Abstract
Introduction
The CGG repeats in the 5′ untranslated region of the fragile X mental retardation 1 gene (FMR1) gene shows increased instability upon maternal transmission. Maternal FMR1 intermediate (45–54 repeats) and premutation (PM: 55–<200 repeats) alleles usually expand to full mutation (>200 repeats) alleles in offspring and consequently, cause fragile X syndrome (FXS) in them.
Methods
In a prospective cohort study, Pakistani pregnant women in prenatal care were first screened for FMR1 expanded alleles. In the follow-up, pregnancy outcomes in women carrying FMR1 expanded alleles were recorded and their newborn offspring were also screened for FXS.
Results
In a total of 1950 pregnant women, 89 (4.6%) were detected carriers for FMR1 expanded alleles; however, rates of detection of expanded alleles were found significantly high in women with a history of FXS. In addition, miscarriages and birth of affected newborns with FXS were significantly more common in women carrying large size PM alleles and had a history of FXS (P = 0.0494 and P = 0.0494, respectively).
Conclusions
The current study provides the first evidence of screening Pakistani pregnant women for FMR1 expanded alleles in prenatal care. Moreover, the miscarriage was also detected as a clinical predictor for FXS.
Impact
-
Offspring would have a higher risk of developing FXS due to maternal FMR1 alleles expansions during transmission.
-
This is the first prospective cohort study in Pakistan for finding FMR1 allelic status of pregnant women and their newborn offspring in follow-up.
-
The robust offspring risk for FXS estimated in this study may be valuable information for genetic counseling of women carriers for FMR1 expanded alleles.
-
The family history and miscarriage were detected as effective indicators for FXS carrier screening in Pakistani women.
Similar content being viewed by others
Introduction
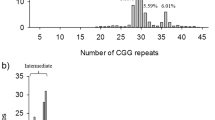
Fragile X syndrome (FXS) is a neurodevelopmental inherited disorder (MIM 300624), with an estimated prevalence of 1:4000 in males and 1:8000 in females1. Individuals with FXS may have clinical features such as intellectual disability, autism spectrum disorder, social anxiety and withdrawal, language deficits, hyperactivity, aggression, and self-injurious behaviors, in addition to the physical features such as hyperextensible finger joints, prominent ears, and macroorchidism in puberty2,3. These features are less common and often less severe in females1. FXS is caused by the cysteine-guanine-guanine (CGG) repeat expansion mutations in the untranslated region of the FMR1 gene, which spans 38 kb region on chromosome Xq27.3. The FMR1 contains 17 exons that encode 632 amino acids repressor protein (FMRP) of 71,174 Da (UniProtKB – Q06787), which has a central role in the induction of synaptic plasticity in the brain and central nervous system. The CGG repeat expansion mutations cause gene methylation and in turn inactivation of the FMR1 gene. This results in a shortage or lack of FMRP and subsequently causes FXS in individuals4. Most individuals in the general population have stable FMR1 alleles with 6–44 CGG repeats. However, in the affected individuals the CGG repeat expansion exceeds beyond 200 known as full mutation (FM) due to inheritance of the unstable premutation (PM) alleles with repeats in a 55–200 range upon maternal transmission5. Importantly, about 1 in 150–300 women carry PM alleles in general population and area increased risk of producing offspring with FXS6. It is noteworthy that the 45–54 CGG repeats intermediate alleles are commonly found in populations (0.8–3.6%), although geographical variability has also been detected7.
The larger CGG repeats size, the greater the risk of expansion of maternal intermediate and PM alleles in subsequent generations8,9 and usually women carriers of FMR1 expanded alleles have a higher risk of producing offspring with FXS. This risk of expansion of PM to FM may be >95% for maternal alleles containing greater than 100 CGG triplets8,10. Importantly, the intermediate and PM alleles with AGG interruptions in CGG repeats do not expand at all in the offspring11. The smallest PM alleles containing either 56 or 59 CGG repeats have been reported to expand to an FM allele in the next generation8,12. Typically, the FMR1 intermediate alleles containing repeats between 45 and 55 may or may not be inherited in an unstable manner upon maternal transmission. The instability increases with larger intermediate alleles containing greater CGG repeats8. Studies have examined that the 50–54 CGG repeats intermediate alleles expansion in PM alleles9 and about 2% expansion in FM alleles in subsequent generations7. Population-based studies have reported transmission and expansion of maternal alleles in fetuses as well as subsequent delivery of affected offspring with FXS7,13,14. Importantly, offspring risk for FXS can be determined if the pregnant women and their fetuses are screened for FMR1 expanded alleles in prenatal care settings15.
