Impact
-
This is an introduction to an article series devoted to the current state and future of pediatric research.
-
The role of public–private partnerships, influencing factors, challenges, and recent trends in pediatric research are described, with emphasis on funding, drug and device development, physician-scientist training, and diversity.
-
Potential solutions and advocacy opportunities are discussed.
Similar content being viewed by others
Introduction
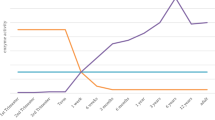
Children have unique and rapidly changing physical, psychosocial, and developmental needs. Addressing early-life diseases and adverse childhood experiences has lifelong benefits for individuals, families and communities. This may also limit or even prevent many chronic adult-onset diseases that originate in early life. However, most pediatric researchers face financial, regulatory, institutional, ethical, and career challenges (Table 1), placing pediatric research at a distinct disadvantage compared to adult investigations (Fig. 1).
Federal research funding
Pediatric research funding from the National Institutes of Health (NIH), the largest public funding agency worldwide, has been historically low compared to funding for adult diseases.1,2 Although pediatric NIH spending has increased over time, the purchasing power of their pediatric and perinatal research portfolio declined by 15.9% and 12.4%, respectively from 2004 to 2015.2 Fortunately, pediatric funding has recently significantly increased due to fiscal and legislative responsiveness requiring NIH to report pediatric research spending annually.3 Nonetheless, high inflation and the COVID-19 pandemic may place future pediatric research funding at risk. Furthermore, priorities for federal pediatric research support may need to be adjusted to account for rapidly changing healthcare needs4 and pediatric disease burden.5
Drug and device development
Pediatric drug and device development continues to lag behind programs addressing adult conditions. Industry-sponsored trials involving children remain limited due to expected lower profitability. Heightened regulatory, ethical, and safety standards for clinical trials involving pregnant women and children, and issues with obtaining parental informed consent and child assent highlight the considerable challenges. Most pediatric diseases are considered rare, which often results in trial prolongation and inadequate enrollment.6 Pre-clinical models for many childhood diseases are lacking and designing pediatric studies requires multiple stakeholders; outcome measures are not uniformly standardized7 and assessing the impact of interventions on neurodevelopmental outcomes can require years of follow-up. Many pediatric clinical research sites do not enroll a single patient, often due to limitations with a highly trained workforce. Consequently, most drugs and devices used in children are not approved by the US Food and Drug Administration (FDA) and approximately two-thirds of FDA-approved drugs and biologics with indications relevant to children are marketed for longer than 5 years without adequate pediatric safety and efficacy labeling.8 Likewise, most FDA approvals of high-risk pediatric devices are based on adult trials, with few children exposed to these devices before market availability.9
To address these shortcomings, several legislative and regulatory changes have been enacted. The Best Pharmaceuticals for Children Act (2002) incentivizes pharmaceutical companies to test drugs in children by giving them an additional 6 months of market exclusivity. The Pediatric Research Equity Act (2003) and the NIH Inclusion Across the Lifespan Policy (2017) mandate the inclusion of participants of all ages in human subject research. Several public–private partnerships and other national/international research collaborations have recently emerged, designed to streamline pediatric clinical trial processes and drug and device development. These include the International Neonatal Consortium (oversight by the Critical Path Institute), a global collaboration that focuses on novel regulatory pathways for evaluating the safety and effectiveness of neonatal therapies,10 the FDA-sponsored System of Hospitals for Innovation in Pediatrics-Medical Devices initiative to accelerate pediatric device development, and the Institute for Advanced Clinical Trials for Children to facilitate multicenter studies for pediatric drug development.
