Abstract
Background
Intrapartum antibiotic prophylaxis (IAP) is widely used, but the evidence of the long-term effects on the gut microbiota and subsequent health of children is limited. Here, we compared the impacts of perinatal antibiotic exposure and later courses of antibiotic courses on gut microbiota.
Methods
This was a prospective, controlled cohort study among 100 vaginally delivered infants with different perinatal antibiotic exposures: control (27), IAP (27), postnatal antibiotics (24), and IAP and postnatal antibiotics (22). At 1 year of age, we performed next-generation sequencing of the bacterial 16S ribosomal RNA gene of fecal samples.
Results
Exposure to the perinatal antibiotics had a clear impact on the gut microbiota. The abundance of the Bacteroidetes phylum was significantly higher in the control group, whereas the relative abundance of Escherichia coli was significantly lower in the control group. The impact of the perinatal antibiotics on the gut microbiota composition was greater than exposure to later courses of antibiotics (28% of participants).
Conclusions
Perinatal antibiotic exposure had a marked impact on the gut microbiota at the age of 1 year. The timing of the antibiotic exposure appears to be the critical factor for the changes observed in the gut microbiota.
Impact
-
Infants are commonly exposed to IAP and postnatal antibiotics, and later to courses of antibiotics during the first year of life.
-
Perinatal antibiotics have been associated with an altered gut microbiota during the first months of life, whereas the evidence regarding the long-term impact is more limited.
-
Perinatal antibiotic exposure had a marked impact on the infant’s gut microbiota at 1 year of age.
-
Impact of the perinatal antibiotics on the gut microbiota composition was greater than that of the later courses of antibiotics at the age of 1 year.
Similar content being viewed by others
Introduction
Neonates are routinely exposed to antibiotics at birth and in the perinatal period.1,2 Intrapartum antibiotic prophylaxis (IAP) is commonly used to prevent severe neonatal bacterial infections, sepsis, and meningitis caused by the Group B Streptococcus (GBS) and Streptococcus agalactiae; a substantial use of IAP has reduced the incidence of the early-onset GBS sepsis by 50–80%.3,4 Universal antenatal screening of pregnant women for GBS colonization, a widely recommended policy in many developed countries, has shown that 20–30% of pregnant women and their newborn infants are exposed to IAP.5,6,7 In addition, an estimated 2–5% of newborns are exposed to the empirical postnatal intravenous antibiotics used to treat suspected neonatal sepsis.8
Several studies have indicated that both IAP and empirical antimicrobial therapy after birth influence the subsequent composition and biodiversity of the gut microbiota in vaginally delivered infants during the first few months.9,10,11 However, it is not known whether these changes persist beyond early infancy. Furthermore, infants and young children frequently receive courses of oral antibiotics for common childhood infections.12,13 In previous epidemiological studies, early exposure to oral antibiotics has been associated with subsequent asthma, allergic diseases, overweight, inflammatory bowel disease, and celiac disease in childhood.14,15,16,17,18,19 In a previous study, oral macrolide use was associated with long-term alterations in the gut microbiota in daycare children.20
In our earlier prospective, controlled study of vaginally delivered newborn infants, perinatal antibiotic exposure rapidly changed the gut microbiota during the first week of life, with changes persisting up to the age of 6 months.11 Our hypothesis was that the observed differences vanish as the infants grow older and are exposed to courses of antimicrobials. We have now followed up the cohort and compared the impacts of perinatal antibiotics and courses of antibiotics on the composition of the gut microbiota at 1 year of age.
Methods
Study design and study population
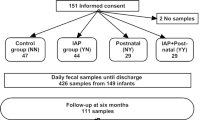
The participants in this prospective, controlled cohort study (Fig. 1) were vaginally delivered term infants born between February 2014 and June 2015 at Oulu University Hospital, Oulu, Finland. The parents of all the recruited infants gave their written informed consent. Only families with decent Finnish language skills were included. Infants born with severe congenital disorders were not included in the study. None of the children had a neurological or developmental problem at the age of 1 year. We have earlier reported the influence of perinatal antibiotics on the early gut microbiota in the same cohort up to 6 months of age.11 We now collected data on antimicrobial consumption after the perinatal period and obtained a fecal sample at the age of 1 year. The research plan was reviewed and approved by the Regional Ethics Committee of the Northern Ostrobothnia Hospital District, Oulu University Hospital, Oulu, Finland (decision number EETTMK 76/2013).
