Abstract
Background
The intrauterine adverse environment during nephrogenesis reduces the nephron number, probably associates with impaired ureteric bud (UB) branching.
Methods
The kidneys in C57/BL6 mice were irradiated with a single dose of 10 gray (10 Gy) as adverse environment on postnatal day 3 (irradiated PND3 kidneys) after UB branching ceased. The renal functions and pathological findings of irradiated PND3 kidneys were compared with those of non-irradiated control and 10 Gy irradiation on PND14 (irradiated PND14 kidney) from 1 to 18 months.
Results
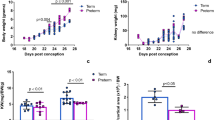
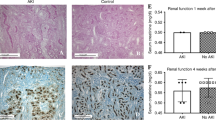
The number and density of glomeruli in irradiated PND3 kidneys were reduced by 1 month with renal dysfunction at 6 months. The morphologically incomplete glomeruli with insufficient capillaries were involuted by 1 month in the superficial cortex. Reduced tubular numbers and developmental disability with shortening renal tubules occurred in irradiated PND3 kidneys with impaired urine concentration at 6 months. Hypertrophy of glomeruli developed, and occasional sclerotic glomeruli appeared in the juxtamedullary cortex with hypertension and albuminuria at 12 to 18 months.
Conclusions
The reduced number of nephrons with shortening renal tubules occurred with impaired renal functions in a postnatal adverse environment after cessation of UB branching, and glomerular hypertrophy with occasional glomerulosclerosis developed accompanied with hypertension and albuminuria in the adulthood.
Impact
-
The reduced number of nephrons with shortening renal tubules occurred with impaired renal functions in a postnatal adverse environment after cessation of ureteric bud branching.
-
The reduced number of glomeruli were associated with not only the impaired formation of glomeruli but also involution of morphologically small incomplete glomeruli after an adverse environment. The insufficiently developed nephrons were characterized by the shortening renal tubules with impaired urine concentration. In addition, glomerular hypertrophy and occasional glomerulosclerosis developed with hypertension and albuminuria in adulthood.
-
The present study can help to understand the risk of alternations of premature nephrons in preterm neonates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Chang, H. H. et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. Lancet 381, 223–234 (2013).
Ancel, P. Y. et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr. 169, 230–238 (2015).
Barker, D. J. & Osmond, C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1, 1077–1081 (1986).
Chantal, C., Evans, R. G., Bertram, J. F. & Moritz, K. M. Effects of dietary protein restriction on nephron number in the mouse. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1768–R1774 (2007).
Walton, S. L. et al. Prolonged prenatal hypoxia selectively disrupts collecting duct patterning and postnatal function in male mouse offspring. J. Physiol. 596, 5873–5889 (2018).
Janot, M., Cortes-Dubly, M. L., Rodriguez, S. & Huynh-Do, U. Bilateral uterine vessel ligation as a model of intrauterine growth restriction in mice. Reprod. Biol. Endocrinol. 12, 62 (2014).
Goodwin, K. & Nelson, C. M. Branching morphogenesis. Development 147, dev184499 (2020).
Short, K. M. et al. Global quantification of tissue dynamics in the developing mouse kidney. Dev. Cell 29, 188–202 (2014).
Hartman, H. A., Lai, H. L. & Patterson, L. T. Cession of renal morphogenesis in mice. Dev. Biol. 310, 379–387 (2007).
Harrison, M. R. et al. Management of the fetus with congenital hydronephrosis. J. Pediatr. Surg. 17, 728–742 (1982).
Chen, J. H., Tarry-Adkins, J. L., Matharu, K., Yeo, G. S. & Ozanne, S. E. Maternal protein restriction affects gene expression profiles in the kidney at weaning with implications for the regulation of renal function and lifespan. Clin. Sci. 119, 373–384 (2010).
Juvet, C. et al. Renal programming by transient postnatal overfeeding: the role of senescence pathways. Front. Physiol. 11, 511 (2020).
Farahani, R. et al. Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr. Pulmonol. 43, 20–28 (2008).
