly controlled type 1 diabetes mellitus: a pilot study. Pediatr. Diabetes 18, 271–276 (2017).
Garcia-Tirado, J. et al. Advanced closed-loop control system improves postprandial glycemic control compared with a hybrid closed-loop system following unannounced meal. Diabetes Care 44, 2379–2387 (2021).
Gomez, A. M. et al. Efficacy and safety of sensor-augmented pump therapy (SAPT) with predictive low-glucose management in patients diagnosed with type 1 diabetes mellitus previously treated with SAPT and low glucose suspend. Endocrinol. Diabetes Nutr. 65, 451–457 (2018).
Gu, W. et al. Multicentre randomized controlled trial with sensor-augmented pump vs multiple daily injections in hospitalized patients with type 2 diabetes in China: time to reach target glucose. Diabetes Metab. 43, 359–363 (2017).
Guilmin-Crepon, S. et al. Is there an optimal strategy for real-time continuous glucose monitoring in pediatrics? A 12-month French multi-center, prospective, controlled randomized trial (Start-In!). Pediatr. Diabetes 20, 304–313 (2019).
Heller, S. et al. A cluster randomised trial, cost-effectiveness analysis and psychosocial evaluation of insulin pump therapy compared with multiple injections during flexible intensive insulin therapy for type 1 diabetes: the repose trial. Health Technol. Assess. 21, 1–278 (2017).
Kariyawasam, D. et al. Hybrid closed-loop insulin delivery versus sensor-augmented pump therapy in children aged 6-12 years: a randomised, controlled, cross-over, non-inferiority trial. Lancet Digit. Health 4, e158–e168 (2022).
Kovatchev, B. et al. Randomized controlled trial of mobile closed-loop control. Diabetes Care 43, 607–615 (2020).
Kovatchev, B. P. et al. Evening and overnight closed-loop control versus 24/7 continuous closed-loop control for type 1 diabetes: a randomised crossover trial. Lancet Digit. Health 2, e64–e73 (2020).
Laffel, L. M. et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical. JAMA 323, 2388–2396 (2020).
Laffel, L. M., Bailey, T. S., Christiansen, M. P., Reid, J. L. & Beck, S. E. Accuracy of a seventh-generation continuous glucose monitoring system in children and adolescents with type 1 diabetes. J. Diabetes Sci. Technol. 17, 962–967 (2022).
Lang, Q., Ye, B., Wang, N. & Li, G. Therapeutic effect of different insulin injections on type 1 diabetes mellitus. Biomed. Res. (India) 28, 9867–9870 (2017).
Lawson, M. L. et al. Timing of CGM initiation in pediatric diabetes: the CGM TIME Trial. Pediatr. Diabetes 22, 279–287 (2021).
Lukka, M., Tillmann, V. & Peet, A. Decreased need for correction boluses with universal utilisation of dual-wave boluses in children with type 1 diabetes. J. Clin. Med. 11, 1689 (2022). (no pagination).
Lynch, J. et al. The insulin-only bionic pancreas pivotal trial extension study: a multi-center single-arm evaluation of the insulin-only configuration of the bionic pancreas in adults and youth with type 1 diabetes. Diabetes Technol. Ther. 24, 726–736 (2022).
Messaaoui, A., Tenoutasse, S., Hajselova, L. & Crenier, L. Comparison between continuous versus flash glucose monitoring in children, adolescents, and young adults with type 1 diabetes: an 8-week prospective randomized trial. Diabetes Ther. 13, 1671–1681 (2022).
Messer, L. H. et al. Positive impact of the bionic pancreas on diabetes control in youth 6-17 years old with type 1 diabetes: a multicenter randomized trial. Diabetes Technol. Ther. 24, 712–725 (2022).
Michalak, A., Pagacz, K., Mlynarski, W., Szadkowska, A. & Fendler, W. Discrepancies between methods of continuous glucose monitoring in key metrics of glucose control in children with type 1 diabetes. Pediatr. Diabetes 20, 604–612 (2019).
Mueller-Godeffroy, E. et al. Psychosocial benefits of insulin pump therapy in children with diabetes type 1 and their families: the pumpkin multicenter randomized controlled trial. Pediatr. Diabetes 19, 1471–1480 (2018).
Nimri, R. et al. MD-Logic overnight type 1 diabetes control in home settings: a multicentre, multinational, single blind randomized trial. Diabetes Obes. Metab. 19, 553–561 (2017).