Antibiotic exposures and samples
The participating vaginally delivered infants were divided into four groups based on their perinatal antibiotic exposure. Detailed perinatal antibiotic exposures were collected from comprehensive hospital medical records by the study physician. The control group was not exposed to IAP during delivery, or to postnatal antibiotics during the first week of life, or to any maternal antibiotics within 1 week before delivery, the IAP group received only IAP, the postnatal group was exposed to empirical postnatal intravenous antibiotics within 24 h after birth, and the IAP + postnatal group received both IAP and postnatal intravenous antibiotics. Inclusion criteria to IAP group and IAP + postnatal group was that maternal antibiotic prophylaxis was administered 2–24 h before the birth and in the postnatal group and IAP + postnatal group infants had to receive postnatal antibiotics in 24 h after the birth to be included in the study. All the infants exposed to postnatal antibiotics received a Lactobacillus reuteri probiotic product with a daily dose of 108 colony-forming units during the first week of life until discharge. The families then collected a fecal sample from the infant’s diaper or potty at 12 months of age and sent these to the laboratory for storage at −20 °C and later analysis.
For oral antibiotic courses, the study nurse contacted all families at the age of 6 months and again at the age of 12 months either by a web-based survey sent by email or by telephone contact. The timing of oral antibiotic courses, including drug names and indications, were recorded (Table 1). The infants were then regrouped based on their antimicrobial consumption after the perinatal period: (1) no courses of antibiotics, (2) one or two courses of antibiotics, and (3) three or more courses of antibiotics.
DNA extraction, PCR, and microbiota analysis
DNA extraction from fecal samples was performed using the QIAamp Fast DNA stool mini kit according to the protocol provided by the manufacturer. The final product was eluted to 50 µl to increase the DNA yield further. The DNA concentration was measured using Nanodrop. The samples were stored at −20 °C until used. We have previously reported the PCR reagents and cycling conditions and analyses of the PCR from the fecal sample in detail.11 Universal R926trP1 and F519 primers with unique barcodes were used to amplify a portion of the bacterial 16S ribosomal RNA (rRNA) gene. PCR was performed on a Veriti 96-Well Thermal Cycler from Applied Biosystems. The reactions were performed in triplicate for all the samples. The PCR products were purified with an AMPure XP PCR clean-up kit from Agencourt Bioscience. DNA concentrations were measured with a Bioanalyzer DNA chip from Agilent Technologies. The samples were pooled in equimolar amounts and the pooled samples were purified again with the AMPure XP kit. The DNA concentration in the final product was measured with a Quant-iT PicoGreen dsDNA Assay kit from Thermo Fisher Scientific. The Ion PGM Hi-Q View OT2 kit for a 400 bp protocol, the Ion PGM Hi-Q View Sequencing kit, and Ion 316 v2 chips were used for sequencing, which was performed with an Ion Torrent PGM instrument.
Bioinformatics analysis
The next-generation sequencing data for the bacterial 16S rRNA gene were processed by the J. Craig Venter Institute (JCVI) 16S annotation pipeline, which was integrated using programs from the Uparse,21 Mothur,22 and Biom23 packages. Raw reads were then filtered using the usearch fastq_filter command (maximum expected error threshold at 1.0). Unique reads were identified and singletons removed were by means of usearch derep_fulllength and sortbysize with default parameters. The operational taxonomic units (OTUs) were then clustered by 97% identity with usearch cluster_otus command, which also performed chimera filtering. The OTUs were annotated by the Mothurclassify.seqs command with the default parameters, using SILVA24 as the reference database. The OTU feature table was converted to biom format using the biom tool. The data were then rarefied to 3300 reads per sample and the alpha and beta diversity (using principal coordinate analysis) was assessed in QIIME2.25 The illustrated microbiota was drawn using the Krona visualization tool.26 All the raw sequences were submitted to GenBank sequence reads archives (SRA) with the Bioproject SRA accession number: PRJNA605735.
