Abstract
Background
Although most children experience mild symptoms during acute SARS-CoV-2 infection, some develop the severe post-COVID-19 complication, Multisystem Inflammatory Syndrome in Children (MIS-C). While acute presentations of COVID-19 and MIS-C have been well immunophenotyped, little is known about the lasting immune profile in children after acute illness.
Methods
Children 2 months–20 years of age presenting with either acute COVID-19 (n = 9) or MIS-C (n = 12) were enrolled in a Pediatric COVID-19 Biorepository at a single medical center. We deeply profiled humoral immune responses and circulating cytokines following pediatric COVID-19 and MIS-C.
Results
Twenty-one children and young adults provided blood samples at both acute presentation and 6-month follow-up (mean: 6.5 months; standard deviation: 1.77 months). Pro-inflammatory cytokine elevations resolved after both acute COVID-19 and MIS-C. Humoral profiles continue to mature after acute COVID-19, displaying decreasing IgM and increasing IgG over time, as well as stronger effector functions, including antibody-dependent monocyte activation. In contrast, MIS-C immune signatures, especially anti-Spike IgG1, diminished over time.
Conclusions
Here, we show the mature immune signature after pediatric COVID-19 and MIS-C, displaying resolving inflammation with recalibration of the humoral responses. These humoral profiles highlight immune activation and vulnerabilities over time in these pediatric post-infectious cohorts.
Impact
-
The pediatric immune profile matures after both COVID-19 and MIS-C, suggesting a diversified anti-SARS-CoV-2 antibody response after resolution of acute illness.
-
While pro-inflammatory cytokine responses resolve in the months following acute infection in both conditions, antibody-activated responses remain relatively heightened in convalescent COVID-19.
-
These data may inform long-term immunoprotection from reinfection in children with past SARS-CoV-2 infections or MIS-C.
Similar content being viewed by others
Introduction
Over 15.3 million children have been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as of January 2023 (https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/), and while many children experience asymptomatic or paucisymptomatic infection, more than 180,000 have been hospitalized for acute coronavirus disease 2019 (COVID-19) (https://services.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/; https://covid.cdc.gov/covid-data-tracker/#datatracker-home) and over 9300 have developed the severe, post-acute illness, Multisystem Inflammatory Syndrome in Children (MIS-C) (https://www.cdc.gov/mis-c/hcp/).1 Additionally, there is an increasing recognition that after COVID-19, many individuals, including children, experience persistent symptoms.2,3,4 While significant advances have been made to define the symptomatology and immunopathology associated with acute COVID-19 and MIS-C,5,6,7,8,9 little is known about lasting humoral or cytokine profiles following acute illness in children.
During acute infection, the induction of functional IgG is central to containment of infection, and the acute pediatric humoral response to SARS-CoV-2 is comparable to profiles observed in mildly ill adults.7,10 However, in MIS-C, a profound, expansive immunoglobulin response is seen.7,11,12 Defining lasting immune signatures to assess whether differences in pediatric humoral signatures following pediatric SARS-CoV-2 infection persist over time is important, not only to probe for signs of dysfunctional inflammation, but also to ascertain immunoprotection against re-infection with SARS-CoV-2, and to gain insight into the immunologic impact of SARS-CoV-2 infection in children.
To define the evolution of immune profiles over time of pediatric patients with acute COVID-19 or MIS-C, we utilized a systems serology approach to deeply profile humoral responses at acute presentation and 6 months post-infection, in addition to circulating cytokine responses. While cytokine profiles resolved following acute illness, humoral signatures displayed increased antibody-activated functional capacity after COVID-19, suggesting a recalibration of immune responses in recovery from acute illness. The post-infection immune profile in MIS-C, however, showed decreased anti-SARS-CoV-2 antibody activation and functional response following acute illness. The resulting data define long-lasting immunoprofiles following acute SARS-CoV-2 infection and MIS-C in children.
