Abstract
Background
This study evaluated the risk of hypertension, major adverse cardiac events (MACE), and all-cause mortality in Kawasaki disease (KD) patients up to young adulthood.
Methods
An inception cohort of 1169 KD patients between 1991 and 2008 from a tertiary-level hospital in Ontario, Canada was linked with health administrative data to ascertain outcomes up to 28 years of follow-up. Their risk was compared with 11,690 matched population comparators. The primary outcome was hypertension and secondary outcomes were MACE and death.
Results
After a median follow-up of 20 years [IQR: 8.3], the cumulative incidence of hypertension and MACE in the KD group was 3.8% (95% CI: 2.5–5.5) and 1.2% (95% CI: 0.6–2.4%), respectively. The overall survival probability in the KD group was 98.6% (95% CI: 97.2–99.3%). Relative to comparators, KD patients were at an increased risk for hypertension [aHR: 2.2 (95% CI: 1.5–3.4)], death [aHR: 2.5 (95% CI: 1.3–5.0)], and MACE [aHR: 10.7 (95% CI: 6.4–17.9)]. For hypertension and MACE, the aHR was the highest following diagnosis and then the excess risk diminished after 16 and 13 years of follow-up, respectively. MACE occurred largely in KD patients with coronary aneurysms [cumulative incidence: 12.8%].
Conclusions
KD patients demonstrated a reassuring cardiac prognosis up to young adulthood with low events and excellent survival. KD patients were at increased risk for hypertension, but this excess risk occurred early and declined with time.
Impact
-
With the current standard of care, KD patients demonstrated favorable cardiac prognosis, with low events of hypertension, MACE, and excellent survival.
-
Hypertension and MACE risk appear to be highest around the time of KD diagnosis.
-
MACE occurred primarily in KD patients with coronary aneurysms.
-
Our findings are reassuring to KD patients, families, and their providers.
-
Our study demonstrated an association between KD exposure and hypertension. This association is relatively novel. Previous studies have remained conflicting if KD contributes to long-term atherosclerotic risk.
Similar content being viewed by others
Introduction
Kawasaki disease (KD) is an acute systemic vasculitis that primarily occurs in children, and is the leading cause of acquired heart disease in childhood in developed countries.1 Without treatment, approximately 25% of children can develop coronary artery aneurysms (CAA).2,3 Complications related to CAA can include rupture, myocardial ischemia, and sudden death.4,5,6 Therefore, KD has the potential to cause severe cardiac complications during childhood and beyond.
While speculated, the long-term cardiac prognosis of all KD children, particularly those with normal coronary arteries, remains unclear. Current long-term prognosis studies have reported greater than 90% survival and a major adverse cardiac event (MACE)-free survival ranging from 36 to 96% at 30 years.6,7,8,9,10,11 However, these results are not generalizable to the majority of children with KD, as these studies frequently exclude children with normal coronary arteries. Eliminating these patients (i.e., those without CAA) results in a limited understanding of the prognosis of the entire KD population, especially considering intravenous immunoglobulin (IVIG) therapy has significantly reduced the incidence of CAA.1,12,13,14
Furthermore, it remains unclear if patients with KD demonstrate ongoing low states of vascular inflammation and/or dysfunction, which may predispose them to earlier or accelerated atherosclerotic disease. Studies have been conflicting; some studies have reported persistent vascular abnormalities including increased arterial wall thickness and stiffness in KD patients when compared to those without a previous history of KD,15,16,17,18,19 while other studies reported no differences in these measures.20,21,22 It remains unknown if these abnormal sonographic findings translate to more clinical diseases later in life, such as higher rates of hypertension, stroke, or myocardial infarctions.
Therefore, the objective of this study was to evaluate whether individuals with KD were at an increased risk for hypertension, all-cause mortality, and MACE over time, when compared to individuals without KD. We hypothesized that individuals with KD were at higher risk for hypertension, MACE, and death relative to those without KD.
