Abstract
Background
Neonatal encephalopathy (NE) is a major cause of mortality and severe neurological disability in the neonatal period and beyond. We hypothesized that the degree of brain injury is reflected in the molecular composition of peripheral blood samples.
Methods
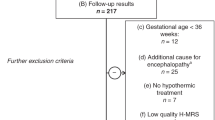
A sub-cohort of 28 newborns included in the HYPOTOP trial was studied. Brain injury was assessed by magnetic resonance imaging (MRI) once per patient and neurodevelopment at 24 months of age was evaluated using the Bayley III Scales of Infant and Toddler Development. The nuclear magnetic resonance (NMR) profile of 60 plasma samples collected before, during, and after cooling was recorded.
Results
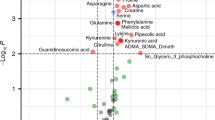
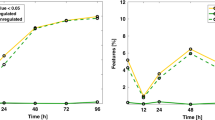
In total, 249 molecular features were quantitated in plasma samples from newborns and postnatal age showed to affect detected NMR profiles. Lactate, beta-hydroxybutyrate, pyruvate, and three triglyceride biomarkers showed the ability to discern between different degrees of brain injury according to MRI scores. The prediction performance of lactate was superior as compared to other clinical and biochemical parameters.
Conclusions
This is the first longitudinal study of an ample compound panel recorded by NMR spectroscopy in plasma from NE infants. The serial determination of lactate confirms its solid position as reliable candidate biomarker for predicting the severity of brain injury.
Impact
-
The use of nuclear magnetic resonance (NMR) spectroscopy enables the simultaneous quantitation of 249 compounds in a small volume (i.e., 100 μL) of plasma.
-
Longitudinal perturbations of plasma NMR profiles were linked to magnetic resonance imaging (MRI) outcomes of infants with neonatal encephalopathy (NE).
-
Lactate, beta-hydroxybutyrate, pyruvate, and three triglyceride biomarkers showed the ability to discern between different degrees of brain injury according to MRI scores.
-
Lactate is a minimally invasive candidate biomarker for early staging of MRI brain injury in NE infants that might be readily implemented in clinical guidelines for NE outcome prediction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated and analyzed during this study are included in this published article and its Supplementary Information files.
References
Douglas-Escobar, M. & Weiss, M. D. Hypoxic-ischemic encephalopathy: a review for the clinician. JAMA Pediatr. 169, 397–403 (2015).
Ma, Q. & Zhang, L. Epigenetic programming of hypoxic-ischemic encephalopathy in response to fetal hypoxia. Prog. Neurobiol. 0, 28–48 (2015).
Lorente-Pozo, S. et al. Oxygen in the neonatal period: oxidative stress, oxygen load and epigenetic changes. Semin. Fetal. Neonatal Med. https://doi.org/10.1016/j.siny.2020.101090 (2020).
Lee, A. C. et al. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 74, 50–72 (2013).
Lehtonen, L., Gimeno, A., Parra-Llorca, A. & Vento, M. Early neonatal death: a challenge worldwide. Semin. Fetal Neonatal Med. 22, 153–160 (2017).
Chalak, L. F. et al. Prospective research in infants with mild encephalopathy identified in the first six hours of life: neurodevelopmental outcomes at 18–22 months. Pediatr. Res. 84, 861–868 (2018).
Wassink, G. et al. Therapeutic hypothermia in neonatal hypoxic-ischemic encephalopathy. Curr. Neurol. Neurosci. Rep. 19, 2 (2019).
Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 33, 696–705 (1976).
Thompson, C. M. et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr. 86, 757–761 (1997).
Merchant, N. & Azzopardi, D. Early predictors of outcome in infants treated with hypothermia for hypoxic-ischaemic encephalopathy. Dev. Med. Child Neurol. 57, 8–16 (2015).
O’Boyle, D. S. et al. Improvement in the prediction of neonatal hypoxic-ischemic encephalopathy with the integration of umbilical cord metabolites and current clinical makers. J. Pediatr. 229, 175.e1–181.e1 (2021).
Mooney, C. et al. Predictive modelling of hypoxic ischaemic encephalopathy risk following perinatal asphyxia. Heliyon 7, e07411 (2021).
Ahearne, C. E., Boylan, G. B. & Murray, D. M. Short and long term prognosis in perinatal asphyxia: an update. World J. Clin. Pediatr. 5, 67–74 (2016).
Groenendaal, F. & de Vries, L. S. Fifty years of brain imaging in neonatal encephalopathy following perinatal asphyxia. Pediatr. Res. 81, 150–155 (2017).
Hou, B. L. & Hu, J. MRI and MRS of human brain tumors. Methods Mol. Biol. 520, 297–314 (2009).
Shibasaki, J. et al. Changes in brain metabolite concentrations after neonatal hypoxic-ischemic encephalopathy. Radiology 288, 840–848 (2018).
Hanrahan, J. D. et al. Persistent increases in cerebral lactate concentration after birth asphyxia. Pediatr. Res. 44, 304–311 (1998).
Lee, W. L. A., Michael-Titus, A. T. & Shah, D. K. Hypoxic-ischaemic encephalopathy and the blood-brain barrier in neonates. Dev. Neurosci. 39, 49–58 (2017).
Douglas-Escobar, M. & Weiss, M. Biomarkers of hypoxic-ischemic encephalopathy in newborns. Front. Neurol. 3, 144 (2012).
Chiesa, C. et al. Umbilical cord interleukin-6 levels are elevated in term neonates with perinatal asphyxia. Eur. J. Clin. Investig. 33, 352–358 (2003).