Statistical analysis
We first analyzed the associations between perinatal antibiotics and the gut microbiota at the age of 12 months, followed by those between the courses of antimicrobials during the first year of life and the gut microbiota at 12 months. The relative abundances of the main bacterial phyla and genera, indices of alpha diversity, and number of OTUs were compared. The independent samples t test was used first to compare the relative abundances and biodiversity indices between the control group and the group of all those exposed to perinatal antibiotics (IAP, postnatal, IAP + postnatal combined) to ensure sufficient statistical power. In a similar manner, we then used the t test for comparisons between those not exposed to antimicrobials and the group exposed to at least one course of antimicrobials (≥1 course). Comparisons between all three perinatal antibiotic groups and the control group were performed first using an analysis of variance followed by post hoc tests with Tukey’s HSD (honestly significant difference) or Games–Howell correction for multiple comparisons. The same was done for the groups formed in terms of antimicrobial courses in the first year of life (none, 1–2 courses, and ≥3 courses). We then performed the linear mixed model adjusted for the different antibiotic exposure groups to control the possible confounding effect of the antibiotic exposure after the perinatal period and the antibiotic exposure later in life.
Results
Fecal follow-up samples were received from 100 of the 150 study participants at the age of 12 months (Fig. 1a, b). Antimicrobial consumption data for the first 12 months were available from 99 infants (Fig. 1b).
Perinatal antibiotics and the gut microbiota at the age of 1 year
Exposure to the perinatal antibiotics had an impact on the gut microbiota at the phylum level at 12 months of age (Table 2 and Figs. 2 and 3). The abundance of the Bacteroidetes phylum was highest in the control group (71%), followed by the IAP group (53%), the postnatal group (50%), and the IAP + postnatal group (45%) (Table 3 and Fig. 2a). The relative abundance of Bacteroidetes differed significantly between the control group and the perinatal antibiotics groups (71% vs. 49%), P < 0.001 (Table 2). When comparing all four groups with each other, the difference was statistically significant between the IAP + postnatal group and the control group (Table 3). The relative abundance of Firmicutes was highest in the IAP + postnatal group (47%), followed by the postnatal group (45%), the IAP group (39%), and the control group (25%) (Table 3 and Fig. 2a).
Bacteroides was the most abundant genus in the samples; its relative abundance being highest in the control group, which differed significantly from the perinatal antibiotic groups (65% vs. 36%) (Table 2 and Fig. 2a). Comparing all four groups, statistically significant differences were seen between the control group (65%) and the IAP group (42%), between the control and the postnatal group (33%), and between the control and the IAP + postnatal group (32%) (Table 3 and Fig. 2a). The relative abundance of the genus Clostridium was lower in the control group than in the any perinatal antibiotic group, that is, IAP, postnatal, and IAP + postnatal groups combined (Table 2). Similarly, Faecalibacterium was less abundant in the control group than in the any perinatal antibiotic group (Table 2). The relative abundance of Prevotella was higher in the any perinatal antibiotic group than in the control group (Table 2). At the species level, the relative abundance of Escherichia coli was significantly lower in the control group than in the any perinatal antibiotic group (Table 2).
The number of OTUs did not differ between the control and perinatal groups (75 vs. 79), P = 0.35 (Table 2), and no differences in the microbial diversity were seen between these groups when measured in terms of Shannon diversity index or Pielou’s evenness index (Table 2). Likewise, Faith’s phylogenetic diversity index was 7.5 in the control group and 8.1 in the perinatal antibiotic group, but this difference was not statistically significant, P = 0.06 (Tables 2 and 4).