Methods
Study design and setting
This was a retrospective closed-inception data linkage study. Patients with KD from a tertiary hospital (The Hospital for Sick Children – SickKids) in Ontario, Canada were linked to health administrative data at ICES (www.ices.on.ca), and then compared with population comparators, in order to ascertain outcomes (Supplementary Fig. 1). Ontario has a single-payer Ontario Health Insurance Plan (OHIP) in which most physician and hospital services are covered. Institutional ethics approval was obtained from SickKids, Toronto, Canada.
Data sources
The SickKids KD cohort was used to identify and describe KD patients. This cohort includes all children diagnosed with KD at SickKids. Collected information included clinical features, treatments, and echocardiogram findings.
The Registered Persons Database (RPDB) includes demographic information of all current and former residents of Ontario and was used to identify comparators. Postal code of residence, when linked to the Canadian Census, was used to derive the neighborhood income quintile data. Rurality was also determined by the RPDB, defined as individuals residing in a community size ≤10,000 residents. All-cause mortality was determined with the RPBD. The registration of deaths within RPDB is virtually complete due to legal requirements.23
To identify and match individuals on ethnicity, the ICES-derived ETHNIC database was used, which includes encoded classifiers for either South Asian, Chinese, or other on the basis of surnames (surnames are not included in the database). Validation studies against self-identified ethnicity indicate specificities of 99.7 and 99.7% and sensitivities of 50.4 and 80.2% for South Asians and Chinese Canadians, respectively.24
The Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD), Same Day Surgery (SDS) database, and the OHIP claims database were used to identify hypertension and MACE. CIHI-DAD and SDS include information from hospital or day surgery records, including hospitalization dates, associated diagnoses, and procedures. The OHIP claims database identifies physician services and includes the date of encounters and associated diagnosis code. The OHIP Claims database uses an adaptation of the International Classification of Diseases (ICD)-8 coding system, and the CIHI-DAD/SDS uses the ICD-9 and ICD-10 system (ICD-10 was used from 2002 onwards).25 Procedures were coded with either the Canadian Classification of Procedures (CCP) or Canadian Classification of Interventions (CCI) systems (CCI was used from 2002 onwards).
Study population
The KD group included patients diagnosed with KD as children (<18 years old) at SickKids from January 1, 1991, until December 31, 2008. The index date was defined as the date of KD diagnosis. We excluded individuals who had missing/invalid health card numbers and could not be linked to health administrative databases.
The general population comparator (non-KD) group included Ontario residents without a diagnosis of KD (ICD-9: 446.1, ICD-10: M30.3) or a hospitalization related to the fever of unknown origin (ICD-9: 672.0, ICD-10: R50.9). The index date for comparators was defined as the index date of the matched KD patient. Ten comparators were matched with each KD patient according to sex, age, ethnicity (Chinese, South Asian, or Other), regional area of residence, and within the same calendar year.
For both groups, we excluded individuals with a previous diagnosis of hypertension, congestive heart failure (CHF), ischemic heart disease (IHD), stroke, or cardiac interventions (determined from administrative data) within 2 years prior to the index date.
Outcomes
The primary outcome, hypertension, was defined as one hospitalization with a hypertension diagnosis (ICD-9 codes: 401.x–405.x, or ICD-10 codes: I10.×–I13.×, I15.×), or at least two physician billing claims related to hypertension within a 2-year period. The date of hypertension was defined as the date of the first hypertension record. This case definition has been previously validated in patients ≥35 years old (specificity: 95%, sensitivity: 72%, positive predictive value: 87%).26
Secondary outcomes included all-cause mortality (defined as the date of death on RPDB) and MACE. MACE was a composite outcome that included validated case definitions of IHD, stroke, cardiac interventions, and CHF27,28,29,30,31 (Supplementary Table 1).
Statistical analysis
The reporting was in compliance with the Reporting of Studies Conducted Using Observational Routinely-Collected Data (RECORD) statement.32 We conducted descriptive analyses to summarize the cohort and standardized differences were calculated to compare characteristics between KD and non-KD groups. A standardized difference >0.10 was statistically significant. Incidence rates for hypertension, MACE, and death [per 1000 person-years (PY)] were calculated by determining the number of events in each group and the overall duration (PY) of follow-up. Incidence rate ratios (IRR) were derived using Poisson regression. The absolute risk increase (ARI) was the difference in incidence rates between groups.
