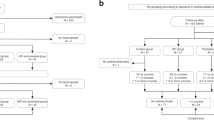
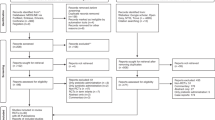
Abstract
Background
Studies investigating neonatal outcomes following intrapartum antibiotic exposure show conflicting results.
Methods
Data were collected prospectively in pregnancy to 1-year-of-age, from 212 mother-infant pairs. Adjusted multivariable regression models estimated relationships following exposure to intrapartum antibiotics among vaginally-born, full-term infants and outcomes related to growth, atopic disease, gastrointestinal symptoms, and sleep at 1-year.
Results
Intrapartum antibiotic exposure (n = 40) was not associated with mass, ponderal index, BMI z-score (1- year), lean mass index (5-months) or height. Antibiotic exposure in labour ≥4-h was associated with increase in fat mass index at 5-months (β 0.42 [95% CI: 0.03, 0.80], p = 0.03). Intrapartum antibiotic was associated with atopy in the first year (OR: 2.93 [95% CI: 1.34, 6.43], p = 0.007). Antibiotic exposure during intrapartum or day 1–7 was associated with newborn fungal infection requiring antifungal therapy (OR 3.04 [95% CI: 1.14, 8.10], p = 0.026), and number of fungal infections (IRR: 2.90 [95% CI: 1.02, 8.27], p = 0.046).
Conclusion
Intrapartum and early life exposure to antibiotics were independently associated with measures of growth, atopy, and fungal infections suggesting that intrapartum and early neonatal antibiotics be used prudently following careful risk-benefit analysis.
Impact
This prospective study:
-
Shows a shift in fat mass index at 5 months associated with antibiotic administration ≥4 h in labour; an earlier age than previously reported;
-
Shows atopy reported less frequently among those not exposed to intrapartum antibiotics;
-
Supports earlier research of increased likelihood of fungal infection following exposure to intrapartum or early-life antibiotics;
-
Adds to growing evidence that antibiotics used intrapartum and in early neonatal periods influence longer-term outcomes for infants.
-
Suggests that use of intrapartum and early neonatal antibiotics should be used prudently after careful consideration of risk and benefit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Access to the data included in this manuscript is available through direct contact with the Corresponding Author.
Change history
09 June 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41390-023-02613-2
References
Gosalbes, M. J. et al. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin. Exp. Allergy 43, 198–211 (2013).
Tsuji, H. et al. Molecular monitoring of the development of intestinal microbiota in Japanese infants. Benef. Microbes 3, 113–125 (2012).
Jaureguy, F. et al. Effects of intrapartum penicillin prophylaxis on intestinal bacterial colonization in infants. J. Clin. Microbiol. 42, 5184–5188 (2004).
Stearns, J. C. et al. Intrapartum antibiotics for GBS prophylaxis alter colonization patterns in the early infant gut microbiome of low risk infants. Sci. Rep. 2017 71 7, 1–9 (2017).
Ainonen, S. et al. Antibiotics at birth and later antibiotic courses: effects on gut microbiota. Pediatr. Res. (2021) https://doi.org/10.1038/S41390-021-01494-7.
Azad, M. et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG 123, 983–993 (2016).
Coker, M. et al. Specific class of intrapartum antibiotics relates to maturation of the infant gut microbiota: a prospective cohort study. BJOG 127, 217–227 (2020).
Corvaglia, L. et al. Influence of intrapartum antibiotic prophylaxis for group B streptococcus on gut microbiota in the first month of life. J. Pediatr. Gastroenterol. Nutr. 62, 304–308 (2016).
Weeks, J. W. et al. Persistence of penicillin G benzathine in pregnant group B streptococcus carriers. Obstet. Gynecol. 90, 240–243 (1997).
Cox, L. M. et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell 158, 705–721 (2014).
Cho, I. et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012 488, 621–626 (2012).
Murk, W., Risnes, K. R. & Bracken, M. B. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics 127, 1125–1138 (2011).
Shao, X. et al. Antibiotic exposure in early life increases risk of childhood obesity: a systematic review and meta-analysis. Front. Endocrinol. (Lausanne). 8, 170 (2017).
Mukhopadhyay, S. et al. Intrapartum group B Streptococcal prophylaxis and childhood weight gain. Arch. Dis. Child. Fetal Neonatal Ed. 106, 649–656 (2021).