Methods
Study design and sample collection
Pediatric patients ranging from 2 months to 20 years of age diagnosed with COVID-19 or MIS-C were enrolled in the Massachusetts General Hospital (MGH) Pediatric COVID-19 Biorepository (IRB #2020P000955).13 Informed consent, and where appropriate, assent, was verbally obtained from participants and/or parents/guardians in accordance with IRB guidelines for blood specimen collection and questionnaire administration. Acute COVID-19 diagnosis was determined by a nasopharyngeal swab positive for SARS-CoV-2 by clinical quantitative PCR testing, and MIS-C diagnosis was confirmed by meeting 2020 CDC criteria (https://www.cdc.gov/mis/mis-c.html). Demographic information was obtained from medical records, and blood was collected by venipuncture. The post-infection follow-up time point was determined by the number of months passed between the date of initial COVID-19 or MIS-C evaluation or diagnosis and post-infection blood specimen collection. All samples were matched, meaning the participant provided a blood specimen during acute illness, as well as at follow-up (6 months post-infection).
IgG1, IgM, and IgA1 titers and Fc binding measured by Luminex
All participants provided matched blood specimens at acute illness and follow-up. SARS-CoV-2-S-specific antibody isotypes were analyzed from sera by Luminex multiplexing as described in Brown—immune methods. The antigens were carboxy-coupled to Luminex microspheres provided by Luminex Corp, Austin, TX and were then incubated with IgG1, IgM, and IgA1 polyclonal plasma samples. A second antibody or FcgR tagged with fluorophore was used to probe the SARS-CoV-2-specific isotypes. Relative concentrations were analyzed by flow cytometry, given by mean fluorescent intensity (MFI).
Antibody effector functions
For the functional assays, antigens were biotinylated and coupled to fluorescent neutravidin beads (Thermo Fisher). Beads were incubated with plasma samples to form immune complexes in 96-well plates for 2 h at 37 °C. Immune complexes were washed and incubated with guinea pig complement factor for 20 min at 37 °C (for antibody-dependent complement deposition (ADCD)), primary neutrophils from ACK lysed blood for 1 h at 37 °C (for antibody-dependent neutrophil phagocytosis (ADNP)) or with THP-1 monocytes for 16 h at 37 °C (for ADNP). ADCD was detected on beads using a polyclonal anti-guinea pig C3-FITC antibody (ADCD) and neutrophils stained for CD66 expression using a respective antibody (Biolegend) after the respective incubation. All samples were fixed with 4% para-formaldehyde prior to analysis on an Ique analyzer.
Statistical analyses
A paired non-parametric t test was used to test for statistical significance between acute and follow-up time points of the same group. An unpaired non-parametric t test was used to test for statistical difference between the groups. Prism software (Prism 9, Graphpad Software, San Diego, CA) was used to analyze and graph data. SystemseRology (v.1.0) and ropls (v.1.22.0) packages in R (v.3.6) and R Studio (v.1.3) were used to perform and visualize LASSO and PLS-DA and the network (v.1.1) package was used for the network analysis.
Results
In-depth humoral profiling, paired with cytokine analysis, was performed on 21 children infected with SARS-CoV-2 during the first wave of the pandemic (April 2020–January 2021), each of whom provided matched blood samples from acute illness and convalescence. All participants met CDC diagnostic criteria for acute COVID-19 (https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/clinical-considerations-diagnosis.html) (n = 9, 100%) and MIS-C (https://www.cdc.gov/mis/mis-c.html) (n = 12, 100%). Children with a history of COVID-19 (n = 9) were an average age of 16.4 years at acute illness, whereas children with past MIS-C (n = 12) were on average 5.9 years old at acute presentation (Table 1). Sex was equally distributed in both groups, and 78% (n = 7) of children with COVID-19 and 58% (n = 7) with MIS-C identified as Hispanic. Convalescent blood samples were collected at a mean 7.3 months post-acute infection for children with COVID-19 (SD 0.8) and 5.9 months post-onset of symptoms of MIS-C (SD 2.1). Clinical characteristics, disease course, and treatments for each participant are detailed in Supplemental Table 1. Of note, none of the participants in this study received a SARS-CoV-2 vaccine prior to sample collection.