Survival time was the length of time from the index date to the date of the primary/secondary outcome or censoring (date of outmigration or death). Patients without a documented date of death or outmigration were right censored at the study end date. For hypertension and MACE, the cumulative incidence was evaluated and compared with Gray’s test. Survival probabilities were derived with Kaplan–Meier curves and compared with the Log-Rank test. As a subgroup analysis, we evaluated the cumulative incidence of hypertension in KD patients with normal coronary arteries relative to their comparators. In addition, for each outcome, we evaluated the cumulative incidence/survival probability in the entire KD group according to the presence/absence of CAA.
Multivariable cause-specific models were performed to determine if KD exposure resulted in a difference in time to hypertension or MACE, while adjusting for income and rurality (as indicators of access to care, a potential confounder). A multivariable Cox model was used to evaluate survival. If the proportional hazards assumption was violated, we performed an expanded Cox model that included the time-dependent interaction between KD status and time.
All statistical analyses were performed using SAS Enterprise (SAS Institute Inc., Cary, NC, 2017, Version 7.15).33 Analyses yielding a cell count of ≤5 subjects were suppressed in accordance with ICES’ privacy policies. Statistical significance was defined as a two-sided p value <0.05.
Sensitivity analysis
Sensitivity analyses were performed for analyses relevant to hypertension. In order to evaluate for surveillance bias, we varied the adjusted hazard ratio (aHR) for hypertension by a range of values that reflects the degree of detection bias (bias factor). We ranged the bias factor from 0.6 to 2.0, with >1 reflecting a higher probability of hypertension detection in the KD group compared to the non-KD group.
In order to ensure that the hypertension events that were captured were not due to transient hypertension diagnoses at the time of KD hospitalization (because hypertension can occur with IVIG or steroid treatments), we conducted a landmark analysis where the index date was revised to 12 months after the initial index date.
Results
Cohort
Based on eligibility, 1169 and 11,690 individuals were included in the KD group and non-KD group, respectively (Supplementary Figs. 2 and 3). Due to matching, most baseline characteristics were comparable across groups (Table 1). The median follow-up duration was 20 years (IQR: 8.3).
The clinical characteristics of the KD group are provided in Table 2. The majority of KD patients exhibited the complete subtype (839, 71.8%) and received IVIG (985, 91.7%). Overall, 781 KD patients (66.8%) had normal coronary arteries, 54 (4.6%) had coronary dilatations, 110 (9.4%) had small CAA, 27 (2.3%) had medium CAA, and 20 (1.7%) had giant CAA. The remaining 117 patients (15.4%) had missing echocardiogram information.
Hypertension
After 21,792 and 218,578 PY of follow-up in the KD group and non-KD group, respectively, the incidence rate of hypertension in the KD group [IR: 1.4/1000 PY (95% CI: 0.9–2.0)] was statistically significantly higher than in the non-KD group [IR: 0.6/1000 PY (95% CI: 0.5–0.7)] with an IRR of 2.2 (95% CI: 1.5–3.3, p < 0.0001) (Table 3). The ARI for hypertension was 0.8/1000 PY (95% CI: 0.4–1.2).
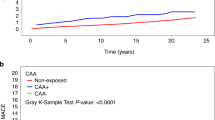
The cumulative incidence of hypertension at 10-, 20-, and 28-year follow-up in the KD group was 0.9, 2.7, and 3.8%, respectively, compared with 0.2, 1.0, and 2.5%, respectively, in the non-KD group (Fig. 1a). This comparative difference was statistically different (Gray’s test p value <0.0001). Within the KD group, there was no statistically significant difference in the cumulative incidence according to CAA status (Supplementary Fig. 4). KD patients with normal coronary arteries continued to demonstrate an increased risk for hypertension relative to their comparators (Supplementary Fig. 5). In our landmark sensitivity analysis, the KD group continued to experience more hypertension events than the non-KD group, and this was statistically significant (Supplementary Fig. 6).