Courses of antibiotics and the gut microbiota at the age of 1 year
Courses of antibiotics were administered to infants in all the perinatal antibiotic groups (Fig. 1b). Altogether, 28 infants (28%) were exposed to a total of 44 such courses during the first 12 months (Fig. 1b and Table 1). β-Lactams (amoxicillin with or without clavulanic acid, cefuroxime, and cephalexin) were the most commonly used antibiotics comprising 35 (80%) of all the courses (Table 1). Macrolides (azithromycin and clindamycin) were used for five courses (11%) and sulfatrimethoprim for three of the courses (7%), with one antimicrobial course remaining unknown (Table 1). The median time between the last antibiotic course and the 12 months fecal sampling was 2 months in the control group (range 8, from 1 to 9) and the IAP group (range 4, from 1 to 5), and 3 months in the postnatal group (range 8, from 1 to 9) and the IAP + postnatal group (range 7, from 1 to 8) (Table 1).
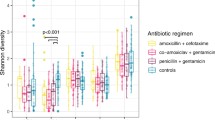
Exposure to antibiotic courses during the first year of life was not associated with the infant’s gut microbiota at 1 year of age (Figs. 2b and 3 and Table 2). The relative abundance of Bacteroidetes was 47% in the group exposed to three or more courses of antibiotics, 55% in the group exposed to one or two courses, and 56% in the control group, the difference not being significant, P = 0.84 (Table 5, Fig. 2b). At the genus level, the relative abundance of Bacteroides was 28% in the group exposed to three or more antibiotic courses, 44% in the group exposed to one or two courses, and 44% in the control group, but again the difference was not statistically significant, P = 0.59 (Table 5 and Fig. 2b). Likewise, the relative abundance of Clostridium was 3.3% in the group exposed to three or more antibiotic courses, 1.3% in the group exposed to one to two antibiotics, and 1.2% in the control group, without the difference being statistically significant, P = 0.20 (Table 5 and Fig. 2b).
No differences were seen in the microbial diversity as measured by the Shannon index, Faith’s phylogenetic diversity index, or Pielou’s evenness index, nor did the number of OTUs differ between the antibiotic course groups (Table 5).
Multivariate analysis of gut microbiome composition adjusted for different antibiotic exposures
Effects of the perinatal antibiotics and courses of antibiotics on the three main bacterial phyla, Bacteroidetes, Firmicutes, and Proteobacteria of the infant’s gut microbiota, were compared using the linear mixed-model analysis adjusted for different antibiotic exposure groups: IAP, postnatal, IAP + postnatal, and courses of oral antibiotics (any vs. none) (Table 6). Relative abundance of the Bacteroidetes was inversely associated with the exposure to IAP (P = 0.045), to postnatal antibiotics (P = 0.02), and to IAP + postnatal antibiotics (P = 0.005). Exposure to courses of oral antibiotics did not show a statistically significant effect (P = 0.68) (Table 6). Relative abundance of the Firmicutes was linearly associated with the postnatal group (P = 0.02) and the IAP + postnatal group (P = 0.01), but no associations were seen in the IAP group (P = 0.09) nor exposing to any courses of antibiotics (P = 0.99). No statistically significant associations were seen in the relative abundance of Proteobacteria and exposures to perinatal antibiotics or courses of antibiotics.
Discussion
In this prospective, controlled cohort study among vaginally delivered infants, the impact of perinatal antibiotic exposure on the gut microbiota composition at the age of 1 year was greater than that of later courses of oral antibiotics. In the case of perinatal antibiotics, there appeared to be a dose-dependent impact, since the greatest difference at the phylum level was seen in infants exposed to both intrapartum antibiotics and intravenous postnatal antibiotics as compared with the control infants.