Perspective of academic institutions
Academic medical institutions face increasing financial constraints due to: (1) external competition, (2) expanded regulatory requirements, (3) limited funding, (4) rising provider costs, (5) the need to educate junior physician-scientists, (6) increased costs of conducting high-quality research, and (7) providing medical care to a diverse population with limited reimbursement.11,12 Pediatric departments are especially impacted by financial burdens due to increasing proportions of Medicaid recipients, heightened consumer expectations and regulatory requirements, limited NIH and industry funding, and escalating medical costs.11 These limitations can reduce support for pediatric research infrastructure and training. New organizational and aligned strategic funding models incorporating departmental research support may help to overcome these challenges.11 Improved federal funding is also essential to train the pediatric physician workforce, as requested by the American Hospital Association and 25 other healthcare organizations.13
Physician-scientist training
Pediatric NIH funding is increasingly concentrated in relatively few research-intensive institutions, challenging diversity in research and further impacting the physician-scientist pipeline. Over a 5-year period, 15 institutions received 63% of all pediatric R01-equivalent NIH awards.14 The majority of R01-funded pediatric physician-scientists were male (63.6%), full professors (58%), and held senior leadership positions (24%). Only 15% of pediatric R01-awards were granted to non-professor physician-scientists.14 Furthermore, the success rate for NICHD career development awards has declined since 2010.14 The limited support for junior pediatric physician-scientists, compounded by individual career choices and competing clinical responsibilities, has created a declining and aging pediatric research workforce. This may limit future discoveries and innovative therapies for children.15 Several recent initiatives are now addressing this gap. One example is the National Pediatric Physician-Scientist Collaborative Workgroup, a collaborative of physician-scientists, graduate medical education leaders, department chairs, and trainees from 19 pediatric programs across the US which aims to strengthen the pediatric physician-scientist pipeline.16 Mentorship at the institutional, regional and national level fosters networking opportunities and support for aspiring pediatric researchers. Another important program includes the NIH Loan Repayment Program to recruit and retain highly qualified health professionals into research careers. Offering early-career formal research education during medical school and physician training can lead to greater future academic productivity and funding success, thus strengthening the physician-scientist workforce.17
Gender and racial/ethnic diversity
Despite comparable enrollment in medical schools, women account for only 18% of hospital chief executive officers and 16% of all deans and department chairs in the US.18 Women remain in the minority as senior authors (10%) and editors-in-chief (7%) at high-ranking medical journals.18 They also comprise less than one-third of NIH-awardees, even though they are as successful as men in obtaining first-time grants.19 Factors contributing to these disparities include implicit gender bias and institutional policies disadvantaging women. Early-stage investigator or career development grants sponsored by NIH or other funders are limited to scientists who finished their training within 10 years, which disproportionately disadvantages women.20 Race and ethnicity also impact career trajectories of physician researchers.21 The Coalition for Pediatric Medical Research is now addressing the need to train the next generation of diverse pediatric researchers. Furthermore, innovative solutions to integrate international medical graduates into the research workforce in addition to increased funding for US-trained physicians represent one strategy to address the current physician-scientist shortage.22 Finally, clinical studies must be designed to improve the participation of underrepresented populations,23 to ensure that drugs and devices are studied in target populations who will benefit most from such interventions. This can be accomplished through community-based participatory research including parental engagement for pediatric trials.24
Dissemination, data sharing and reuse
Timely dissemination of trial results through peer-reviewed publications, registries, and data depositories are imperative to facilitate evidence-based care and decision-making. The FDA Amendments Act (2007) and the NIH require that trials are prospectively registered in CinicalTrials.gov and that summary results of FDA-regulated or NIH-funded interventional trials are made available within 12 months of primary study completion. However, only 39% of registered pediatric trials reported results in peer-reviewed publications and 23.5% in the ClinicalTrials.gov registry by 3 years.25 Notably, 11% of trials were discontinued early, with recruitment failure as the most common cause.25 The NIH Policy on Data Sharing (2003) requires a data-sharing plan in all grant applications and the International Committee of Medical Journal Editors (LCMJE) requires a data-sharing statement. However, less than a third of LCMJE-affiliated journals have implemented a data-sharing policy and only a few published trials provided individual patient data in repositories.26,27 Improved monitoring and incentives for data sharing and timely dissemination of trial results may overcome these problems.