Overall, the KD group demonstrated a two-times higher relative hazard for hypertension [aHR: 2.3 (95% CI: 1.5–3.4), p < 0.0001] (Table 4A). With the proportional hazard assumption violated, the aHR was evaluated over follow-up time. The aHR was highest at the time of diagnosis [aHR: 8.3 (95% CI: 3.5–19.8)] and then diminished over time (Table 4B). The excess risk was observed for 16 years (Fig. 2).
Our sensitivity analysis demonstrated that a 53% higher outcome detection probability in the KD group compared to the non-KD group could explain the observed association of hypertension in KD (Supplementary Fig. 7).
Major adverse cardiac events (MACE)
The incidence rate of MACE in the KD group was 1.30/1000 PY (95% CI: 0.86–1.87) and in the non-KD group was 0.12/1000 PY (95% CI: 0.08–0.18) (Table 3). The ARI for MACE was 1.18/1000 PY (95% CI: 0.78–1.69).
The cumulative incidence of MACE at 20 and 28 years of follow-up in the KD group was 0.7 and 1.2% compared with 0.2 and 0.3% in the non-KD group (Fig. 1b). This difference was statistically significant (Gray’s test p value <0.0001). MACE events within the KD group were largely driven by cardiac interventions (25/28 patients with MACE experienced cardiac interventions). The cumulative incidence of MACE was higher in patients with CAA compared to those without CAA (Supplementary Fig. 8).
Overall, KD exposure resulted in a 10-time increased hazard risk [aHR: 10.7 (95% CI: 6.4–17.9), p < 0.0001] for MACE (Table 4A). In the expanded Cox model, the initial aHR for MACE was 28.4 (95% CI: 13.0–61.8, p < 0.0001) and then decreased with time (Table 4B). The excess risk was observed for 13 years (Supplementary Fig. 9).
All-cause mortality
The incidence rate of mortality in the KD group was 0.45/1000 PY (95% CI: 0.45–0.83), compared to 0.19/1000 PY (95% CI: 0.14–0.26) in the non-KD group (Table 3). The ARI remained low [ARI: 0.3/1000 PY (95% CI: 0.1–0.6)].
While survival probabilities were excellent in both groups [KD group: 98.6% (95% CI: 97.2–99.3), non-KD group: 99.2% (95% CI: 98.9–99.5)], the KD group demonstrated a lower survival (log-rank test p = 0.01; Fig. 1c). KD exposure resulted in a 2.5-time increase in the hazard for death [aHR: 2.5 (95% CI: 1.3–5.0), p < 0.009], after adjusting for income quintile and rurality (Table 4A). There was no statistically significant difference in survival according to CAA status (Supplementary Fig. 10).
Discussion
This study examined the long-term prognosis amongst patients with a confirmed diagnosis of KD from a large tertiary hospital in Canada. This study demonstrated a very reassuring prognosis with low event rates for hypertension and MACE, as well as excellent survival. Relative to comparators, KD patients appeared to have an increased risk for hypertension and MACE, but the excess risk occurred early after the diagnosis and diminished over follow-up time. MACE events were largely driven by cardiac interventions in KD patients with CAA.
In our study, KD patients, including those with normal coronary arteries, were at an increased risk for hypertension relative to comparators. Only a limited number of studies previously explored this association, of which only one study, to our knowledge, demonstrated a higher incidence of hypertension in KD patients.34,35,36,37,38 The explanation for this increased risk remains unclear, but may be due to several mechanisms including functional or structural damage of the affected vessels,2,39,40,41 inherent differences (genetic or behavioral) in the traditional risk factors for hypertension,42,43,44,45,46,47 or ongoing low-grade systemic inflammation.15 More studies are required to evaluate this association. Regardless, in our study, the clinical relevance of this increased relative risk is questioned once absolute measures are taken into consideration. We determined that for every 1250 PY, the KD group had one additional case of hypertension, which we interpreted as a low-risk increase.