Several previous cross-sectional studies have reported an impact of IAP on the gut microbiota in vaginally delivered infants, with IAP being associated with a lower relative abundance of Bacteroidetes and higher relative abundance of Firmicutes at an age of 10 days27 and up to 3–6 months of age.10,11 At the genus level, IAP has been linked to lower relative abundance of Bacteroides and Bifidobacterium, and higher relative abundance of Enterococcus and Clostridium.10,11,28,29 Regarding the long-term impact of perinatal antibiotics, the evidence is more limited. One earlier study of 266 infants exposed to IAP showed that the relative abundance of Bacteroides, Clostridium, Bifidobacterium, and Blautia had decreased in the infants exposed to the most commonly used IAP, penicillin, by comparison with the non-exposed group at 1 year of age.30 In our previous study, at 6 months of age, perinatal antibiotic exposure was associated with a lower relative abundance of Bacteroidetes and Bacteroides as well.11 Thus, our present results at the age of 1 year are consistent with our present results at the age of 6 months. In the present study, we expand the earlier findings and show that perinatal antibiotic exposure appeared to have a long-term effect on the gut microbiota at the age of 12 months, which persisted even though infants had received courses of oral antibiotics for common childhood infections in particular between 6 and 12 months of age.
The previous evidence concerning oral antimicrobial consumption and the gut microbiota in children is limited, but in the case of adults, studies with 10–20 participants per group have reported alterations in the intestinal microbiota composition and decreased microbial diversity in groups exposed to vancomycin,31 clindamycin,32 and ciprofloxacin32,33 as compared with a control group. One large observational study among 200 daycare children showed that macrolide exposure was linked to an altered microbial composition, with a decrease in Actinobacteria and an increase in Bacteroidetes and Proteobacteria, but that the changes were less clear after the commonly used β-lactam antibiotics.20 In addition, in one earlier randomized, double-blinded and placebo-controlled study of 59 children, a 3-day oral exposure to azithromycin caused a reduced gut microbial diversity, richness, and relative abundance of Actinobacteria 14 days after the randomization, but these differences were no longer seen in the samples taken 13–39 months after the treatment.34 On the contrary, one recent study of 808 adolescents showed differences in the salivary microbiota according to lifetime antibiotic use,35 and in another observational study among 40 antibiotic-naive infants exposed to even a single amoxicillin course disrupted gut microbiota composition with a decreased relative abundance of Bifidobacteria persisting after 6 months.36
The majority of our infants were exposed to courses of β-lactam antibiotics and the fecal samples were taken on average 2 or 3 months after the exposure to antimicrobials. In our study, the impact of courses of antimicrobials was not as great as the perinatal antibiotics, and analogously to the two previous studies,20,34 a single course of an antimicrobial did not seem to have a clear long-term impact on the infant’s gut microbiota, but the number of children with three or more courses of antibiotics was too low to allow any conclusions to be made on the influence of repeated antibiotic exposure on the gut microbiota. However, the current research concerning the long-term impact of even a single course of oral antibiotics is contradictory and more research in this topic is highly needed.
The clinical significance of the gut microbiota changes described here is not yet known. Despite the considerable benefits of IAP in preventing GBS sepsis, some countries, including the United Kingdom, for example, have not adopted a routine screening for GBS in pregnant women due to concerns regarding overtreatment and a lack of knowledge of the long-term effects.37 Although no association between IAP and a higher early childhood BMI was found in one earlier study of 4825 mother/infant dyads,38 the observed gut microbiota changes warrant further epidemiological research into the long-term health effects of IAP and perinatal antibiotics, as has earlier been done in the case of the long-term outcome after Cesarean section (C-section).39,40,41,42,43 Interestingly, C-section has also been reported to result in decreased numbers of Bacteroides and higher numbers of Clostridium in the intestinal microbiota.44,45,46
Perinatal antibiotics were found here to be associated with a subsequent increase in E. coli in the gut microbiota at 1 year of age. Urinary tract infections (UTIs) are common infections in children and are most often caused by E. coli, which belongs to the phylum Proteobacteria.47 The relative abundance of E. coli in the gut microbiota has recently been shown to be associated with subsequent symptomatic E. coli UTI,48,49 and an increased relative abundance of Proteobacteria has been reported earlier to be more abundant in the gut microbiota in obese patients and those with Crohn’s disease.50,51,52
The strength of our study lies in the prospective cohort study design, which allowed comparisons to be made between the impacts of different exposures to perinatal antibiotics and courses of antimicrobials. Only vaginally delivered term infants were included in the present study, in order to reduce the confounding effects of prematurity and the mode of delivery. One possible confounding factor was that the infants exposed to postnatal antibiotics receiving Lactobacilli reuteri probiotic product during their stay in the hospital. However, in our earlier study, the early life difference in the relative abundance of Lactobacilli had vanished by the age of 6 months,11 and similarly, no such differences were seen in the present study. In addition, breastfeeding, maternal diet, solid foods, pets, and family size may modify or confound the observed effects of the antibiotics. The limitation of the present study was that we were not able to include them all in a multivariate model with the present sample size.