Palisaitis, E., El Fathi, A., von Oettingen, J. E., Haidar, A. & Legault, L. A meal detection algorithm for the artificial pancreas: a randomized controlled clinical trial in adolescents with type 1 diabetes. Diabetes Care 44, 604–606 (2021).
Petrovski, G. et al. 10-Day structured initiation protocol from multiple daily injection to hybrid closed-loop system in children and adolescents with type 1 diabetes. Acta Diabetol. 57, 681–687 (2020).
Petrovski, G. et al. Glycemic outcomes of advanced hybrid closed loop system in children and adolescents with type 1 diabetes, previously treated with multiple daily injections (MiniMed 780G System in T1D individuals, previously treated with MDI). BMC Endocr. Disord. 22, 80 (2022).
Pinsker, J. E. et al. Clinical evaluation of a novel CGM-informed bolus calculator with automatic glucose trend adjustment. Diabetes Technol. Ther. 24, 18–25 (2022).
Piona, C. et al. Non-adjunctive flash glucose monitoring system use during summer-camp in children with type 1 diabetes: the free-summer study. Pediatr. Diabetes 19, 1285–1293 (2018).
Raviteja, K. V., Kumar, R., Dayal, D. & Sachdeva, N. Clinical efficacy of professional continuous glucose monitoring in improving glycemic control among children with type 1 diabetes mellitus: an open-label randomized control trial. Sci. Rep. 9, 6120 (2019).
Renard, E. et al. Closed-loop driven by control-to-range algorithm outperforms threshold-low-glucose-suspend insulin delivery on glucose control albeit not on nocturnal hypoglycaemia in prepubertal patients with type 1 diabetes in a supervised hotel setting. Diabetes Obes. Metab. 21, 183–187 (2019).
Renard, E. et al. Outcomes of hybrid closed-loop insulin delivery activated 24/7 versus evening and night in free-living prepubertal children with type 1 diabetes: a multicentre, randomized clinical trial. Diabetes Obes. Metab. 24, 511–521 (2022).
Renard, E., Riveline, J. P., Hanaire, H. & Guerci, B. Reduction of clinically important low glucose excursions with a long-term implantable continuous glucose monitoring system in adults with type 1 diabetes prone to hypoglycaemia: the France Adoption Randomized Clinical Trial. Diabetes Obes. Metab. 24, 859–867 (2022).
Roberts, A. et al. Hybrid closed-loop therapy with a first-generation system increases confidence and independence in diabetes management in youth with type 1 diabetes. Diabet. Med 39, e14907 (2022). (no pagination).
Rose, S. et al. Use of intermittently scanned continuous glucose monitoring in young people with high-risk type 1 diabetes—Extension phase outcomes following a 6-month randomized control trial. Diabet. Med. 39, e14756 (2022).
Russell, S. J. et al. Multicenter, randomized trial of a bionic pancreas in type 1 diabetes. N. Engl. J. Med. 387, 1161–1172 (2022).
Schierloh, U. et al. Intermittent scanning glucose monitoring or predicted low suspend pump treatment: does it impact time in glucose target and treatment preference? The QUEST randomized crossover study. Front. Endocrinol. 13, 870916 (2022).
Schoelwer, M. J. et al. Safety and efficacy of initializing the control-IQ artificial pancreas system based on total daily insulin in adolescents with type 1 diabetes. Diabetes Technol. Ther. 22, 594–601 (2020).
Scott, E. S. et al. Continuous subcutaneous insulin infusion alters microRNA expression and glycaemic variability in children with type 1 diabetes. Sci. Rep. 11, 16656 (2021).
Seget, S., Rusak, E., Polanska, J. & Jarosz-Chobot, P. Prospective open-label single-arm, single-center follow-up study of the application of the advanced hybrid closed loop system in well-controlled children and adolescents with type 1 diabetes. Diabetes Technol. Ther. 24, 824–831 (2022).
Strategies to Enhance New CGM Use in Early Childhood (SENCE) Study Group. A randomized clinical trial assessing continuous glucose monitoring (CGM) use with standardized education with or without a family behavioral intervention compared with fingerstick blood glucose monitoring in very young children with type 1 diabetes. Diabetes care 44, 464–472 (2021).
Sherr, J. L. et al. Safety and performance of the Omnipod hybrid closed-loop system in adults, adolescents, and children with type 1 diabetes over 5 days under free-living conditions. Diabetes Technol. Ther. 22, 174–184 (2020).
Slover, R. H. et al. Accuracy of a fourth-generation continuous glucose monitoring system in children and adolescents with type 1 diabetes. Diabetes Technol. Ther. 20, 576–584 (2018).