KD patients were also at higher risk for MACE, but the majority of these events were driven by cardiac interventions in KD patients with known CAA. Few studies have reported on the cumulative incidence of MACE, for comparison. Previous studies have reported MACE-free survival estimates between 25 and 68% at 25 years of follow-up.6,7,8,10 Given that we had to adjust for the competing risk of death, we could not derive survival probabilities. In general, it appeared that MACE occurrences were lower in our study. This is likely attributed to previous studies only including KD patients with CAA, while a large proportion of our population had normal coronary arteries.
It may be possible that our results for both hypertension and MACE, but particularly for hypertension, may be influenced by surveillance bias. Surveillance bias occurs when patients in one group have a higher probability of ascertaining an outcome due to increased monitoring or testing. Given that hypertension is largely asymptomatic, it is possible that frequent surveillance after KD diagnosis can increase opportunities to pick up blood pressure abnormalities. Our sensitivity analysis demonstrated that hypertension would need to be recognized at least 1.5 times more frequently in the KD group to explain the observed increase in hypertension risk.
The overall survival of our KD patients into early adulthood was excellent. This is an improved survival estimate in contrast to the majority of published studies on mortality in the KD population. Studies that have explored survival over follow-up time in KD patients (mostly in those with CAA) have ranged from 63 to 98% beyond 20 years.6,7,8,10,11
We demonstrated that KD patients continued to demonstrate a relative increased risk for death, which contrasts with the conclusions generated from a longitudinal cohort of 6576 Japanese children.48,49,50,51,52 Results from the Japanese cohort reported similar mortality rates in the KD population compared to the general population after 27 years of follow-up. This difference in conclusions may be related to the statistical methods or the genetic/ethnic composition of the two study populations. Card et al. demonstrated that indirect standardization with national population death statistics can underestimate mortality risk.53 Furthermore, the Japanese cohort comprised only individuals of Japanese nationality while our cohort was multiethnic. Studies have previously demonstrated differences in treatment responses and CAA outcomes according to ethnicity.54,55,56,57,58
We utilized the strengths of a large cohort with rich clinical data and a universal healthcare system that captures data on Ontario residents. Linkage with administrative data offered us several advantages. First, we were able to identify patients with a confirmed diagnosis of KD, minimizing exposure misclassification. Second, we were able to explore the impact of CAA status on outcomes. Third, we were able to evaluate cardiac surveillance for KD patients up to early adulthood. Considering that the majority of KD patients do not experience active surveillance for this length of duration, the use of health administrative data to identify outcomes was critical.
In addition to surveillance bias, our conclusions must be considered in light of additional limitations. First, the measurement of some of our covariates was imperfect that may result in residual confounding. For instance, while our study adjusted for ethnicity with a validated surnames algorithm, the algorithm only specified Chinese and South Asian surnames and was not inclusive of other East Asian surnames. As such, it is possible that the prevalence of East Asian patients in the KD group remained underestimated. If East Asian patients are at lower risk of hypertension due to their ethnicity,59 this may then bias our results towards the null. Second, while this study utilized validated case definitions in order to improve the accuracy of identifying outcomes, our study is still prone to outcome misclassification, resulting in imprecision. Furthermore, we had limited clinical characterization of our outcomes, such as whether there were clinical symptoms attributed to hypertension, any associated/relevant risk factors, and treatments used.
Third, while we ensured that both KD patients and population comparators did not have any preceding cardiac disease within the 2 years prior to the index date, it may be possible that individuals who were much older at the index date may have had a remote history of cardiac disease (such as congenital heart disease) that could alter their future cardiac risk. If the proportion of missed congenital heart disease occurred significantly more in the KD group compared to the non-KD group, then this may potentially falsely attribute increased cardiac risk with KD exposure. However, given that the median age at the index date was 2 years old, we do not expect this concern to significantly impact our overall findings or conclusions. Furthermore, we do not expect KD patients to be at a higher risk for congenital heart disease relative to their comparators. Finally, the results of our study may not be generalizable to all Canadians with KD. Our KD patients were selected from the largest pediatric hospital in the largest city in Canada. Thus, it may be possible that our KD population may not be representative of the patients diagnosed with KD from other jurisdictions where the quality of care may be different.
Conclusion
In conclusion, the long-term prognosis of KD patients remained favorable with excellent survival and low incidence of all cardiovascular outcomes. Further studies are needed to determine if KD patients are truly at increased risk for hypertension compared to population comparators.