One limitation of the study is the small number of participants receiving three or more courses of antibiotics, which makes the statistical power of the findings insufficient for assessing the impact of repeated antibiotic courses. Also, since we did not have a fecal sample obtained shortly before and after the later courses of antibiotics, the short-term impact of these could not be studied. The methods used in the present study were limited to the relative gut microbiome composition created by the next-generation sequencing of bacterial 16S marker gene. In the future, functional metabolic profiling, proteomic analysis, metagenome sequencing for antimicrobial resistance genes, and characterization of the fungal microbiome, mycobiome, might add valuable insights to elucidate the antibiotic impact on the gut microbiome in children. Currently, there are limited data on the clinical correlates of the observed changes in the gut microbiome composition after perinatal antibiotic exposure.
In conclusion, this prospective cohort study shows that perinatal antibiotics, including IAP and postnatal intravenous antibiotics, have a marked, long-term impact on the composition of the infant’s gut microbiota at the age of 1 year. Since this impact did not vanish after the unexposed infants received courses of antibiotics for common childhood infections, the timing of the antibiotic exposure appears to be the critical factor for the changes observed in the gut microbiota.
References
Chai, G. et al. Trends of outpatient prescription drug utilization in US children, 2002–2010. Pediatrics 130, 23–31 (2012).
Persaud, R. R. et al. Perinatal antibiotic exposure of neonates in Canada and associated risk factors: a population-based study. J. Matern. Fetal Neonatal Med. 28, 1190–1195 (2015).
Le Doare, K. & Heath, P. T. An overview of global GBS epidemiology. Vaccine 31, D7–D12 (2013).
Moore, M. R., Schrag, S. J. & Schuchat, A. Effects of intrapartum antimicrobial prophylaxis for prevention of group-B-streptococcal disease on the incidence and ecology of early-onset neonatal sepsis. Lancet Infect. Dis. 3, 201–213 (2003).
Le Doare, K. et al. Intrapartum antibiotic chemoprophylaxis policies for the prevention of group B streptococcal disease worldwide: systematic review. Clin. Infect. Dis. 65, S143–S151 (2017).
Schrag, S. J. et al. A population-based comparison of strategies to prevent early-onset group B streptococcal disease in neonates. N. Engl. J. Med. 347, 233–239 (2002).
Van Dyke, M. K. et al. Evaluation of universal antenatal screening for group B streptococcus. N. Engl. J. Med. 360, 2626–2636 (2009).
Fjalstad, J. W. et al. Early-onset sepsis and antibiotic exposure in term infants: a nationwide population based study in Norway. Pediatr. Infect. Dis. J. 35, 1–6 (2016).
Zimmermann, P. & Curtis, N. Effect of intrapartum antibiotics on the intestinal microbiota of infants: a systematic review. Arch. Dis. Child Fetal Neonatal Ed. 105, 201–208 (2020).
Azad, M. B. et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG 123, 983–993 (2016).
Tapiainen, T. et al. Impact of intrapartum and postnatal antibiotics on the gut microbiome and emergence of antimicrobial resistance in infants. Sci. Rep. 9, 10635 (2019).
