Abstract
Background
Characterization of brain injury and neurodevelopmental (ND) outcomes in critical congenital heart disease (cCHD) has primarily focused on hypoplastic left heart syndrome (HLHS) and transposition of the great arteries (TGA). This study reports brain injury and ND outcomes among patients with heterogeneous cCHD diagnoses beyond HLHS and TGA.
Methods
This prospective cohort study included infants with HLHS, TGA, or heterogenous “Other cCHD” including left- or right-sided obstructive lesions, anomalous pulmonary venous return, and truncus arteriosus. Brain injury on perioperative brain MRI and ND outcomes on the Bayley-II at 30 months were compared.
Results
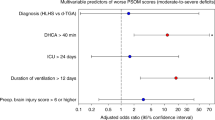
A total of 218 participants were included (HLHS = 60; TGA = 118; “Other cCHD” = 40, including 8 with genetic syndromes). Pre-operative (n = 209) and post-operative (n = 189) MRI showed similarly high brain injury rates across groups, regardless of cardiopulmonary bypass exposure. At 30 months, participants with “Other cCHD” had lower cognitive scores (p = 0.035) compared to those with HLHS and TGA, though worse ND outcome in this group was driven by those with genetic disorders.
Conclusions
Frequency of brain injury and neurodevelopmental delay among patients with “Other cCHD” is similar to those with HLHS or TGA. Patients with all cCHD lesions are at risk for impaired outcomes; developmental and genetic screening is indicated.
Impact
-
This study adds to literature on risk of brain injury in patients with critical congenital heart disease (cCHD) diagnoses other than hypoplastic left heart syndrome (HLHS) and transposition of the great arteries (TGA), a heterogenous cohort of patients that has often been excluded from imaging studies.
-
Children with cCHD beyond HLHS and TGA have similarly high rates of acquired brain injury.
-
The high rate of neurodevelopmental impairment in this heterogenous group of cCHD diagnoses beyond HLHS and TGA is primarily driven by patients with comorbid genetic syndromes such as 22q11.2 deletion syndrome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are not publicly available due to protected health information that, if shared, would compromise the privacy of the individuals. Data are available from the corresponding author on reasonable request.
References
Spillmann, R. et al. Congenital heart disease in school-aged children: cognition, education, and participation in leisure activities. Pediatr. Res. https://doi.org/10.1038/s41390-021-01853-4 (2021).
Miller, S. P. et al. Abnormal brain development in newborns with congenital heart disease. N. Engl. J. Med. 357, 1928–1938 (2007).
Limperopoulos, C. et al. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation 121, 26–33 (2010).
Rollins, C. K. et al. Regional brain growth trajectories in fetuses with congenital heart disease. Ann. Neurol. 89, 143–157 (2021).
Khalil, A. et al. Brain abnormalities and neurodevelopmental delay in congenital heart disease: systematic review and meta-analysis: brain abnormalities and neurodevelopmental delay in CHD. Ultrasound Obstet. Gynecol. 43, 14–24 (2014).
Dimitropoulos, A. et al. Brain injury and development in newborns with critical congenital heart disease. Neurology 81, 241–248 (2013).
Peyvandi, S. et al. Association of prenatal diagnosis of critical congenital heart disease with postnatal brain development and the risk of brain injury. JAMA Pediatr. 170, e154450 (2016).
Miller, S. P. et al. Preoperative brain injury in newborns with transposition of the great arteries. Ann. Thorac. Surg. 77, 1698–1706 (2004).
Peyvandi, S. et al. Neonatal brain injury and timing of neurodevelopmental assessment in patients with congenital heart disease. J. Am. Coll. Cardiol. 71, 1986–1996 (2018).
Lim, J. M. et al. Associations between age at arterial switch operation, brain growth, and development in infants with transposition of the great arteries. Circulation 139, 2728–2738 (2019).
Andropoulos, D. B. et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J. Thorac. Cardiovasc. Surg. 139, 543–556 (2010).
Bonthrone, A. F. et al. Individualized brain development and cognitive outcome in infants with congenital heart disease. Brain Commun. 3, fcab046 (2021).
Claessens, N. H. P. et al. Brain injury in infants with critical congenital heart disease: insights from two clinical cohorts with different practice approaches. J. Pediatr. 215, 75.e2–82.e2 (2019).
Feldmann, M. et al. Delayed maturation of the structural brain connectome in neonates with congenital heart disease. Brain Commun. 2, fcaa209 (2020).
Ortinau, C. et al. Congenital heart disease affects cerebral size but not brain growth. Pediatr. Cardiol. 33, 1138–1146 (2012).
Lynch, J. M. et al. Preoperative cerebral hemodynamics from birth to surgery in neonates with critical congenital heart disease. J. Thorac. Cardiovasc. Surg. 156, 1657–1664 (2018).
Ceschin, R., Zahner, A., Reynolds, W., Beluk, N. & Panigrahy, A. Reduced cerebellar volume in term infants with complex congenital heart disease: correlation with postnatal growth measurements. Diagnostics 12, 1644 (2022).
Williams, W. G. et al. Outcomes of 829 neonates with complete transposition of the great arteries 12-17 years after repair. Eur. J. Cardio-Thorac. Surg. 24, 1–9 (2003). discussion 9-10.
Sananes, R. et al. Six-year neurodevelopmental outcomes for children with single-ventricle physiology. Pediatrics 147, e2020014589 (2021).
Holland, J. E. et al. Psychiatric disorders and function in adolescents with tetralogy of Fallot. J. Pediatr. 187, 165–173 (2017).