Implications for patient outcomes
High-level evidence from clinical studies remains limited for many pediatric diseases and interventions. Most pediatric studies registered in ClinicalTrials.gov are small-scale, single-center, and not funded by industry or the federal government, which translates into fewer drugs being studied over time.28 Published pediatric studies involve significantly fewer randomized controlled trials (RCTs), systematic reviews, and therapeutic trials compared to adults.29 This has significant implications for child health with preterm birth and neonatal infections remaining the leading causes of mortality during the first month of life, accounting for approximately half of the 2.4 million neonatal deaths annually worldwide; there has been limited progress over the past 2 decades due in part to a lack of quality RCTs in this area.30,31,32
Advocacy
There remains an urgent need to communicate33 and advocate healthcare institutions, elected officials, funders, and the public that promoting research focused on fetal and early life has lifelong benefits for children, adults, and society.34 The COVID-19 pandemic has proven that advances in pediatric and adult research can be achieved expediently, especially when governments promote the development of public–private partnerships and global collaboration. Broad support for NIH-sponsored pediatric and perinatal research, enforcement of existing NIH and FDA mandates related to clinical trial reporting, data sharing and reuse, inclusion of children in clinical research, collaborative science, and advocacy hold great promise to advance research and benefit children and future adults.
Data availability
All data pertaining to this report are contained in this special article.
References
Gitterman, D. P., Greenwood, R. S., Kocis, K. C., Mayes, B. R. & McKethan, A. N. Did a rising tide lift all boats? The NIH budget and pediatric research portfolio. Health Aff. 23, 113–124 (2004).
Gitterman, D. P., Langford, W. S. & Hay, W. W. Jr The uncertain fate of the National Institutes of Health (NIH) pediatric research portfolio. Pediatr. Res. 84, 328–332 (2018).
Gitterman, D. P., Hay, W. W., Jr., Langford, W. S. Making the case for pediatric research: a life-cycle approach and the return on investment. Pediatr. Res. 2022. Epub ahead of print.
Grummitt, L. R. et al. Association of childhood adversity with morbidity and mortality in US adults. A systematic review. JAMA Pediatr. 175, 1269–1278 (2021).
Rees, C. A., Monuteaux, M. C., Herdell, V., Fleegler, E. W. & Bourgeois, F. T. Correlation between National Institutes of Health funding for pediatric research and pediatric disease burden in the US. JAMA Pediatr. 175, 1236–1243 (2021).
Pica, N. & Bourgeois, F. Discontinuation and nonpublication of randomized clinical trials conducted in children. Pediatrics 138, e20160223 (2016).
Kahn, M. G., Bailey, L. C., Forrest, C. B., Padula, M. A. & Hirschfeld, S. Building a common pediatric research terminology for accelerating child health research. Pediatrics 133, 516–525 (2014).
Carmack, M., Hwang, T. & Bourgeois, F. T. Pediatric drug policies supporting safe and effective use of therapeutics in children: a systematic analysis. Health Aff. (Millwood) 39, 1799–1805 (2020).
Hwang, T. J., Kesselheim, A. S. & Bourgeois, F. T. Postmarketing trials and pediatric device approvals. Pediatrics 133, e1197–e1202 (2014).
Turner, M. A. et al. The International Neonatal Consortium: collaborating to advance regulatory science for neonates. Pediatr. Res. 80, 462–464 (2016).
Lakshminrusimha, S., et al. “Funds Flow” implementation at academic health centers: unique challenges to pediatric departments. J. Pediatr. 249, 6–10.e4 (2022).
Montoya-Williams, D., Peña, M. M., Fuentes-Afflick, E. In pursuit of health equity in pediatrics. J. Pediatr. X. 5, 100045 (2020).
Bailey, V. Xtelligent Healthcare Media. https://revcycleintelligence.com/news/aha-requests-funding-increase-to-support-pediatric-workforce (2022).
Good, M., McElroy, S. J., Berger, J. N. & Wynn, J. L. Name and characteristics of National Institutes of Health R01-funded pediatric physician-scientists: hope and challenges for the vanishing pediatric physician-scientists. JAMA Pediatr. 172, 297–299 (2018).