Cytokine profiles of pediatric COVID-19 and MIS-C normalize following acute illness
Distinct plasma diagnostic markers and cytokine profiles are associated with severity in acute phases of both pediatric COVID-19 and MIS-C.12,14 IP-10, IL-8, and D-Dimer plasma concentrations are notably elevated in acute pediatric COVID-19 in comparison to convalescence, while IL-6, TNFα, and MIP-1b are the most elevated in acute MIS-C and completely resolve post-illness (Fig. 1a). Using all cytokine data in a principal component analysis (PCA), a clear separation was seen between acute and convalescent COVID-19 and MIS-C (Fig. 1b), driven largely by the distinct cytokine storm11 that takes place during acute MIS-C. Even in those who experienced pediatric COVID-19, the cytokine profile during acute illness also separated from its respective follow-up samples (Fig. 1b). IP-10, one of the most elevated cytokines in MIS-C, demonstrates a steady cytokine decline at follow-up in both children with COVID-19 and MIS-C (Fig. 1d), and there is not a clear separation of the two groups in the PCA (Fig. 1b). IL-6, the highest cytokine seen in acute COVID-19, shows a similar trend, where a significant decline is seen in both groups of children during recovery (Fig. 1c). At the acute time point, we found IL-8, IL-18, IL-10 and TNFα to be elevated in MIS-C, albeit not significantly for the latter two, in our MIS-C cohort. No difference was observed for other tested cytokines or chemokines including IL-1b, MIP-1b, GM-CSF, RANTES, as well as for D-Dimer, and myeloperoxidase (MPO) (Supplemental Fig. 1). While innate activation and cytokine activation contribute to acute presentation of pediatric COVID-19 and MIS-C, the resolution of most cytokines at convalescence indicates a restructuring and restabilization of immune responses.
Maturing humoral profiles display ongoing effector activation following COVID-19
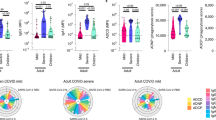
SARS-CoV-2-specific antibodies seen in pediatric COVID-19 can mediate protection from severe disease and may prevent re-infection. Beyond binding or neutralization, antibodies can mediate additional Fc effector functions including the ability to fix complement (ADCD) or recruit neutrophil (ADNP) or monocyte (antibody-dependent cellular (monocyte) phagocytosis (ADCP)) phagocytosis, which might be critical in the protection from disease or re-infection. To begin to understand the longevity of antibodies in pediatric COVID-19 as compared to antibodies in MIS-C, we deeply profiled the SARS-CoV-2-Spike (S)-specific titer, Fc receptor profiling, and Fc-mediated antibody function at acute disease and convalescence. While the days to symptom onset at sample collection for acute disease were comparable between COVID-19 and MIS-C, the former developed immune responses rather quickly after infection, while the initial viral exposure for MIS-C happened presumably weeks before disease onset. IgM followed expected trajectories (Fig. 2a); at the acute time point, a few days after infection, the COVID-19 group was split with some individuals already seroconverted for IgG and IgA, whereas others did not yet seroconvert, marked by the absence of antibodies of all subclasses (Fig. 2b, c). In contrast, all MIS-C patients had detectable S-specific antibodies during the acute presentation pointing to the temporal differences between MIS-C and COVID-19. However, no difference between S-specific IgM and IgA antibodies was noted at the acute time point. We did observe higher ADCD and ADCP activity to SARS-CoV-2-S, which was likely driven by IgG1 antibodies in acute MIS-C (Fig. 2d, f).
Anti-Spike IgM, IgA1, and IgG1 were profiled during acute COVID-19 and MIS-C and at follow-up (a–c). Effector function of antibody responses were also characterized at acute disease and convalescence were also analyzed (d–f). ADCD antibody-dependent complement deposition, ADNP antibody-dependent neutrophil phagocytosis, ADCP antibody-dependent cellular (monocyte) phagocytosis.
While the temporal difference was obvious at the acute time point, we expected the follow-up time points to be more comparable between the groups, and indeed, no significant difference between SARS-CoV-2-S-specific responses was observed between the groups (Fig. 2a–f). However, children with MIS-C tended to have lower titers, Fc receptor-binding profiles, and mediated functions. IgG wanes over time in MIS-C but remains relatively elevated at convalescence in COVID-19 (Fig. 2c).