Spaic, T. et al. Predictive hyperglycemia and hypoglycemia minimization: in-home evaluation of safety, feasibility, and efficacy in overnight glucose control in type 1 diabetes. Diabetes Care 40, 359–366 (2017).
Tauschmann, M. et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 392, 1321–1329 (2018).
Tauschmann, M. et al. Home use of day-and-night hybrid closed-loop insulin delivery in very young children: a multicenter, 3-week, randomized trial. Diabetes Care 42, 594–600 (2019).
Thabit, H. et al. Use of factory-calibrated real-time continuous glucose monitoring improves time in target and HbA(1c) in a multiethnic cohort of adolescents and young adults with type 1 diabetes: the MILLENNIALS study. Diabetes Care 43, 2537–2543 (2020).
Van Name, M. A. et al. Long-term continuous glucose monitor use in very young children with type 1 diabetes: one-year results from the sence study. J. Diabetes Sci. Technol. 17, 976–987 (2022).
Verbeeten, K. C. et al. Fear of hypoglycemia in children with type 1 diabetes and their parents: effect of pump therapy and continuous glucose monitoring with option of low glucose suspend in the CGM time trial. Pediatr. Diabetes 22, 288–293 (2021).
Wadwa, R. P., Laffel, L. M., Shah, V. N. & Garg, S. K. Accuracy of a factory-calibrated, real-time continuous glucose monitoring system during 10 days of use in youth and adults with diabetes. Diabetes Technol. Ther. 20, 395–402 (2018).
Ware, J. et al. Cambridge hybrid closed-loop algorithm in children and adolescents with type 1 diabetes: a multicentre 6-month randomised controlled trial. Lancet Digit. Health 4, e245–e255 (2022).
Ware, J. et al. Randomized trial of closed-loop control in very young children with type 1 diabetes. N. Engl. J. Med. 386, 209–219 (2022).
Weinzimer, S. A. et al. A comparison of postprandial glucose control in the medtronic advanced hybrid closed-loop system versus 670g. Diabetes Technol. Ther. 24, 573–582 (2022).
Wheeler, B. J. et al. Improved technology satisfaction and sleep quality with Medtronic MiniMed Advanced Hybrid Closed-Loop delivery compared to predictive low glucose suspend in people with Type 1 diabetes in a randomized crossover trial. Acta Diabetol. 59, 31–37 (2022).
Yi, Q. et al. Comparison of effectiveness of continuous subcutaneous insulin infusion with daily insulin injection in a Chinese population of Type I diabetic patients. Trop. J. Pharm. Res. 20, 1517–1522 (2021).
Aydın Şahin, D., Başpınar, O., Sülü, A., Karslıgil, T. & Kul, S. A comparison of the in vivo neoendothelialization and wound healing processes of three atrial septal defect occluders used during childhood in a nonrandomized prospective trial. Anatol. J. Cardiol. 18, 229–234 (2017).
Baird, C. W., Chavez, M., Backer, C. L., Galantowicz, M. E. & Del Nido, P. J. Preliminary results with a novel expanded polytetrafluoroethylene-based pulmonary valved conduit. Ann. Thorac. Surg. 114, 2314–2321 (2022).
Bergersen, L. et al. Harmony feasibility trial: acute and short-term outcomes with a self-expanding transcatheter pulmonary valve. JACC Cardiovasc. Inter. 10, 1763–1773 (2017).
Bhattacharjya, S. et al. Prospective concurrent head-to-head comparison of three different types of nitinol occluder device for transcatheter closure of secundum atrial septal defects. EuroIntervention 15, E321–E328 (2019).
Choi, Y. et al. The advantage of the mini-electrode-equipped catheter for the radiofrequency ablation of paroxysmal supraventricular tachycardia. J. Cardiovasc. Electrophysiol. 33, 2164–2171 (2022).
Delaney, J. W. et al. Covered CP stent for treatment of right ventricular conduit injury during melody transcatheter pulmonary valve replacement: results from the PARCS study. Circ: Cardiovasc. Inter. 11, e006598 (2018). (no pagination).
Gillespie, M. J., Javois, A. J., Moore, P., Forbes, T. & Paolillo, J. A. Use of the GORE® CARDIOFORM Septal Occluder for percutaneous closure of secundum atrial septal defects: results of the multicenter U.S. IDE trial. Catheter Cardiovasc. Inter. 95, 1296–1304 (2020).