Stoll, B. J. & Taegtmeyer, H. Challenges for today’s pediatric physician-scientists. JAMA Pediatr. 172, 220–221 (2018).
Forster, C. S. et al. Perspectives from the Society for Pediatric Research: advice on sustaining science and mentoring during COVID-19. Pediatr. Res. 90, 738–743 (2021).
Hsieh, H. et al. Formal research training during surgical residency: scaffolding for academic success. Am. J. Surg. 207, 141–145 (2014).
Mangurian, C., Linos, E., Sarkar, U., Rodriguez, C., Jagsi, R. What’s holding women in medicine back from leadership. Harv. Bus. Rev., June 2018, updated 7 November 2018.
Hechtman, L. A. et al. NIH funding longevity by gender. Proc. Natl Acad. Sci. USA. 115, 7943–7948 (2018).
Jones, R. D. et al. The most valuable resource is time: Insights from a novel national program to improve retention of physician-scientists with caregiving responsibilities. Acad. Med. 94, 1746–1756 (2019).
Siebert, A. L. et al. Factors associated with underrepresented minority physician scientist trainee career choices. BMC Med. Educ. 20, 422 (2020).
Puljak, L. An overlooked source of physician-scientists. J. Investig. Med. 55, 402–405 (2007).
FDA News Release April 13, 2022. FDA takes important steps to increase racial and ethnic diversity in clinical trials. https://www.fda.gov/news-events/press-announcements/fda-takes-important-steps-increase-racial-and-ethnic-diversity-clinical-trials.
Javier, J. R. et al. Recruiting Filipino immigrants in a randomized controlled trial promoting enrollment in an evidence-based parenting intervention. J. Immigr. Minor. Health 21, 324–331 (2019).
Brewster, R. et al. Early discontinuation, results reporting, and publication of pediatric clinical trials. Pediatrics 149, e2021052557 (2022).
Danchev, V., Min, Y., Borghi, J., Baiocchi, M. & Ioannidis, J. P. A. Evaluation of data sharing after implementation of the International Committee of Medical Journal Editors data sharing statement requirement. JAMA Netw. Open 4, e2033972 (2021).
Bourgeois, F. T. Data-driven approaches to maximize the impact of pediatric clinical trials. Pediatrics 149, e2021055815 (2022).
Zhong, Y., Zhang, X., Zhou, L., Li, L. & Zhang, T. Updated analysis of pediatric clinical studies registered in ClinicalTrials.gov, 2008–2019. BMC Pediatr. 21, 212 (2021).
Martinez-Castaldi, C., Silversten, M. & Bauchner, H. Child versus adult research: the gap in high-quality study design. Pediatrics 122, 52–57 (2008).
Lawn, J. E., Cousens, S. & Zupan, J. Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: When? Where? Why? Lancet 365, 891–900 (2005).
World Health Organization. Newborns: improving survival and well-being. Fact sheet, September. https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-mortality (2020).
Wynn, J. L. Defining neonatal sepsis. Curr. Opin. Pediatr. 28, 135–140 (2016).
Brownell, S. E., Price, J. V. & Steinman, L. Science communication to the general public: why we need to teach undergraduate and graduate students this skill as part of their formal scientific training. J. Undergrad. Neurosci. Educ. 12, E6–E10 (2013).
Cheng, T. L., Russo, C., Cole, C. & Williams, D. A. Advocacy for research starting early in the life course. Pediatr. Res. 91, 1312–1314 (2022).
Author information
Authors and Affiliations
Contributions
E.M.S. wrote the initial draft of the manuscript. All authors substantially contributed to the conception and content of the article, critically revised the manuscript for important intellectual content, and approved the final version for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Speer, E.M., Lee, L.K., Bourgeois, F.T. et al. The state and future of pediatric research—an introductory overview. Pediatr Res (2023). https://doi.org/10.1038/s41390-022-02439-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-022-02439-4