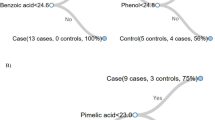
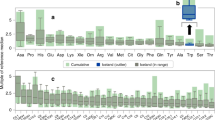
Abstract
Background
The biochemical variations occurring in intrauterine growth restriction (IUGR), when a fetus is unable to achieve its genetically determined potential, are not fully understood. The aim of this study is to compare the urinary metabolomic profile between IUGR and non-IUGR very preterm infants to investigate the biochemical adaptations of neonates affected by early-onset-restricted intrauterine growth.
Methods
Neonates born <32 weeks of gestation admitted to neonatal intensive care unit (NICU) were enrolled in this prospective matched case–control study. IUGR was diagnosed by an obstetric ultra-sonographer and all relevant clinical data during NICU stay were captured. For each subject, a urine sample was collected within 48 h of life and underwent untargeted metabolomic analysis using mass spectrometry ultra-performance liquid chromatography. Data were analyzed using multivariate and univariate statistical analyses.
Results
Among 83 enrolled infants, 15 IUGR neonates were matched with 19 non-IUGR controls. Untargeted metabolomic revealed evident clustering of IUGR neonates versus controls showing derangements of pathways related to tryptophan and histidine metabolism and aminoacyl-tRNA and steroid hormones biosynthesis.
Conclusions
Neonates with IUGR showed a distinctive urinary metabolic profile at birth. Although results are preliminary, metabolomics is proving to be a promising tool to explore biochemical pathways involved in this disease.
Impact
-
Very preterm infants with intrauterine growth restriction (IUGR) have a distinctive urinary metabolic profile at birth.
-
Metabolism of glucocorticoids, sexual hormones biosynthesis, tryptophan-kynurenine, and methionine-cysteine pathways seem to operate differently in this sub-group of neonates.
-
This is the first metabolomic study investigating adaptations exclusively in extremely and very preterm infants affected by early-onset IUGR.
-
New knowledge on metabolic derangements in IUGR may pave the ways to further, more tailored research from a perspective of personalized medicine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Blencowe, H. et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 379, 2162–2172 (2012).
Crump, C., Sundquist, J., Winkleby, M. A. & Sundquist, K. Gestational age at birth and mortality from infancy into mid-adulthood: a national cohort study. Lancet Child Adolesc. Health 3, 408–417 (2019).
Deter, R. L. et al. Individualized growth assessment: conceptual framework and practical implementation for the evaluation of fetal growth and neonatal growth outcome. Am. J. Obstet. Gynecol. 218, 656–678 (2018).
Nardozza, L. M. et al. Fetal growth restriction: current knowledge. Arch. Gynecol. Obstet. 295, 1061–1077 (2017).
Gordijn, S. J., Beune, I. M. & Ganzevoort, W. Building consensus and standards in fetal growth restriction studies. Best. Pract. Res. Clin. Obstet. Gynaecol. 49, 117–126 (2018).
Carducci, B. & Bhutta, Z. A. Care of the growth-restricted newborn. Best. Pract. Res. Clin. Obstet. Gynaecol. 49, 103–116 (2018).
Longo, S. et al. Short-term and long-term sequelae in intrauterine growth retardation (IUGR). J. Matern. Fetal Neonatal Med. 26, 222–225 (2013).
Moco, S. et al. Metabolomics perspectives in pediatric research. Pediatr. Res. 73, 570–576 (2013).
Mayneris-Perxachs, J. & Swann, J. R. Metabolic phenotyping of malnutrition during the first 1000 days of life. Eur. J. Nutr. 58, 909–930 (2019).
Souza, R. T. et al. Metabolomics applied to maternal and perinatal health: a review of new frontiers with a translation potential. Clin. (Sao Paulo) 74, e894 (2019).
Priante, E. et al. Intrauterine growth restriction: new insight from the metabolomic approach. Metabolites 9, 267 (2019).
Leite, D. F. B. & Cecatti, J. G. New approaches to fetal growth restriction: the time for metabolomics has come. Rev. Bras. Ginecol. Obstet. 41, 454–462 (2019).
Leite, D. F. B. et al. Examining the predictive accuracy of metabolomics for small-for-gestational-age babies: a systematic review. BMJ Open 9, e031238 (2019).
Dessì, A., Marincola, F. C. & Fanos, V. Metabolomics and the great obstetrical syndromes-GDM, PET, and IUGR. Best. Pract. Res. Clin. Obstet. Gynaecol. 29, 156–164 (2015).
Dessì, A. et al. Metabolomics in newborns with intrauterine growth retardation (IUGR): urine reveals markers of metabolic syndrome. J. Matern. Fetal Neonatal Med. 24, 35–39 (2011).