Several studies have reported significantly higher rates of FMR1 expanded alleles in pregnant women with a family history of FXS compared to those without a family history of FXS.16,17,18,19 The American College of Obstetricians and Gynecologists (ACOG) recommends screening for FMR1 expanded alleles in pregnant women with a family history of FXS20. However, pregnant women without a family history of FXS have also been detected as carriers for FMR1 expanded alleles previously14,21 so recommendations of ACOG do not identify all pregnant women at risk for delivering offspring with FXS. For this reason, clinicians recommend screening for FMR1 expanded alleles of a substantial number of pregnant women who do not meet ACOG screening criteria22.
Here, we report the first study of screening Pakistani pregnant women with or without a history of FXS for FMR1 expanded alleles during their prenatal care visits and finding the risk of maternal alleles CGG repeat expansion in offspring during transmission through newborn screening.
Materials and methods
Subjects
The present study was approved by the Ethical Committee and Advanced Studies Research Board of Kohat University of Science and Technology, Kohat, Khyber Pakhtunkhwa, Pakistan. The study subjects were pregnant women, who were visiting primary health care centers for prenatal care in Khyber Pakhtunkhwa region of Pakistan between April 2019 and October 2021. Before recruitment, informed written consent was obtained from each participating pregnant woman. The recruitment process adopted for this study is illustrated in Fig. 1. Recruitment of subjects was random, those pregnant women with a family history of FXS were recruited in the risk group whereas those pregnant women who had no family history of FXS were included in the control group. A considerable number of women with or without a family history of FXS declined to participate in this study. Information on demographics, family medical history, health status, and clinical investigations were collected from recruited pregnant women with help of obstetricians and gynecologists who are providing prenatal care at primary health care centers.
The “N” is the total number of pregnant women with or without a family history of Fragile X Syndrome (FXS) and were approached at primary health care centers in Khyber Pakhtunkhwa region of Pakistan for participation in the study. The risk group women had a family history of FXS and control group women had no such history. The “n” represents number of women who were recruited, declined participation in the study and screened for FMR1 expanded alleles in risk group and control group. The “%” refers to frequency of FMR1 premutation (PM) and intermediate alleles detected in women of both groups.
Pregnant women screening for FMR1 expanded alleles
Gnomic DNA was extracted from the peripheral blood of all participating pregnant women using the standard phenol-chloroform method. Polymerase chain reactions (PCR) were initially optimized for screening of FMR1 expanded alleles in recruited pregnant women in both the risk group and control group. In the first step, specifically designed CGG repeat amplification primers forward 5’-TCAGCTCCGTTTCGGTTTC-3’ and reverse 5’-CCTTGTAGAAAGCGCCATTG-3’ were utilized in amplification reactions by optimizing PCR standard conditions as described previously23. In the second step, primers particularly designed for the random amplification of CGG repeats (c primer: 5′-GCTCAGCTCCGTTTCGGTTTCACTTCCGGT-3′ and CGG-chimeric primer:5′-AGCGTCTACTGTCTCGGCACTTGCCCGCCGCCGCCG-3′ were used in PCR reactions by optimizing amplification conditions as described previously24. Furthermore, the CGG repeats number in FMR1 expanded alleles of carrier women were determined by molecular assays using services of commercial diagnostic laboratories in Islamabad.
Collection of pregnancy outcome records
To find the association of pregnancy outcomes with FMR1 CGG repeat number, in follow-up, pregnancy outcome records were obtained from primary health centers that were opted for delivery services by participating women who were detected carriers for FMR1 expanded alleles. Those participating carrier women who opted for other health care centers for delivery services were visited at their residence sites for recording outcomes of pregnancies.
Newborns screening for FMR1 expanded alleles
Although pregnant women detected carriers for FMR1 expanded alleles were offered a prenatal diagnosis of fetuses, women who declined prenatal diagnosis of fetuses for FXS, their newborns were screened for FMR1 expanded alleles to determine the risk of maternal alleles’ expansions in offspring upon transmission. For this purpose, the heel prick method was used to collect blood from newborns up to about three or four weeks of age during their postnatal care visit to primary health care centers, then DNA was extracted from blood samples of newborns using the standard phenol-chloroform method and utilized in molecular diagnostic assays. In addition, the services of diagnostic laboratories in Islamabad were used to confirm FMR1 expanded alleles in offspring that were born from PM carrier mothers.