Favilla, E. et al. Early evaluation and the effect of socioeconomic factors on neurodevelopment in infants with tetralogy of Fallot. Pediatr. Cardiol. 42, 643–653 (2021).
Peyvandi, S. et al. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J. Thorac. Cardiovasc. Surg. 155, 291–300.e3 (2018).
Moore, T., Johnson, S., Haider, S., Hennessy, E. & Marlow, N. Relationship between Test Scores Using the Second and Third Editions of the Bayley Scales in Extremely Preterm Children. J. Pediatr. 160, 553–558 (2012).
Johnson, S., Moore, T. & Marlow, N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr. Res. 75, 670–674 (2014).
IBM SPSS Statistics for Windows (IBM Corp., 2020).
Miller-Hance, W. C. & Tacy, T. A. Gender differences in pediatric cardiac surgery: the cardiologist’s perspective. J. Thorac. Cardiovasc. Surg. 128, 7–10 (2004).
Algra, S. O. et al. Minimizing the risk of preoperative brain injury in neonates with aortic arch obstruction. J. Pediatr. 165, 1116.e3–1122.e3 (2014).
Marino, B. S. et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation 126, 1143–1172 (2012).
Simon, B. V. et al. Neurodevelopmental delay after the neonatal repair of coarctation and arch obstruction. Ann. Thorac. Surg. 108, 1416–1422 (2019).
Long, S. H., Galea, M. P., Eldridge, B. J. & Harris, S. R. Performance of 2-year-old children after early surgery for congenital heart disease on the Bayley Scales of Infant and Toddler Development, Third Edition. Early Hum. Dev. 88, 603–607 (2012).
Goldstone, A. B. et al. The Bayley-III scale may underestimate neurodevelopmental disability after cardiac surgery in infants. Eur. J. Cardiothorac. Surg. 57, 63–71 (2020).
Claessens, N. H. P. et al. Perioperative neonatal brain injury is associated with worse school-age neurodevelopment in children with critical congenital heart disease. Dev. Med. Child Neurol. 60, 1052–1058 (2018).
Gaynor, J. W. et al. Patient characteristics are important determinants of neurodevelopmental outcome at one year of age after neonatal and infant cardiac surgery. J. Thorac. Cardiovasc. Surg. 133, 1344–1353 (2007).
Fuller, S. et al. Predictors of impaired neurodevelopmental outcomes at one year of age after infant cardiac surgery. Eur. J. Cardiothorac. Surg. 36, 40–48 (2009).
Zaidi, S. & Brueckner, M. Genetics and genomics of congenital heart disease. Circ. Res. 120, 923–940 (2017).
Patel, A. et al. Prevalence of noncardiac and genetic abnormalities in neonates undergoing cardiac operations: analysis of The Society of Thoracic Surgeons Congenital Heart Surgery Database. Ann. Thorac. Surg. 102, 1607–1614 (2016).
Landis, B. J., Cooper, D. S. & Hinton, R. B. CHD associated with syndromic diagnoses: peri-operative risk factors and early outcomes. Cardiol. Young 26, 30–52 (2016).
Carotti, A. et al. Cardiac defects and results of cardiac surgery in 22q11.2 deletion syndrome. Dev. Disabil. Res. Rev. 14, 35–42 (2008).
Alsoufi, B. et al. The effect of noncardiac and genetic abnormalities on outcomes following neonatal congenital heart surgery. Semin. Thorac. Cardiovasc. Surg. 28, 105–114 (2016).
Yi, J. J. et al. Contribution of congenital heart disease to neuropsychiatric outcome in school-age children with 22q11.2 deletion syndrome. Am. J. Med. Genet. B Neuropsychiatr. Genet. 165B, 137–147 (2014).
Gaynor, J. W. et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics 135, 816–825 (2015).
Naef, N. et al. Neurodevelopmental profiles of children with congenital heart disease at school age. J. Pediatr. 188, 75–81 (2017).
Homsy, J. et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science 350, 1262–1266 (2015).
Jin, S. C. et al. Contribution of rare inherited and de novo variants in 2,871 congenital heart disease probands. Nat. Genet. 49, 1593–1601 (2017).
Bucholz, E. M. et al. Socioeconomic status and long-term outcomes in single ventricle heart disease. Pediatrics 146, e20201240 (2020).
Acknowledgements
We would like to acknowledge the neuroradiologic contributions by Dr. A. James Barkovich (A.J.B.) and Dr. Kenneth Poskitt (K.P.).
Funding
This work was supported by the NINDS grant K23 NS099422 and P01 NS082330 from the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
R.V.: substantial contributions to analysis and interpretation of data; drafting of article, revising article critically for important intellectual content; final approval of the version to be published. S.P. and P.M.: substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data; drafting/revising article critically for important intellectual content; final approval of the version to be published. D.G.: substantial contributions to conception and design, analysis and interpretation of data; revising article critically for important intellectual content; final approval of the version to be published. S.C.: substantial contributions to acquisition of data, analysis and interpretation of data; revising the article critically for important intellectual content; final approval of the version to be published. Y.Z.: substantial contributions to acquisition of data; final approval of the version to be published. S.M.: substantial contributions to conception and design, acquisition of data; final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All subjects included in this research project consented to participation in this IRB-approved study. Specific consent for inclusion in this sub-analysis was not performed as no direct contact with participants was required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vassar, R., Peyvandi, S., Gano, D. et al. Critical congenital heart disease beyond HLHS and TGA: neonatal brain injury and early neurodevelopment. Pediatr Res 94, 691–698 (2023). https://doi.org/10.1038/s41390-023-02490-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02490-9