Cascant-Vilaplana, M. M. et al. Do levels of lipid peroxidation biomarkers reflect the degree of brain injury in newborns? Antioxid. Redox Signal. https://doi.org/10.1089/ars.2021.0168 (2021).
Massaro, A. N. et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 194, 67.e1–75.e1 (2018).
Shah, D. K. et al. Raised plasma neurofilament light protein levels are associated with abnormal MRI outcomes in newborns undergoing therapeutic hypothermia. Front. Neurol. 9, 86 (2018).
Negro, S. et al. Early prediction of hypoxic-ischemic brain injury by a new panel of biomarkers in a population of term newborns. Oxid. Med. Cell. Longev. 2018, 7608108 (2018).
Sánchez-Illana, Á., Piñeiro-Ramos, J. D. & Kuligowski, J. Small molecule biomarkers for neonatal hypoxic ischemic encephalopathy. Semin. Fetal. Neonatal Med. https://doi.org/10.1016/j.siny.2020.101084 (2020).
Soininen, P., Kangas, A. J., Würtz, P., Suna, T. & Ala-Korpela, M. Quantitative serum nuclear magnetic resonance metabolomics in cardiovascular epidemiology and genetics. Circ. Cardiovasc. Genet. 8, 192–206 (2015).
Emwas, A.-H. et al. NMR spectroscopy for metabolomics research. Metabolites 9, 123 (2019).
Reinke, S. N. et al. 1H NMR derived metabolomic profile of neonatal asphyxia in umbilical cord serum: implications for hypoxic ischemic encephalopathy. J. Proteome Res. 12, 4230–4239 (2013).
Ahearne, C. E. et al. Early cord metabolite index and outcome in perinatal asphyxia and hypoxic-ischaemic encephalopathy. Neonatology 110, 296–302 (2016).
Piñeiro-Ramos, J. D. et al. Metabolic phenotypes of hypoxic-ischemic encephalopathy with normal vs. pathologic magnetic resonance imaging outcomes. Metabolites 10, 109 (2020).
Piñeiro-Ramos, J. D. et al. Noninvasive monitoring of evolving urinary metabolic patterns in neonatal encephalopathy. Pediatr. Res. https://doi.org/10.1038/s41390-021-01553-z (2021).
Nuñez-Ramiro, A. et al. Topiramate plus cooling for hypoxic-ischemic encephalopathy: a randomized, controlled, multicenter, double-blinded trial. Neonatology 116, 76–84 (2019).
Rutherford, M. et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 9, 39–45 (2010).
Shankaran, S. et al. Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 167, 987.e3–993.e3 (2015).
Bayley, N. Bayley Scales of Infant and Toddler Development 3rd edn (Harcourt Assessment Inc., 2006).
Soininen, P. et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst 134, 1781–1785 (2009).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 57, 289–300 (1995).
Pang, Z. et al. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 49, W388–W396 (2021).
Wu, T.-W. et al. Cerebral lactate concentration in neonatal hypoxic-ischemic encephalopathy: in relation to time, characteristic of injury, and serum lactate concentration. Front. Neurol. 9, 293 (2018).
Yum, S. K., Moon, C.-J., Youn, Y.-A. & Sung, I. K. Changes in lactate dehydrogenase are associated with central gray matter lesions in newborns with hypoxic-ischemic encephalopathy. J. Matern. Fetal Neonatal Med. 30, 1177–1181 (2017).
Sánchez-Illana, Á. et al. Evolution of energy related metabolites in plasma from newborns with hypoxic-ischemic encephalopathy during hypothermia treatment. Sci. Rep. 7, 1–12 (2017).
Chiang, M.-C. et al. Serum lactate, brain magnetic resonance imaging and outcome of neonatal hypoxic ischemic encephalopathy after therapeutic hypothermia. Pediatr. Neonatol. 57, 35–40 (2016).
Lee, I.-C., Wong, S.-H., Wang, X.-A. & Yu, C.-S. Identifying early diagnostic biomarkers associated with neonatal hypoxic-ischemic encephalopathy. Diagnostics 11, 897 (2021).
Saugstad, O. D. Is lactate a reliable indicator of tissue hypoxia in the neonatal period? Acta Paediatr. 91, 17–19 (2002).
Acknowledgements
The authors would like to express their gratitude to the parents and their newborns who participated in the study.
Funding
This work was supported by the Instituto Carlos III, Ministry of Economy and Competitiveness, Spain [grant numbers CM20/00187, CD19/00037, CPII21/00003, PI17/00127, EC11-046, and PI20/00964] (Co-funded by European Regional Development Fund “A way to make Europe”) and the HYPOTOP study group acknowledges RETICS funded by the PN 2018-2021 (Spain), ISCIII-Sub-Directorate General for Research Assessment and Promotion and the European Regional Development Fund (FEDER), reference RD16/0022/001.
Author information
Authors and Affiliations
Consortia
Contributions
J.K. and M.V. were responsible for the conception and design of the study; M.M.C.-V., I.L.-C., A.N.-R., A.S.-G., and R.L.-S. acquired the data; J.K., G.Q., M.M.C.-V., I.L.-C., R.L.-S., and A.S.-G. analyzed and interpreted the data; J.K. drafted the article; G.Q., M.M.C.-V., and M.V. revised the manuscript critically and all authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent was obtained from legal representatives of infants and all methods were performed in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cascant-Vilaplana, M.M., Lara-Cantón, I., Núñez-Ramiro, A. et al. Longitudinal perturbations of plasma nuclear magnetic resonance profiles in neonatal encephalopathy. Pediatr Res 94, 331–340 (2023). https://doi.org/10.1038/s41390-023-02464-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02464-x