Rossignoli, A., Clavenna, A. & Bonati, M. Antibiotic prescription and prevalence rate in the outpatient paediatric population: analysis of surveys published during 2000–2005. Eur. J. Clin. Pharm. 63, 1099–1106 (2007).
Vaz, L. E. et al. Recent trends in outpatient antibiotic use in children. Pediatrics 133, 375–385 (2014).
Murk, W., Risnes, K. R. & Bracken, M. B. Prenatal or early-life exposure to antibiotics and risk of childhood asthma: a systematic review. Pediatrics 127, 1125–1138 (2011).
Kronman, M. P., Zaoutis, T. E., Haynes, K., Feng, R. & Coffin, S. E. Antibiotic exposure and IBD development among children: a population-based cohort study. Pediatrics 130, e794–e803 (2012).
Saari, A., Virta, L. J., Sankilampi, U., Dunkel, L. & Saxen, H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics 135, 617–626 (2015).
Zven, S. E., Susi, A., Mitre, E. & Nylund, C. M. Association between use of multiple classes of antibiotic in infancy and allergic disease in childhood. JAMA Pediatr. 174, 199–200 (2020).
Chelimo, C., Camargo, C. A. Jr, Morton, S. M. B. & Grant, C. C. Association of repeated antibiotic exposure up to age 4 years with body mass at age 4.5 years. JAMA Netw. Open 3, e1917577 (2020).
Dydensborg Sander, S. et al. Association between antibiotics in the first year of life and celiac disease. Gastroenterology 156, 2217–2229 (2019).
Korpela, K. et al. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat. Commun. 7, 10410 (2016).
Edgar, R. C. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998 (2013).
Schloss, P. D. et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
McDonald, D. et al. The Biological Observation Matrix (BIOM) format or: how I learned to stop worrying and love the ome-ome. Gigascience 12, 7 (2012).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Bolyen, E. et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Ondov, B. D., Bergman, N. H. & Phillippy, A. M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 12, 385–385 (2011).
Nogacka, A. et al. Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginally delivered full-term neonates. Microbiome 5, 93 (2017).
Mazzola, G. et al. Early gut microbiota perturbations following intrapartum antibiotic prophylaxis to prevent group B streptococcal disease. PLoS ONE 11, e0157527 (2016).
Corvaglia, L. et al. Infuence of intrapartum antibiotic prophylaxis for group B streptococcus on gut microbiota in the first month of life. J. Pediatr. Gastroenterol. Nutr. 62, 304–308 (2016).
Coker, M. O. et al. Specific class of intrapartum antibiotics relates to maturation of the infant gut microbiota: a prospective cohort study. BJOG 127, 217–227 (2020).
Vrieze, A. et al. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J. Hepatol. 60, 824–831 (2014).
Rashid, M. U. et al. Determining the long-term effect of antibiotic administration on the human normal intestinal microbiota using culture and pyrosequencing methods. Clin. Infect. Dis. 60, S77–S84 (2015).
Stewardson, A. J. et al. Collateral damage from oral ciprofloxacin versus nitrofurantoin in outpatients with urinary tract infections: a culture-free analysis of gut microbiota. Clin. Microbiol Infect. 21, 344.e1–344e11 (2015).
Wei, S. et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine 38, 265–272 (2018).
Raju, S. C. et al. Antimicrobial drug use in the first decade of life influences saliva microbiota diversity and composition. Microbiome 8, 121 (2020).
Korpela, K. et al. Antibiotics in early life associate with specific gut microbiota signatures in a prospective longitudinal infant cohort. Pediatr. Res. 88, 438–443 (2020).
Seedat, F. et al. Universal antenatal screening for group B streptococcus may cause more harm than good. BMJ 364, I463 (2019).
Metz, T. D. et al. Exposure to group B Streptococcal antibiotic prophylaxis and early childhood body mass index in a vaginal birth cohort. J. Matern. Fetal Neonatal Med. 7, 1–6 (2019).