Higo, S. et al. Acute graft-versus-host disease of the kidney in allogeneic rat bone marrow transplantation. PLoS ONE 26, e115399 (2014).
Aratani, S. et al. Radiation-induced premature cellular senescence involved in glomerular diseases in rats. Sci. Rep. 14, 16812 (2018).
Mii, A. et al. Renal thrombotic microangiopathy associated with chronic graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Pathol. Int. 61, 518–527 (2011).
Phifer, C. B. & Terry, L. M. Use of hypothermia for general anesthesia in preweanling rodents. Physiol. Behav. 38, 887–890 (1986).
Dijkman, H. B. et al. Glomerular involution in children with frequently relapsing minimal change nephrotic syndrome: An unrecognized form of glomerulosclerosis? Kidney Int. 71, 44–52 (2007).
Emery, J. L. & Macdonald, M. S. Involuting and scarred glomeruli in the kidneys of infants. Am. J. Pathol. 36, 713–723 (1960).
Nonoguchi, H., Tomita, K. & Marumo, F. Effects of atrial natriuretic peptide and vasopressin on chloride transport in long- and short-looped medullary thick ascending limbs. J. Clin. Investig. 90, 349–357 (1992).
Leung, R. S. Radiation protection of the child from diagnostic imaging. Curr. Pediatr. Rev. 11, 235–242 (2015).
Ikezumi, Y. et al. Low birthweight and premature birth are risk factors for podocytopenia and focal segmental glomerulosclerosis. Am. J. Nephrol. 38, 149–157 (2013).
Barker, D. J. The developmental origins of chronic adult disease. Acta Paediatr. 446, 26–33 (2004).
Spitzer, A. & Brandis, M. Functional & morphologic maturation of the superficial nephrons. Relationship to total kidney function. J. Clin. Investig. 53, 279–287 (1974).
Kim, W. Y. et al. Descending thin limb of the intermediate loop expresses both aquaporin 1 and urea transporter A2 in the mouse kidney. Histochem. Cell Biol. 146, 1–12 (2016).
Endo, T. et al. Exploring the origin and limitation of kidney regeneration. J. Pathol. 236, 251–263 (2015).
Cosset, J. M. et al. Single dose versus fractionated total body irradiation before bone marrow transplantation: radiobiological and clinical considerations. Int. J. Radiat. Oncol. Biol. Phys. 30, 477–492 (1994).
Nielsen, S., Digiovanni, S. R., Christensen, E. I., Knepper, M. A. & Harris, H. W. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc. Natl Acad. Sci. USA 90, 11663–11667 (1993).
Thöny, H. C. et al. Histological features of glomerular immaturity in infants and small children with normal or altered tubular function. Eur. J. Pediatr. 154, S65–S68 (1995).
Acknowledgements
We express special thanks to Mr. Takashi Arai, Ms. Kyoko Wakamatsu, Ms. Arimi Ishikawa, Ms. Naomi Kuwahara, and Ms. Haruna Shimizu for expert technical assistance.
Funding
This work was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (KAKENHI) [grant number 20K08620 (A.S.)].
Author information
Authors and Affiliations
Contributions
Masako Tagawa., A.M., Y.T., and A.S. designed the research. Mika Terasaki and A.S. directed the study. Masako Tagawa, Mika Terasaki, A.M., E.T., Y.K., S.K., Y.T., and A.S. performed the experiments and analyzed the data and pathology. Masako Tagawa, Mika Terasaki, A.M., and E.T. drafted the manuscript. S.K., Y.T., and A.S. approved the final version of the manuscript. A.S. acquired the research funding. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All procedures performed in studies involving experimental animals were in accordance with the ethical standards of the institutional research committee at which the studies were conducted (approval number: 2009-048) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tagawa, M., Terasaki, M., Mii, A. et al. The reduced number of nephrons with shortening renal tubules in mouse postnatal adverse environment. Pediatr Res 93, 1873–1882 (2023). https://doi.org/10.1038/s41390-022-02332-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02332-0