Statistical analysis
The characteristics of the participating pregnant women were summarized and analyzed using the SPSS 21.0 software. The FMR1 alleles’ frequencies were calculated and χ2 test as recommended previously 25,26 was used to compare the frequencies of expanded alleles in the women from the risk group and control group. A P value < 0.05 was considered significant to find the association between family history with FXS and pregnancy outcomes with FMR1 CGG repeat expansions.
Results
A total of 1950 pregnant women from Khyber Pakhtunkhwa region were screened for FMR1 CGG repeat expansion mutations. Overall, 89 (4.6%) pregnant women were detected carrying FMR1 expanded alleles. Forty-three (7.7%) women from the risk group and one (0.07%) woman from the control group were found to carry PM alleles. In addition, 32 (5.7%) women from the risk group and 13 (0.94%) women from the control group were found to carry intermediate alleles. The association between FMR1 alleles and the history of FXS is shown in Table 1. The rate of detection of intermediate alleles was found higher in the women with a family history of FXS than the women with no family history of FXS, and this difference was highly significant (P ≤ 0.0001). Similarly, the rate of detection of PM alleles was also found significantly high (P ≤ 0.0001) in the women with a family history of FXS.
Prenatal screening of pregnant women and later on screening of newborns for FMR1 expanded alleles revealed that in eleven cases maternal intermediate alleles in the range of 50–54 repeats expanded to 60–93 repeats PM alleles in newborn offspring. Similarly, in 18 cases maternal PM alleles in the range of 90–106 CGG repeats expanded to >200 repeats FM alleles in newborn offspring during transmission (Table 2).
In study follow-up, pregnancy outcomes were recorded in women that were detected carriers for FMR1 intermediate and PM alleles and are presented in Table 2. Out of 89 pregnant women carrying FMR1 allelic expansions, 24 women carrying intermediate alleles in the range of 45 and 49 repeats, fortunately, delivered normal offspring. Importantly, the birth of carrier offspring, miscarriage, and perinatal mortality were recorded as unexpected pregnancy outcomes of women who were detected carriers for intermediate alleles with CGG repeats in the range of 50 and 54 repeats. However, the rates of these pregnancy outcomes were not found significantly different in women of the risk group and control group. Evidently, in total 26 (29.2%) women carrying PM alleles in the range of 61–85 CGG repeats experienced pregnancy losses due to miscarriage. However, the miscarriage rate was found significantly high in PM carrier women from the risk group than in the control group (P = 0.0494). Similarly, a significantly high proportion of PM carrier women with a history of FXS delivered affected offspring than the PM carrier women with no specific history (P = 0.0487) as shown in Table 2.
Discussion
The CGG repeats in the FMR1 gene exhibit remarkable instability upon transmission from mothers with FMR1 expanded alleles, thus, the women carrier for expanded alleles are at an increased risk for producing offspring with FXS27. To reduce the burden of FXS-affected individuals, the accurate diagnosis of mother and fetus for FXS in the prenatal setting is available to determine whether a pregnant woman has an affected pregnancy by fragile X that is important in decision making in relation to continue or terminate pregnancy28. However, the ACOG recommends fragile X carrier screening for pregnant women with a family history of FXS in the prenatal settings20,29 that may only identify about 50% of women carrying FMR1 expanded alleles30. Therefore, population-based studies suggested and health care professionals also recommended fragile X carrier screening for pregnant women even when they do not meet ACOG screening criteria in order to identify all those women who may be at risk of an affected pregnancy22,31,32,33,34.
According to our knowledge, this is the first study from Pakistan that recruited a large number of pregnant women with or without a history of FXS in prenatal care at primary health care centers in Khyber Pakhtunkhwa region and were offered fragile X carrier screening. About 4.6% of pregnant women were detected carrying FMR1 expanded alleles and among them, 2.3% of pregnant women were found carriers for PM alleles in the present study. Population-based studies detected 0.12% Korean35, 0.3–0.2% French-Canadian36, 0.6–0.2% American37, 0.08–0.13% Chinese14,18, 0.04–0.27% Australian38,39, 0.6% Israeli31,40, 0.9% in Spanish41, and 0.4% Finnish42 pregnant women carriers for FMR1 PM alleles. A family history of FXS was found the most common contributor to the high rate of PM alleles in Pakistani pregnant women in the present study in comparison to previous studies. As the current study data analysis revealed that about 7.7% of risk group women in comparison to 0.07% of control group women were detected as PM carriers. In addition, 5.7% of risk group women in comparison to 0.94% of control group women were found carriers for FMR1 intermediate alleles. The significantly higher prevalence rates of FMR1 PM and intermediate alleles in pregnant women with a family history of FXS compared to those without any family history of FXS are in agreement with the studies conducted previously16,17,18,19. However, detection of a substantial number of pregnant women carrying FMR1 expanded alleles had no family history of FXS in the present and previous studies14,21 also suggest that fragile X carrier screening should be available to all pregnant women with or without a history of FXS in primary health care centers22.