Jones, T. K. et al. Long-term outcomes after melody transcatheter pulmonary valve replacement in the US investigational device exemption trial. Circ: Cardiovasc. Inter. 15, e010852 (2022).
Kenny, D. et al. 3-year outcomes of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position from the COMPASSION multicenter clinical trial. JACC: Cardiovasc. Inter. 11, 1920–1929 (2018).
Kenny, D. et al. A randomized, controlled, multi-center trial of the efficacy and safety of the Occlutech Figulla Flex-II Occluder compared to the Amplatzer Septal Occluder for transcatheter closure of secundum atrial septal defects. Catheter. Cardiovasc. Inter. 93, 316–321 (2019).
Morray, B. H. et al. Implantation of the Melody transcatheter pulmonary valve PB1016 in patients with dysfunctional right ventricular outflow tract conduits. Catheter. Cardiovasc. Inter. 93, 474–480 (2019).
Prodan, Z. et al. Initial clinical trial of a novel pulmonary valved conduit. Semin. Thorac. Cardiovasc. Surg. 34, 985–991 (2022).
US Food and Drug Administration. Pediatric Medical Devices, https://www.fda.gov/medical-devices/products-and-medical-procedures/pediatric-medical-devices (2023).
Pathak, K., Narang, C., Hwang, T. J., Espinoza, J. C. & Bourgeois, F. T. High-risk therapeutic devices approved by the US Food and drug administration for use in children and adolescents from 2016 to 2021. JAMA Pediatr. 177, 98–100 (2023).
Simmons, K. M. & Michels, A. W. Type 1 diabetes: a predictable disease. World J. Diabetes 6, 380–390 (2015).
Zhang, B., Guo, J. & Zhang, H. Design and analysis of crossover trials for investigating high-risk medical devices: a review. Contemp. Clin. Trials Commun. 30, 101004 (2022).
Mercieca-Bebber, R., King, M. T., Calvert, M. J., Stockler, M. R. & Friedlander, M. The importance of patient-reported outcomes in clinical trials and strategies for future optimization. Patient Relat. Outcome Meas. 9, 353–367 (2018).
Brinjikji, W. & Kallmes, D. F. How everybody wins when playing by the rules: the benefits of investigator-initiated industry-sponsored clinical trials. AJNR Am. J. Neuroradiol. 32, 427–429 (2011).
Biomedical Alliance Europe. Clinicians Concerned About Limited Availability of Medical Devices: Report of the Biomed Alliance Survey Conducted in Cooperation with the ESC and EFFORT, https://www.biomedeurope.org/images/news/2023/Report_survey_results_v3.pdf (2023).
Guerlich, K. et al. European Expert Recommendations on Clinical Investigation and Evaluation of High-Risk Medical Devices for Children. Acta Paediatr. (2023). https://doi.org/10.1111/apa.16919. Online ahead of print.
Hausner, E., Metzendorf, M. I., Richter, B., Lotz, F. & Waffenschmidt, S. Study filters for non-randomized studies of interventions consistently lacked sensitivity upon external validation. BMC Med. Res. Methodol. 18, 171 (2018).
Acknowledgements
We are grateful to all CORE-MD consortium members, who provided us with important insights into regulatory research and overall support to perform this project Task. We thank Dr. Benjamin Glicksberg and his co-authors for sharing with us the list of paediatric devices identified in the study by ref. 21, for the purpose of this review. We also thank the members of the European Commission Medical Device Coordination Group Task Force on Orphan Devices for helpful discussions and comments.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 965246 (CORE-MD Project). BK is the Else Kröner Senior Professor of Paediatrics at LMU—University of Munich, financially supported by Else Kröner-Fresenius-Foundation, LMU Medical Faculty and LMU University Hospitals. BPG is supported by a grant from the Alexander von Humboldt Foundation, Bonn, Germany. Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
KG, BPG, AGF, TM and BK designed the review. KG, BPG, MK and PD performed the screening and data extraction. KG, BPG and MK analysed the material. KG and BPG drafted and finalized the manuscript. All authors critically reviewed the manuscript and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
TM has engaged in paid clinical training for a notified body, National Standards Authority of Ireland.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guerlich, K., Patro-Golab, B., Dworakowski, P. et al. Evidence from clinical trials on high-risk medical devices in children: a scoping review. Pediatr Res 95, 615–624 (2024). https://doi.org/10.1038/s41390-023-02819-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02819-4
This article is cited by
-
Evidence from clinical trials on high-risk medical devices in children: a scoping review
Pediatric Research (2023)