Next, we aimed to compare temporal SARS-CoV-2 responses more comprehensively, including responses to Nucelocapsid (N) and receptor-binding domain (RBD) across the groups. Overall, the anti-S response dampens in MIS-C for most IgG subgroups, with the exception of IgG4, where an expansion in response is seen post-infection (Fig. 3a). The resolution of anti-SARS-CoV-2 antibody responses seen in convalescent MIS-C contrasted with those of children with past COVID-19, where rising levels of anti-S IgG are seen at follow-up (Fig. 3a). A paralleled decline in anti-N Ig is seen in both COVID-19 and MIS-C (Fig. 3a). Interestingly, IgA to Nucleocapsid (N) antigen was elevated in acute pediatric COVID-19 (Fig. 3a), and consequently, so were the N-specific antibody-activated neutrophils, which express the IgA binding Fcα-receptor (FcαR) (Fig. 3b). However, this nucleocapsid, neutrophil-activating mucosal immune response declined in follow-up and was not observed in MIS-C (Fig. 3b). Anti-S Fc effector functions such as monocyte phagocytosis (ADCP) and the ability to fix complement (ADCD) diversified in convalescence in COVID-19 (Fig. 3c). Interestingly, the MIS-C immune profile, particularly Fc effector responses, diminish at follow-up, likely due to the delayed onset of symptoms in acute illness, a compensation for dramatic acute responses as well as anti-inflammatory treatments during illness15,16 (Fig. 3c). However, S-specific monocyte and complement activation remain relatively prominent in comparison to acute illness (Fig. 3c). Using a simple linear regression model, we were unable to detect any impact of age or sex on changes in humoral profiles over time for either pediatric COVID-19 or MIS-C.
Changes in anti-SARS-CoV-2-S antibody responses were visualized to show the expansion and/or contraction of anti-SARS-CoV-2 specific immunoglobulin titers (a), functional Fc-binding capacity (b), and effector responses (c) in acute presentation of pediatric COVID-19 or MIS-C and six-month follow-up.
Pediatric COVID-19 and MIS-C display distinct humoral profiles over time
We next used multivariate machine learning tools to investigate whether distinct humoral antibody features can distinguish between MIS-C and COVID-19 in children. We built a partial least-squares discriminant analysis (PLS-DA) model with a minimal set of least absolute shrinkage and selection operator (LASSO) selected features (Fig. 4a, b). LASSO selects features that are highly correlated to non-selected features and these features explain the highest variability in the data set. Of the immune features explored that comprise the responses in acute pediatric COVID-19 and MIS-C, only five were necessary to reveal distinct immunologic compositions. Of the selected features, monocyte phagocytosis and Fc receptor binding, involved in the activation of macrophages, were enriched in MIS-C (Fig. 4a) and might play a significant role in the systemic inflammatory overreaction seen in acute illness. In contrast, IgM and FcαR binding were enriched in pediatric COVID-19 (Fig. 4a), pointing to the early time point in the disease course. LASSO only selects non-correlated features; therefore, to gain a deeper understanding of the associations of the selected immune features to the overall humoral response, a co-correlation network was built. For the acute time point, two clusters of IgA and IgM features both enriched in COVID-19 appeared (Fig. 4a), highlighting the acute phase of the response. In contrast, a large cluster of FcγR-binding titer and antibody functions to different antigens and all enriched in MIS-C was formed (Fig. 4a), reflecting the hyperinflammatory response of MIS-C. Monocytes have been associated with disease pathology in MIS-C, and interestingly, ADCP to Spike and Nucleocapsid, both of which are enriched in MIS-C, created a separate cluster (Fig. 4a).
In follow-up, the immune responses appear less coordinated, but still display a separate distribution in the PLS-DA (Fig. 4b). Interestingly, neutrophil phagocytosis and neutrophil-recruiting IgA specific to Nucleocapsid were selected by the LASSO and enriched in COVID-19, whereas no features were selected in MIS-C at the follow-up time point (Fig. 4b). This highlights a diverging post-acute response in COVID-19 and MIS-C.
Next, to further explore the coordination of the humoral immune response in COVID-19 and MIS-C at both the acute and follow-up time points, we analyzed correlations of antibody titer and FcγR recruitment. During acute illness for both groups, titers to the three analyzed SARS-CoV-2 antigens were highly correlated to the ability to recruit and bind to FcγR (Fig. 5a, b). In acute pediatric COVID-19, these associations were SARS-CoV-2 specific, as there were no strong associations seen for other respiratory pathogens (Fig. 5a). In the MIS-C group, however, we observed an increased inflammatory potential marked by strong FcγR binding to a variety of pathogens during acuity (Fig. 5b). This may indicate a nonspecific humoral immune response that contributes to disease pathology.7 At follow-up, antibody associations decline in MIS-C as expected with subsiding inflammation both from natural resolution of illness as well as from the use of anti-inflammatory treatments (Fig. 5b), indicating resolution of disease and the predominance of less inflammatory antibodies.