Metz, T. D. et al. Exposure to group B Streptococcal antibiotic prophylaxis and early childhood body mass index in a vaginal birth cohort. J. Matern. Fetal Neonatal Med. 33, 3318–3323 (2020).
Koebnick, C. et al. Intrapartum antibiotic exposure and body mass index in children. Clin. Infect. Dis. 73, e938–e946 (2021).
Dhudasia, M. B. et al. Intrapartum group B streptococcal prophylaxis and childhood allergic disorders. Pediatrics 147, e2020012187 (2021).
Wohl, D. L., Curry, W. J., Mauger, D., Miller, J. & Tyrie, K. Intrapartum antibiotics and childhood atopic dermatitis. J. Am. Board Fam. Med. 28, 82–89 (2015).
May, S. M., Hartz, M. F., Joshi, A. Y. & Park, M. A. Intrapartum antibiotic exposure for group B Streptococcus treatment did not increase penicillin allergy in children. Ann. Allergy, Asthma Immunol. 116, 134–138 (2016).
Dowhower Karpa, K. et al. A retrospective chart review to identify perinatal factors associated with food allergies. Nutr. J. 11, 87 (2012).
Dinsmoor, M. J., Viloria, R., Lief, L. & Elder, S. Use of intrapartum antibiotics and the incidence of postnatal maternal and neonatal yeast infections. Obstet. Gynecol. 106, 19–22 (2005).
Seedat, F. et al. Adverse events in women and children who have received intrapartum antibiotic prophylaxis treatment: a systematic review. BMC Pregnancy Childbirth 17, 247 (2017).
Nogacka, A. M. et al. Early microbiota, antibiotics and health. Cell. Mol. Life Sci. 75, 83–91 (2018).
Le Doare, K. et al. Uncertainties in screening and prevention of group B streptococcus disease. Clin. Infect. Dis. 69, 720–725 (2019).
Zimmerman, P. & Curtis, N. Effect of intrapartum antibiotics on the intestinal microbiota of infants: a systematic review. Arch. Dis. Child. Fetal Neonatal Ed. 105, 201–208 (2020).
Spaetgens, R. et al. Perinatal antibiotic usage and changes in colonization and resistance rates of group B streptococcus and other pathogens. Obstet. Gynecol. 100, 525–533 (2002).
Persaud, R. R. et al. Perinatal antibiotic exposure of neonates in Canada and associated risk factors: a population-based study. J. Matern. Fetal Neonatal Med. 28, 1190–1195 (2015).
Seale, A. C. et al. Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin. Infect. Dis. 65, S200–S219 (2017).
Money, D. & Allen, V. M. Infectious Diseases Committee. The prevention of early-onset neonatal group B streptococcal disease. J. Obstet. Gynaecol. Can. 35, 939–948 (2013).
Prevention of Group B. Streptococcal early-onset disease in newborns: ACOG committee opinion summary, number 782. Obstet. Gynecol. 134, 206–210 (2019).
Verani, J. R., McGee, L. & Schrag, S. J. Division of bacterial diseases, national center for immunization and respiratory diseases, centers for disease control and prevention (CDC). Prevention of perinatal group B streptococcal disease–revised guidelines from CDC. MMWR Recomm. Rep. 59, 1–32 (2010).
Edmond, K. M. et al. Group B streptococcal disease in infants aged younger than 3 months: Systematic review and meta-analysis. Lancet 379, 547–556 (2012).
Simioni, J. et al. A comparison of intestinal microbiota in a population of low-risk infants exposed and not exposed to intrapartum antibiotics: The Baby & Microbiota of the Intestine cohort study protocol. BMC Pediatr. 16, 183 (2016).
WHO Multicentre Growth Reference Study Group:WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 450, 76–85 (2006).
Muraro, A. et al. Dietary prevention of allergic diseases in infants and small children. Part II. Evaluation of methods in allergy prevention studies and sensitization markers. Definitions and diagnostic criteria of allergic diseases. Pediatr. Allergy Immunol. 15, 196–205 (2004).
Asher, M. I. et al. International study of asthma and allergies in childhood (ISAAC): rationale and methods. Eur. Respir. J. 8, 483–491 (1995).
Sadeh, A. A brief screening questionnaire for infant sleep problems: validation and findings for an Internet sample. Pediatrics 113, e570–e577 (2004).