Data availability
The study data set is held securely in the coded form at ICES. Although legal data sharing agreements between ICES and data providers (e.g., healthcare organizations and governments) prohibit ICES from making the data set publicly available, access might be granted to those who meet prespecified criteria for confidential access, available at www.ices.on.ca/DAS or via email (das@ices.on.ca). The full data set creation plan and underlying analytical code are available from the authors on request, understanding that the computer programs might rely on coding templates or macros that are unique to ICES and are therefore either inaccessible or require modification.
References
McCrindle Brian, W. et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 135, e927–e999 (2017).
Kato, H. et al. Long-term consequences of Kawasaki disease. A 10- to 21-year follow-up study of 594 patients. Circulation 94, 1379–1385 (1996).
Daniels, L. B. et al. Prevalence of Kawasaki disease in young adults with suspected myocardial ischemia. Circulation 125, 2447–2453 (2012).
Lega, J. C. et al. Extracoronary echocardiographic findings as predictors of coronary artery lesions in the initial phase of Kawasaki disease. Arch. Dis. Child 98, 97–102 (2013).
Kato, H., Ichinose, E. & Kawasaki, T. Myocardial infarction in Kawasaki disease: clinical analyses in 195 cases. J. Pediatr. 108, 923–927 (1986).
Tsuda, E. et al. The 30-year outcome for patients after myocardial infarction due to coronary artery lesions caused by Kawasaki disease. Pediatr. Cardiol. 32, 176–182 (2011).
Kitamura, S. et al. Twenty-five-year outcome of pediatric coronary artery bypass surgery for Kawasaki disease. Circulation 120, 60–68 (2009).
Suda, K. et al. Long-term prognosis of patients with Kawasaki disease complicated by giant coronary aneurysms: a single-institution experience. Circulation 123, 1836–1842 (2011).
Tsuda, E., Tsujii, N. & Hayama, Y. Cardiac events and the maximum diameter of coronary artery aneurysms in Kawasaki disease. J. Pediatr. 188, 70–74.e1 (2017).
Tadokoro, N. et al. Multiple coronary artery bypass grafting for Kawasaki disease-associated coronary artery disease. Ann. Thorac. Surg. 108, 799–805 (2019).
Chih, W. L. et al. Progressive coronary dilatation predicts worse outcome in Kawasaki disease. J. Pediatr. 171, 78–82.e1 (2016).
Furusho, K. et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet 2, 1055–1058 (1984).
Durongpisitkul, K. et al. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis on the efficacy of aspirin and immunoglobulin treatment. Pediatrics 96, 1057–1061 (1995).
Newburger, J. W. et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N. Engl. J. Med. 315, 341–347 (1986).
Cheung, Y. F. et al. Increased high sensitivity C reactive protein concentrations and increased arterial stiffness in children with a history of Kawasaki disease. Heart 90, 1281–1285 (2004).
Ishikawa, T. & Iwashima, S. Endothelial dysfunction in children within 5 years after onset of Kawasaki disease. J. Pediatr. 163, 1117–1121 (2013).
Cho, H. J. et al. Cardiovascular risk factors of early atherosclerosis in school-aged children after Kawasaki disease. Korean J. Pediatr. 57, 217–221 (2014).
Cheung, Y. F., Wong, S. J. & Ho, M. H. K. Relationship between carotid intima-media thickness and arterial stiffness in children after Kawasaki disease. Arch. Dis. Child. 92, 43–47 (2007).
Niboshi, A. et al. Endothelial dysfunction in adult patients with a history of Kawasaki disease. Eur. J. Pediatr. 167, 189–196 (2008).
McCrindle, B. W. et al. Are patients after Kawasaki disease at increased risk for accelerated atherosclerosis? J. Pediatr. 151, 244 (2007).
Parihar, M. et al. Mid-term risk for subclinical atherosclerosis and chronic myocarditis in children with Kawasaki disease and transient coronary abnormalities. Pediatr. Cardiol. 38, 1123–1132 (2017).