Segers, K., Declerck, S., Mangelings, D., Heyden, Y. V. & Eeckhaut, A. V. Analytical techniques for metabolomic studies: a review. Bioanalysis 11, 2297–2318 (2019).
Beale, D. J. et al. Review of recent developments in GC-MS approaches to metabolomics-based research. Metabolomics 14, 152 (2018).
Carraro, S., Giordano, G., Reniero, F., Perilongo, G. & Baraldi, E. Metabolomics: a new frontier for research in pediatrics. J. Pediatr. 154, 638–644 (2009).
Bardanzellu, F. & Fanos, V. How could metabolomics change pediatric health? Ital. J. Pediatr. 46, 37 (2020).
Fanos, V., Pintus, R. & Dessì, A. Clinical metabolomics in neonatology: from metabolites to diseases. Neonatology 113, 406–413 (2018).
Ryan, D. & Robards, K. Metabolomics: the greatest omics of them all? Anal. Chem. 78, 7954–7958 (2006).
Kosmides, A. K., Kamisoglu, K., Calvano, S. E., Corbett, S. A. & Androulakis, I. P. Metabolomic fingerprinting: challenges and opportunities. Crit. Rev. Biomed. Eng. 41, 205–221 (2013).
Pecks, U. et al. Mass spectrometric profiling of cord blood serum proteomes to distinguish infants with intrauterine growth restriction from those who are small for gestational age and from control individuals. Transl. Res. 164, 57–69 (2014).
Stocchero, M. et al. PLS2 in metabolomics. Metabolites 9, 51 (2019).
Wishart, D. S. et al. HMDB 4.0—the Human Metabolome Database for 2018. Nucleic Acids Res. 46, 486–494 (2018).
Guijas, C. et al. METLIN: a technology platform for identifying knowns and unknowns. Anal. Chem. 90, 3156–3164 (2018).
Sumner, L. W., Urbanczyk-Wochniak, E. & Broeckling, C. D. Metabolomics data analysis, visualization, and integration. Methods Mol. Biol. 406, 409–436 (2007).
Comai, S., Bertazzo, A., Brughera, M. & Crotti, S. Tryptophan in health and disease. Adv. Clin. Chem. 95, 165–218 (2020).
Kudo, Y. & Boyd, C. A. Characterisation of L-tryptophan transporters in human placenta: a comparison of brush border and basal membrane vesicles. J. Physiol. 531, 405–416 (2001).
Regnault, T. R., de Vrijer, B. & Battaglia, F. C. Transport and metabolism of amino acids in placenta. Endocrine 19, 23–41 (2002).
Favretto, D. et al. Cord blood metabolomic profiling in intrauterine growth restriction. Anal. Bioanal. Chem. 402, 1109–1121 (2012).
Hernandez-Rodriguez, J., Meneses, L., Herrera, R. & Manjarrez, G. Another abnormal trait in the serotonin metabolism path in intrauterine growth-restricted infants. Neonatology 95, 125–131 (2009).
Cosmi, E. et al. Selective intrauterine growth restriction in monochorionic twin pregnancies: Markers of endothelial damage and metabolomic profile. Twin Res. Hum. Genet. 16, 816–826 (2013).
Moros, G. et al. Insights into intrauterine growth restriction based on maternal and umbilical cord blood metabolomics. Sci. Rep. 11, 7824 (2021).
Murthi, P., Wallace, E. M. & Walker, D. W. Altered placental tryptophan metabolic pathway in human fetal growth restriction. Placenta 52, 62–70 (2017).
Bahado-Singh, R. O. et al. Artificial intelligence and the analysis of multi-platform metabolomics data for the detection of intrauterine growth restriction. PLoS One 14, e0214121 (2019).
Wang, L. et al. Metabolic biomarkers of monochorionic twins complicated with selective intrauterine growth restriction in cord plasma and placental tissue. Sci. Rep. 8, 15914 (2018).
Liu, J., Chen, X. X., Li, X. W., Fu, W. & Zhang, W. Q. Metabolomic research on newborn infants with intrauterine growth restriction. Medicine (Baltimore) 95, e3564 (2016).
Horgan, R. P. et al. Changes in the metabolic footprint of placental explant-conditioned medium cultured in different oxygen tensions from placentas of small for gestational age and normal pregnancies. Placenta 31, 893–901 (2010).
Kalhan, S. C. One carbon metabolism in pregnancy: Impact on maternal, fetal and neonatal health. Mol. Cell Endocrinol. 435, 48–60 (2016).
Kalhan, S. C. & Marczewski, S. E. Methionine, homocysteine, one carbon metabolism and fetal growth. Rev. Endocr. Metab. Disord. 13, 109–119 (2012).