Tollanes, M. C., Moster, D., Daltveit, A. K. & Irgens, L. M. Cesarean section and risk of severe childhood asthma: a population-based cohort study. J. Pediatr. 153, 112–116 (2008).
Huh, S. Y. et al. Delivery by caesarean section and risk of obesity in preschool age children: a prospective cohort study. Arch. Dis. Child 97, 610–616 (2012).
Sevelsted, A., Stokholm, J., Bønnelykke, K. & Bisgaard, H. Cesarean section and chronic immune disorders. Pediatrics 135, e92–e98 (2015).
Yuan, C. et al. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr. 170, e162385 (2016).
Mitselou, N. et al. Cesarean delivery, preterm birth, and risk of food allergy: Nationwide Swedish cohort study of more than 1 million children. J. Allergy Clin. Immunol. 142, 1510–1514.e2 (2018).
Jakobsson, H. E. et al. Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by Caesarean section. Gut 63, 559–566 (2014).
Penders, J. et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 118, 511–521 (2006).
Bokulich, N. A. et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 8, 343ra82 (2016).
Freedman, A. L. Urologic diseases in America Project: urologic diseases in North America project: trends in resource utilization for urinary tract infections in children. J. Urol. 173, 949–954 (2005).
Magruder, M. et al. Gut uropathogen abundance is a risk factor for development of bacteriuria and urinary tract infection. Nat. Commun. 10, 5521 (2019).
Paalanne, N. et al. Intestinal microbiome as a risk factor for urinary tract infections in children. Eur. J. Clin. Microbiol. Infect. Dis. 37, 1881–1891 (2018).
Michail, S. et al. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol. Ecol. 91, 1–9 (2014).
Zhu, L. et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 57, 601–609 (2013).
Kaakoush, N. O. et al. Microbial dysbiosis in pediatric patients with Crohn’s disease. J. Clin. Microbiol. 50, 3258–3266 (2012).
Acknowledgements
This work was financially supported by the Academy of Finland, Finnish Pediatric Research Foundation and Oulu University Hospital, Finland.
Funding
Open access funding provided by University of Oulu including Oulu University Hospital.
Author information
Authors and Affiliations
Contributions
S.A. performed the statistical analyses, drafted the initial manuscript. and critically revised the manuscript. M.V.T. and M.R.M. conducted the microbiota analysis, performed the bioinformatics analysis, and critically reviewed the manuscript for important intellectual content. N.P. conceptualized and designed the study, supervised the data analysis, drafted the initial manuscript. and critically reviewed the manuscript for important intellectual content. T.P. planned and performed the statistical analyses and critically reviewed the manuscript for important intellectual content. A.M.P., W.L., and K.E.N. supervised the microbiota and bioinformatics analyses and critically reviewed the manuscript for important intellectual content. J.S. and M.R. conceptualized and designed the study, organized the data collection, supervised the data analysis, and critically reviewed the manuscript for important intellectual content. P.V. participated in the microbiota analysis and critically reviewed the manuscript for important intellectual content. T.T. conceptualized and designed the study, planned the data collection, and analysis and co-wrote the manuscript. All the authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of consent
All families gave written informed consent for the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ainonen, S., Tejesvi, M.V., Mahmud, M.R. et al. Antibiotics at birth and later antibiotic courses: effects on gut microbiota. Pediatr Res 91, 154–162 (2022). https://doi.org/10.1038/s41390-021-01494-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01494-7
This article is cited by
-
The individual response to antibiotics and diet — insights into gut microbial resilience and host metabolism
Nature Reviews Endocrinology (2024)
-
Increased number of children in households may protect against inflammatory bowel disease
Pediatric Research (2023)
-
Associations of intrapartum antibiotics and growth, atopy, gastrointestinal and sleep outcomes at one year of age
Pediatric Research (2023)
-
Development of gut mycobiome in infants and young children: a prospective cohort study
Pediatric Research (2023)
-
Microbiota succession throughout life from the cradle to the grave
Nature Reviews Microbiology (2022)