Discussion
As cases of SARS-CoV-2 infection in children continue to rise, efforts are needed to define the lasting immunologic impact of SARS-CoV-2 infection on children. This study presents detailed findings from a long-term follow-up pediatric COVID-19 biospecimen repository, describing in-depth humoral responses of children and adolescents with prior acute COVID-19 and MIS-C. While children with acute COVID-19 display humoral activation distinct from the highly inflamed profile seen in MIS-C, immunoglobulin levels followed expected trajectories and convalescent stages of both COVID-19 and MIS-C display resolving inflammation with recalibration of the humoral responses. These findings provide insight into longevity and lasting functionality of anti-SARS-CoV-2 antibody responses in children and offer insight into distinct immune profiles following pediatric COVID-19 and MIS-C.
Following infection with SARS-CoV-2, antibody levels increase in a predictable fashion in an immunocompetent individuals.10,17 In the acute illness of MIS-C, however, excessive humoral responses are seen,7,11 driven by trafficking of SARS-CoV-2 Spike/S1 superantigen18 across a permeable gastrointestinal mucosal barrier.14 Interestingly, an augmented contraction of anti-SARS-CoV-2 immunoglobulins, along with a reduction in antibody-mediated functional and effector responses, is seen in MIS-C, differing from humoral trajectories following acute COVID-19 in children. This contracture of antibody responses in MIS-C may result from the high dose steroids, intravenous immunoglobulin or other immunosuppressive therapy used during the acute MIS-C illness or immune exhaustion following superantigen exposure.
Several studies have shown that SARS-CoV-2 can be shed in the stool for months after the acute illness in both children and adults,14,19 suggesting that persistent antigen exposure in the gastrointestinal tract could remain present even months after resolution of disease. While we did not study mucosal immune responses, we did not detect elevated circulating levels of IgA in the follow-up period for either acute COVID-19 or MIS-C. However, persistent respiratory and gastrointestinal symptoms have been commonly reported by both children with prior COVID-19 and MIS-C20,21 warranting further investigation of changes in mucosal immunity post infection.
Interestingly, while antibody levels and Fc-binding, for the most part, decrease in convalescence, antibody-mediated complement activation plus antibody-mediated monocyte and neutrophil phagocytosis are increased after COVID-19. During acute COVID-19, there is a robust anti-nucleocapsid IgA-mediated neutrophil activation. Following acute COVID-19, however, anti-Spike-mediated cellular activation predominates, which is interesting especially as many patients with long COVID-19 have detectable Spike antigen in their blood.22 It is possible that following COVID-19, circulating Spike-immune complexes, if present, may result in cellular responses that could contribute to lasting symptomatology.
Although serologic responses after COVID-19 are sustained in recovery, as seen by increased SARS-CoV-2-specific IgG in convalescence, the degree and durability of pediatric humoral responses after natural SARS-CoV-2 infection are lower when compared to those induced by SARS-CoV-2 mRNA vaccination.23 Further, it has been shown that vaccination confers better cross-reactivity against variants of concern than natural infection, and re-infection following natural infection is not uncommon. This suggests that children carry incomplete and waning immunoprotection after natural infection with SARS-CoV-2. In MIS-C, we show a notable dampening in the immune response, potentially due to immune-suppressive treatments such as steroids used to address complications during acute illness, that further highlights this vulnerability in the pediatric population to reinfection after COVID-19 and MIS-C. SARS-CoV-2 vaccines are now recommended for children ages 6 months and older, and completion of a mRNA vaccine series is highly effective in preventing COVID-19 and MIS-C, as well as progression to severe disease and post-acute sequelae of COVID-19 in children.24,25,26 Vaccination will continue to be a strong public health strategy targeted to protect adult and pediatric populations from infection, transmission, and the long-term complications from COVID-19.