Abd El-Wahed, M. A., El-Farghali, O. G., ElAbd, H. S. A., El-Desouky, E. D. & Hassan, S. M. Metabolic derangements in IUGR neonates detected at birth using UPLC-MS. Egypt. J. Med. Hum. Genet. 18, 281–287 (2017).
Holeček, M. Histidine in health and disease: metabolism, physiological importance, and use as a supplement. Nutrients 12, 848 (2020).
Brownlee, K. G. et al. Catabolic effect of dexamethasone in the preterm baby. Arch. Dis. Child. 67, 1–4 (1992).
Perrone, S., Laschi, E. & Buonocore, G. Biomarkers of oxidative stress in the fetus and in the newborn. Free Radic. Biol. Med. 142, 23–31 (2019).
Rashid, C. S., Bansal, A. & Simmons, R. A. Oxidative stress, intrauterine growth restriction, and developmental programming of type 2 diabetes. Physiology (Bethesda) 33, 348–359 (2018).
Cong, J. et al. Effects of dietary supplementation with carnosine on growth performance, meat quality, antioxidant capacity and muscle fiber characteristics in broiler chickens. J. Sci. Food Agric. 97, 3733–3741 (2017).
Zhang, L. et al. Carnosine pretreatment protects against hypoxia-ischemia brain damage in the neonatal rat model. Eur. J. Pharmacol. 667, 202–207 (2011).
Alcántara-Alonso, V., Panetta, P., de Gortari, P. & Grammatopoulos, D. K. Corticotropin-releasing hormone as the homeostatic rheostat of feto-maternal symbiosis and developmental programming in utero and neonatal life. Front. Endocrinol. (Lausanne) 8, 161 (2017).
Wadhwa, P. D. et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: a prospective investigation. Am. J. Obstet. Gynecol. 191, 1063–1069 (2004).
Johnston, R. C. et al. Associations between placental corticotropin-releasing hormone, maternal cortisol, and birth outcomes, based on placental histopathology. Reprod. Sci. 27, 1803–1811 (2020).
Zhu, P., Wang, W., Zuo, R. & Sun, K. Mechanisms for establishment of the placental glucocorticoid barrier, a guard for life. Cell Mol. Life Sci. 76, 13–26 (2019).
Economides, D., Nicolaides, K. & Campbell, S. Metabolic and endocrine findings in appropriate and small for gestational age fetuses. J. Perinat. Med. 19, 97–105 (1991).
Su, Q. et al. Maternal stress in gestation: birth outcomes and stress-related hormone response of the neonates. Pediatr. Neonatol. 56, 376–381 (2015).
Nixon, M., Upreti, R. & Andrew, R. 5α-Reduced glucocorticoids: a story of natural selection. J. Endocrinol. 212, 111–127 (2012).
Hennebert, O., Chalbot, S., Alran, S. & Morfin, R. Dehydroepiandrosterone 7α-hydroxylation in human tissues: possible interference with type 1 11β-hydroxysteroid dehydrogenase-mediated processes. J. Steroid Biochem. Mol. Biol. 104, 326–333 (2007).
Allvin, K., Ankarberg-Lindgren, C., Niklasson, A., Jacobsson, B. & Dahlgren, J. Altered umbilical sex steroids in preterm infants born small for gestational age. J. Matern. Fetal Neonatal Med. 33, 4164–4170 (2020).
Mansell, T. et al. The newborn metabolome: associations with gestational diabetes, sex, gestation, birth mode, and birth weight. Pediatr. Res. 91, 1864–1873 (2022).
Scalabre, A. et al. Evolution of newborns' urinary metabolomic profiles according to age and growth. J. Proteome Res. 16, 3732–3740 (2017).
Ernst, M., Rogers, S. & Lausten-Thomsen, U. et al. Gestational age-dependent development of the neonatal metabolome. Pediatr. Res. 89, 1396–1404 (2021).
Author information
Authors and Affiliations
Contributions
E.P. contributed to study conception and design, acquisition, and interpretation of data and drafted the article. G.V. and E.B. contributed to study conception and design, interpretation of data, and critically reviewed the manuscript. M.S., G.G., and P.P. contributed to study conception and design, analysis, and interpretation of data, and critically reviewed the manuscript. L.B., S.V., and L.M. critically reviewed the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
A written informed consent was collected from all parents/guardians before the enrollment of the neonates in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Priante, E., Verlato, G., Stocchero, M. et al. Metabolomic profiling of intrauterine growth-restricted preterm infants: a matched case–control study. Pediatr Res 93, 1599–1608 (2023). https://doi.org/10.1038/s41390-022-02292-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02292-5
This article is cited by
-
Intrauterine growth restriction alters kidney metabolism at the end of nephrogenesis
Nutrition & Metabolism (2023)