Maternal intermediate and PM alleles contain greater CGG repeats that usually expand to PM and FM alleles upon transmission from mothers to offspring respectively8,43. The FMR1 CGG repeats' status of pregnant women and then in this study, follow-up in newborns was determined to find the effect of maternal transmissions of intermediate and PM alleles in offspring. In the previously conducted studies7,8,44 and in the present study, it has been observed that the majority of pregnant women carried intermediate alleles with repeats between 45 and 49 that were stably transmitted into offspring. However, maternal intermediate alleles in the range of 50–54 repeats in a substantial number of cases (12.4%) were found to expand to PM alleles in offspring. Consistent with our findings, a previous study has also examined the expansion of intermediate alleles in the range of 50–54 CGG repeats into PM alleles9. On the contrary, Madrigal et al.7 observed expansion of about 2% intermediate alleles in a range of 50–54 CGG repeats into FM alleles in subsequent generations. However, the risk of expansion was estimated at about 6.4% for small PM alleles with CGG repeats between 55 and 59 into FM alleles (>200 repeats) in offspring9. One possible explanation for the unstable transmissions and expansions of FMR1 alleles in future generations may be the absence of AGG interruptions within CGG repeats11,43 that could not be determined in the current study due to the utilization of commercial services for molecular diagnosis and this is the limitation of our study. In the present study, unstable transmissions and expansions of maternal PM alleles with repeats 90 and 106 into FM alleles in fetuses could not be determined as the pregnant women declined cost-effective invasive prenatal diagnosis and that is another limitation of our study. However, diagnostic laboratory reports revealed that newborns were found affected with FXS as they carried FM alleles. Importantly, prenatal diagnosis is essential if pregnant women are detected PM carriers, as it identifies 98% of fragile X affected fetuses15. In this context, studies conducted in developed countries have reported a substantial number of pregnant women carrying PM alleles, and their fetuses were detected carriers for FM alleles during prenatal FXS diagnosis9,13,14,35,45. However, in the current study fetuses’ risk for FXS could not be determined due to the high cost and non-willingness of parents to test their fetuses for fragile X expanded alleles in prenatal care. In this study follow-up, newborn screening detected a significantly high rate of FM alleles in offspring of PM carrier women with a family history of FXS, thus, the present study highlights the fact that the PM alleles have a clear risk for expansion to FM alleles in offspring during maternal transmission.
To determine the risk of expansion of maternal FMR1 alleles in offspring during transmission, pregnancy outcomes of women carrying FMR1 expanded alleles were recorded in the study follow-up. Among them, the miscarriage was observed as a common outcome of pregnancies among women carrying FMR1 expanded alleles in the present and previous studies46,47. However, large size expanded alleles were found unstable and lead to miscarriages more commonly in women with a history of FXS in the present study than in the sizes of alleles that were previously detected in women experiencing miscarriages. In addition, we found that a significant proportion of women carrying PM alleles with CGG repeats between 61 and 85 experienced miscarriages. In contrast, a study conducted in the UK recorded a non-significant proportion of women who had fragile X expansions experienced miscarriage, stillbirths, or therapeutic abortions48. Recording significantly high miscarriage rate among PM carrier women in the current study thus confirmed the association of miscarriage with FXS and may be considered a clinical predictor for FXS.
This is the first report from Pakistan presenting the findings of screening for FMR1 CGG repeat expansions in pregnant women in prenatal care. We observed that the high cost of the fetal diagnosis for FXS in Pakistan has raised prenatal counseling challenges. Screening of newborn offspring of women carrying expanded alleles revealed that the maternal PM alleles expanded to FM alleles in a significant number of offspring and thus, were diagnosed affected with FXS. The family history was found as an effective indicator for FXS carrier screening in Pakistani pregnant women. In addition, miscarriage was determined as a significant clinical predictor for FXS in Pakistani women. We recommend cost-effective prenatal screening of both mother and fetus for FMR1 expanded alleles in health care settings in Pakistan to reduce the risk of affected pregnancies, particularly of those with a family history of FXS.