Chen, K. Y. et al. Evidence of microvascular changes in the retina following Kawasaki disease. Sci. Rep. 7, 40513 (2017).
ICES. Living and Dying in Ontario (accessed 26 May 2021); https://www.ices.on.ca/flip-publication/living-and-dying-in-ontario/files/assets/basic-html/page16.html (2008).
Shah, B. R. et al. Surname lists to identify South Asian and Chinese ethnicity from secondary data in Ontario, Canada: a validation study. BMC Med. Res. Methodol. 10, 42 (2010).
Canadian Institute for Health Information. Data Quality Documentation, National Ambulatory Care Reporting System—Multi-Year Information (2012).
Tu, K. et al. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 1, e18–e26 (2007).
Hall, R. et al. Accuracy of administrative data for the coding of acute stroke and TIAs. Can. J. Neurol. Sci. 43, 765–773 (2016).
Lee, D. S. et al. Administrative hospitalization database validation of cardiac procedure codes. Med. Care 51, e22–e26 (2013).
Schultz, S. E. et al. Identifying cases of congestive heart failure from administrative data: a validation study using primary care patient records. Chronic Dis. Inj. Can. 33, 160–166 (2013).
Tu, K. et al. Validation of physician billing and hospitalization data to identify patients with ischemic heart disease using data from the Electronic Medical Record Administrative data Linked Database (EMRALD). Can. J. Cardiol. 26, e225–e228 (2010).
Tu, J. V. et al. The Cardiovascular Health in Ambulatory Care Research Team (CANHEART): using big data to measure and improve cardiovascular health and healthcare services. Circ. Cardiovasc. Qual. Outcomes 8, 204–12. (2015).
Benchimol, E. I. et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 12, e1001885 (2015).
SAS. SAS Enterprise Guide; https://support.sas.com/en/software/enterprise-guide-support.html#documentation (2021).
Holve, T. J. et al. Long-term cardiovascular outcomes in survivors of Kawasaki disease. Pediatrics 133, e305–e311 (2014).
Novelli, V. M. et al. Cardiovascular abnormalities in Kawasaki disease. Arch. Dis. Child. 59, 405–409 (1984).
Noto, N. et al. Noninvasive assessment of the early progression of atherosclerosis in adolescents with kawasaki disease and coronary artery lesions. Pediatrics 107, 1095–1099 (2001).
Ou, C. Y. et al. Significant relationship between serum high-sensitivity C-reactive protein, high-density lipoprotein cholesterol levels and children with Kawasaki disease and coronary artery lesions. J. Formos. Med. Assoc. 108, 719–724 (2009).
McCrindle, B. W. et al. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation 116, 174–179 (2007).
Jiang, L. C., Cao, J. Y. & Chen, M. Coronary artery aneurysm combined with other multiple aneurysms at multiple locations: a case report and systematic review. Medicine (Baltimore) 96, e9230 (2017).
Roy, S. & Biswas, M. K. Multiple systemic aneurysms in a case of neglected Kawasaki disease. J. Paediatr. Child Health 55, 117–117 (2019).
Zhao, Q.-m et al. Systemic artery aneurysms and Kawasaki disease. Pediatrics 144, e20192254 (2019).
Onouchi, Y. The genetics of Kawasaki disease. Int J. Rheum. Dis. 21, 26–30 (2018).
Onouchi, Y. et al. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat. Genet 44, 517–521 (2012).
Yorifuji, T., Tsukahara, H. & Doi, H. Early childhood exposure to maternal smoking and Kawasaki disease: a longitudinal survey in Japan. Sci. Total Environ. 655, 141–146 (2019).
Portman, M. A. et al. Soy isoflavone intake is associated with risk of Kawasaki disease. Nutr. Res. 36, 827–834 (2016).
Baker, A. L. et al. Physical and psychosocial health in children who have had Kawasaki disease. Pediatrics 111, 579–583 (2003).
Fukuda, S. et al. Exposures associated with the onset of Kawasaki disease in infancy from the Japan Environment and Children’s Study. Sci. Rep. 11, 13309 (2021).