Our study has several limitations: samples reflect immune responses at the acute phase of illness, where the pediatric profiles are still maturing, and 6–7 months following initial disease. While we show durability of humoral responses, we likely have missed the true peak of humoral response and are underestimating the decline in antibodies over time. Additionally, this study consists of a modest sample size, as all samples were collected during the first 6 months of the COVID-19 pandemic, making collecting matched samples challenging. Furthermore, SARS-CoV-2 viral sequences were not analyzed, therefore variant-specific immunogenicity was not considered in our discussion of long-term humoral effects of SARS-CoV-2 infection and MIS-C. T and memory B cell responses were not measured in this analysis, nor were direct associations between humoral responses and persistent symptoms after COVID-19 and MIS-C. Further, protective and pathogenic autoantibody patterns have been reported to impact the development of post-COVID-19 conditions such as Long COVID.27,28,29 We did not assess for the development of autoantibodies in our cohort, but this would be an important area of future study, particularly in children who develop post-COVID-19 conditions. Additional studies analyzing the changes in immune responses after SARS-CoV-2 infections of different variants, the characteristics of autoimmunity after SARS-CoV-2 infection, the correlations with ongoing symptomatology, as well as the long-term cellular immune responses after COVID-19 and MIS-C are needed.
Although this is a small study from a single medical center, matched pediatric samples provide early and critical insights into long-term impact of natural SARS-CoV-2 infection, which is crucial to understand as pediatric COVID-19 cases are rising amidst another surge. Additionally, samples were collected following the first wave of the COVID-19 pandemic, prior to the availability of mRNA vaccines, which may impact immunologic responses over time. Subsequent waves of the pandemic, including those caused by SARS-CoV-2 variants Delta and Omicron, may produce distinct immune signatures that need to be studied over time, and the impact of vaccination on these infected immunoprofiles, as well as post-infectious symptoms, needs to be characterized.
Conclusion
Our findings provide key insights into the immunological disease course and clinical characteristics of children with prior COVID-19 and MIS-C. Immune recovery suggests resolution of acute illness, but possible antibody-mediated cellular activation, especially following COVID-19, which warrants further investigation to determine if there is any association with long COVID-19 symptomatology. The underlying humoral immune responses at follow-up in children with past-SARS-CoV-2 infection and MIS-C ultimately wane over time and vaccination will be needed to bolster immune responses.
Data availability
All relevant data are included in this manuscript. Data is available upon reasonable request.
References
Feldstein, L. R. et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 383, 334–346 (2020).
Stephenson, T. et al. Long COVID and the mental and physical health of children and young people: national matched cohort study protocol (the CLoCk study). BMJ Open 11, e052838 (2021).
Soriano, J. B. et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 22, e102–e107 (2022).
Buonsenso, D. et al. Preliminary evidence on long COVID in children. Acta Paediatr. 110, 2208–2211 (2021).
Sacco, K. et al. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat. Med. 28, 1050–1062 (2022).
Yonker, L. M. et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J. Pediatr. 227, 45.e5–52.e5 (2020).
Bartsch, Y. C. et al. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat. Med. 27, 454–462 (2021).
Cotugno, N. et al. Virological and immunological features of SARS-CoV-2-infected children who develop neutralizing antibodies. Cell Rep. 34, 108852 (2021).
Feldstein, L. R. et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 325, 1074–1087 (2021).
Weisberg, S. P. et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 22, 25–31 (2021).
Porritt, R. A. et al. The autoimmune signature of hyperinflammatory multisystem inflammatory syndrome in children. J. Clin. Investig. 131, e151520 (2021).
Vella, L. A. et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci. Immunol. 6, eabf7570 (2021).
Lima, R. et al. Establishment of a pediatric COVID-19 biorepository: unique considerations and opportunities for studying the impact of the COVID-19 pandemic on children. BMC Med. Res. Methodol. 20, 228 (2020).
Yonker, L. M. et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J. Clin. Investig. 131, e149633 (2021).
Ouldali, N. et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA 325, 855–864 (2021).
Lapp, S. A. et al. Serologic and cytokine signatures in children with multisystem inflammatory syndrome and coronavirus disease 2019. Open Forum Infect. Dis. 9, ofac070 (2022).
Long, Q. X. et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 26, 845–848 (2020).