Kleinman, L. et al. The infant gastroesophageal reflux questionnaire revised: development and validation as an evaluative instrument. Clin. Gastroenterol. Hepatol. 4, 588–596 (2006).
Benninga, M. A. et al. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology 150, 1443–1455.e2 (2016).
Schmidt, F. L. The relative efficiency of regression and simple unit predictor weights in applied differential psychology. Educ. Psychol. Meas. 31, 699–714 (1971).
Ogundimu, E. O., Altman, D. G. & Collins, G. S. Adequate sample size for developing prediction models is not simply related to events per variable. J. Clin. Epidemiol. 76, 175–182 (2016).
Schroeder, M. A. Diagnosing and dealing with multicollinearity. West. J. Nurs. Res. 12, 175–187 (1990).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, (2020).
McKeever, T. M., Lewis, S. A., Smith, C. & Hubbard, R. The importance of prenatal exposures on the development of allergic disease: a birth cohort study using the West Midlands General Practice Database. Am. J. Respir. Crit. Care Med. 166, 827–832 (2002).
Dom, S. et al. Pre- and post-natal exposure to antibiotics and the development of eczema, recurrent wheezing and atopic sensitization in children up to the age of 4 years. Clin. Exp. Allergy 40, 1378–1387 (2010).
Batool, T. et al. Prenatal and early-life predictors of atopy and allergic disease in Canadian children: results of the Family Atherosclerosis Monitoring In earLY life (FAMILY) Study. J. Dev. Orig. Health Dis. 7, 665–671 (2016).
Acknowledgements
We would like to thank the parents and their babies who participated in this study for their valuable time. We gratefully acknowledge the midwives and administrators at The Hamilton Midwives, Community Midwives of Hamilton, Access Midwives, Burlington and Area Midwives, Community Midwives of Brantford, Lincoln Community Midwives, Niagara Midwives and Renaissance Midwifery for the time they committed to recruiting participants and collecting data. We would like to thank Laura Rossi and Michelle Shah for their work in processing the stool samples for analysis as well as Efrah Yousuf and Niels Rochow for computing the body composition imputations.
Funding
This study is funded by grants from the Hamilton Academic Health Sciences Organization and the Canadian Institutes of Health Research (MOP-136811).
Author information
Authors and Affiliations
Consortia
Contributions
E.K.H., J.C.S., and K.M.M. had full access to all of the data. E.K.H., J.C.S., L.T., and K.M.M. conceptualised the study design. E.K.H., J.C.S., and K.M.M. were responsible for the investigation and conduct of this study. J.C.S. acquired the data and conducted the analysis with the methodological support from L.T., E.K.H. and K.M.M. E.K.H., J.C.S., and K.M.M. wrote the original draft of the manuscript and all authors revised and edited the manuscript for critical content and methodology. E.K.H., J.C.S., L.T., and K.M.M. contributed to funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Written informed consent was provided by all participants prior to enrolment in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: firstly, the sentence “Antibiotic exposure in labour ≥4-h was associated with increase in fat mass index at 5-months (β 0.42 [95% CI: −0.03, 0.80], p = 0.03)” in the abstract was corrected to read “Antibiotic exposure in labour ≥4-h was associated with increase in fat mass index at 5-months (β 0.42 [95% CI: 0.03, 0.80], p = 0.03)”. In the ‘Results’ section, the sentence “However intrapartum exposure to antibiotic ≥4-h duration was independently associated with fat mass index at 5 months (β 0.342 [95% CI: 0.032, 0.80], p = 0.03) (Table 3)” was corrected to “However intrapartum exposure to antibiotic ≥4-h duration was independently associated with fat mass index at 5 months (β 0.42 [95% CI: 0.03, 0.80], p = 0.03) (Table 3)”. Finally in table 3, the data in the row ‘Fat mass index at 5 months’ and under the column ‘Sensitivity analysis: IAP ≥ 4 h exposure’ was corrected from “0.42 (−0.030, 0.80)” to “0.42 (0.03, 0.80)”.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hutton, E.K., Simioni, J.C., Thabane, L. et al. Associations of intrapartum antibiotics and growth, atopy, gastrointestinal and sleep outcomes at one year of age. Pediatr Res 94, 1026–1034 (2023). https://doi.org/10.1038/s41390-023-02525-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02525-1