Nakamura, Y. et al. Mortality among patients with a history of Kawasaki disease: the third look. The Kawasaki Disease Follow-up Group. Acta Paediatr. Jpn. 40, 419–423 (1998).
Nakamura, Y. et al. Mortality among Japanese with a history of Kawasaki disease: results at the end of 2009. J. Epidemiol. 23, 429–434 (2013).
Nakamura, Y. et al. Mortality among persons with a history of Kawasaki disease in Japan: existence of cardiac sequelae elevated the mortality. J. Epidemiol. 10, 372–375 (2000).
Nakamura, Y. et al. Mortality among persons with a history of Kawasaki disease in Japan: the fifth look. Arch. Pediatr. Adolesc. Med. 156, 162–165 (2002).
Nakamura, Y. et al. Mortality among persons with a history of Kawasaki disease in Japan mortality among males with cardiac sequelae is significantly higher than that of the general population. Circ. J. 72, 134–138 (2008).
Card, T. R. et al. Is an internal comparison better than using national data when estimating mortality in longitudinal studies? J. Epidemiol. Community Health 60, 819–821 (2006).
Holman, R. C. et al. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr. Infect. Dis. J. 29, 483–488 (2010).
Clark, D. E. et al. Predictors of intravenous immunoglobulin nonresponse and racial disparities in Kawasaki disease. Pediatr. Infect. Dis. J. 37, 1227–1234 (2018).
Skochko, S. M. et al. Kawasaki disease outcomes and response to therapy in a multiethnic community: a 10-year experience. J. Pediatr. 203, 408–415.e3 (2018).
Dionne, A. et al. Impact of socioeconomic status on outcomes of patients with Kawasaki disease. J. Pediatr. 212, 87–92 (2019).
Padilla, L. A. et al. Kawasaki disease and clinical outcome disparities among black children. J. Pediatr. 229, 54–60.e2 (2021).
Leenen, F. H. H. et al. Results of the Ontario survey on the prevalence and control of hypertension. CMAJ 178, 1441–1449 (2008).
Acknowledgements
We would like to thank and acknowledge Dr Eleanor Pullenayegum who provided additional statistical advice for the results of this study. This study was supported by ICES that is funded by annual grants from the Ontario Ministry of Health and the Ministry of Health and Long-Term Care. Parts of this material are based on data and/or information compiled and provided by the Canadian Institutes of Health Information, and the Ontario Ministry of Health. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Funding
J.J.Y.L. received support from the Clinician Scientist Training Program, SickKids Research Institute/University of Toronto. J.W. received support from the Arthritis Society Stars Career Development Award (STAR-19-0610).
Author information
Authors and Affiliations
Contributions
J.J.Y.L. designed the study, completed the statistical analyses, interpreted the data, and drafted the manuscript. B.W.M. and R.S.M.Y. designed the study, acquired the data from the original patient cohort, provided guidance on the analysis and interpretation of data, provided feedback on the manuscript, and revised it critically for important intellectual content. B.M.F. and J.W. designed the study, provided guidance on the analysis and interpretation of data, provided feedback on the manuscript, and revised it critically for important intellectual content. P.L. provided feedback on the study design, prepared the data sets and initial statistical analyses, provided statistical support, and revised the manuscript critically for important intellectual content. All authors were involved in the critical analysis of the final version of the manuscript. All authors approved the manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Institutional ethics approval was obtained from SickKids, Toronto, Canada. The use of the data was approved by the ICES Privacy and Legal Office. Informed consent to participate in this project was not required for this study. ICES is a “Prescribed Entity” under the Personal Health Information Protection Act (PHIPA), which permits the collection of information without patient consent as ICES has policies and procedures in place to protect the privacy and confidentiality of patients as required by the Act (s.45(3)).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, J.J.Y., Feldman, B.M., McCrindle, B.W. et al. Evaluating the time-varying risk of hypertension, cardiac events, and mortality following Kawasaki disease diagnosis. Pediatr Res 93, 1439–1446 (2023). https://doi.org/10.1038/s41390-022-02273-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02273-8
This article is cited by
-
Cerebrovascular involvement in systemic childhood vasculitides
Clinical Rheumatology (2023)