Porritt, R. A. et al. HLA class I-associated expansion of TRBV11-2 T cells in multisystem inflammatory syndrome in children. J. Clin. Investig. 131, e146614 (2021).
Natarajan, A. et al. Gastrointestinal symptoms and fecal shedding of SARS-CoV-2 RNA suggest prolonged gastrointestinal infection. Medicine 3, 371–387.e379 (2022).
Ashkenazi-Hoffnung, L. et al. Long COVID in children: observations from a designated pediatric clinic. Pediatr. Infect. Dis. J. 40, e509–e511 (2021).
Borch, L., Holm, M., Knudsen, M., Ellermann-Eriksen, S. & Hagstroem, S. Long COVID symptoms and duration in SARS-CoV-2 positive children - a nationwide cohort study. Eur. J. Pediatr. 181, 1597–1607 (2022).
Swank, Z. et al. Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin. Infect. Dis. 76, e487–e490 (2023).
Bartsch, Y. C. et al. SARS-CoV-2 mRNA vaccination elicits robust antibody responses in children. Sci. Transl. Med. 14, eabn9237 (2022).
Levy, M. et al. Multisystem inflammatory syndrome in children by COVID-19 vaccination status of adolescents in France. JAMA 327, 281–283 (2022).
Olson, S. M. et al. Effectiveness of BNT162b2 vaccine against critical Covid-19 in adolescents. N. Engl. J. Med. 386, 713–723 (2022).
Zambrano, L. D. et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA vaccination against multisystem inflammatory syndrome in children among persons aged 12-18 years - United States, July-December 2021. MMWR Morb. Mortal. Wkly Rep. 71, 52–58 (2022).
Wallukat, G. et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 4, 100100 (2021).
Son, K. et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur. Respir. J. 61, 2200970 (2023).
Muri, J. et al. Autoantibodies against chemokines post-SARS-CoV-2 infection correlate with disease course. Nat. Immunol. 24, 604–611 (2023).
Acknowledgements
We thank the children and their families for participating in this research and Nancy Zimmerman, Mark and Lisa Schwartz, Terry and Susan Ragon, and the SAMANA Kay MGH Research Scholars award for their support.
Funding
We acknowledge support from Massachusetts General Hospital for Children, the Ragon Institute of MGH, MIT, and Harvard, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, and the March of Dimes. We also received support from an anonymous donor (financial support), the NIH (3R37AI080289-11S1, R01AI146785, U19AI42790-01, U19AI135995-02, 1U01CA260476-01, CIVIC75N93019C00052, 5K08HL143183, R01HD100022-02S2) and the Gates Foundation Global Health Vaccine Accelerator Platform funding (OPP1146996 and INV-001650).
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data or analysis and interpretation of data: M.D.B., Y.C.B., J.P.D., B.P.B., M.L., J.K., A.S.K., A.G.E., A.F., G.A., L.M.Y. Drafting the article or revising it critically for important intellectual content: M.D.B., Y.C.B., A.S.K., A.G.E., A.F., G.A., L.M.Y. Final approval of the version to be published: M.D.B., Y.C.B., J.P.D., B.P.B., M.L., J.K., A.S.K., A.G.E., A.F., G.A., L.M.Y.
Corresponding author
Ethics declarations
Competing interests
G.A. is a V.P. at Moderna, a founder and equity holder of Seromyx Systems, and an employee and equity holder of Leyden Labs. G.A.’s interests were reviewed and are managed by MGH and Partners HealthCare in accordance with their conflict-of-interest policies. A.G.E. reported serving as a medical advisor for Mirvie, Inc. and receiving research funding from Merck & Co. outside of the scope of the submitted work. No other disclosures were reported. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Ethics approval and consent to participate
Informed consent, and where appropriate, assent, was verbally obtained from participants and/or parents/guardians involved in the study. The study was approved by the Institutional Review Board of Massachusetts General Brigham (IRB #2020P000955, approved 12/14/20).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Burns, M.D., Bartsch, Y.C., Davis, J.P. et al. Long-term humoral signatures following acute pediatric COVID-19 and Multisystem Inflammatory Syndrome in Children. Pediatr Res 94, 1327–1334 (2023). https://doi.org/10.1038/s41390-023-02627-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02627-w