Data availability
All the data used to support the findings of this study are included within the article and are available on request from the corresponding author.
References
Hagerman, R. J. & Hagerman, P. J. Fragile X Syndrome: Diagnosis, Treatment, and Research (Taylor & Francis US, 2002).
Omar, H. A. H., Kamal, T. M., Abd-Alkhalek, H. S., El Nady, G. H. & Salem, M. Molecular characterization of X chromosome fragility in idiopathic mental retardation. Egyptian. J. Med. Hum. Genet. 17, 165–172 (2016).
Wheeler, A., Raspa, M., Bishop, E. & Bailey, D. Jr Aggression in fragile X syndrome. J. Intellect. Disabil. Res. 60, 113–125 (2016).
Brackett, D. M. et al. FMR1 transcript isoforms: association with polyribosomes; regional and developmental expression in mouse brain. PloS One 8, e58296 (2013).
Alvarez-Mora, M. I. et al. Paternal transmission of a FMR1 full mutation allele. Am. J. Med. Genet. Part A 173, 2795–2797 (2017).
Hunter, J. et al. Epidemiology of fragile X syndrome: a systematic review and meta‐analysis. Am. J. Med. Genet. Part A 164, 1648–1658 (2014).
Madrigal, I. et al. Intermediate FMR1 alleles and cognitive and/or behavioural phenotypes. Eur. J. Hum. Genet. 19, 921–923 (2011).
Nolin, S. L. et al. Expansion of the fragile X CGG repeat in females with premutation or intermediate alleles. Am. J. Hum. Genet. 72, 454–464 (2003).
Tejada, M.-I., et al. Molecular testing for fragile X: analysis of 5062 tests from 1105 fragile X families—performed in 12 clinical laboratories in spain. Biomed. Res. Int. 2014, 195793 (2014).
Jacquemont, S. et al. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to the mGluR5 antagonist AFQ056. Sci. Transl. Med. 3, 64ra1–64ra1 (2011).
Villate, O. et al. Effect of AGG interruptions on FMR1 maternal transmissions. Front. Mol. Biosci. 7, 135 (2020).
Fernandez-Carvajal, I. et al. Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. J. Mol. Diagnostics 11, 324–329 (2009).
Seneca, S. et al. Evaluation of a CGG repeat primed PCR system designed for detection of Fragile X expanded alleles in clinical prenatal samples. Abstract book of the 12th Annual Meeting of the Belgian Society of Human Genetics (2012).
Hung, C.-C. et al. Fragile X syndrome carrier screening in pregnant women in Chinese Han population. Sci. Rep. 9, 1–7 (2019).
Musci, T. J. & Caughey, A. B. Cost-effectiveness analysis of prenatal population-based fragile X carrier screening. Am. J. Obstet. Gynecol. 189, S117 (2003).
Niu, M. et al. Fragile X syndrome: prevalence, treatment, and prevention in China. Front. Neurol. 8, 254 (2017).
Kidd, S. A. et al. Fragile X syndrome: a review of associated medical problems. Pediatrics 134, 995–1005 (2014).
Cheng, Y. K. et al. Identification of fragile X pre-mutation carriers in the Chinese obstetric population using a robust FMR1 polymerase chain reaction assay: implications for screening and prenatal diagnosis. Hong. Kong Med J. 23, 110–116 (2017).
Johansen Taber, K., Lim‐Harashima, J., Naemi, H. & Goldberg, J. Fragile X syndrome carrier screening accompanied by genetic consultation has clinical utility in populations beyond those recommended by guidelines. Mol. Genet. Genom. Med. 7, e1024 (2019).
Genetics, G. C. ACOG Committee opinion no. 469: carrier screening for fragile X syndrome. Obstet. Gynecol. 116, 1008–1010 (2010).
Cronister, A., DiMaio, M., Mahoney, M. J., Donnenfeld, A. E. & Hallam, S. Fragile X syndrome carrier screening in the prenatal genetic counseling setting. Genet. Med. 7, 246–250 (2005).
Nolin, S. L. et al. Fragile X full mutation expansions are inhibited by one or more AGG interruptions in premutation carriers. Genet. Med. 17, 358–364 (2015).
Tassone, F., Pan, R., Amiri, K., Taylor, A. K. & Hagerman, P. J. A rapid polymerase chain reaction-based screening method for identification of all expanded alleles of the fragile X (FMR1) gene in newborn and high-risk populations. J. Mol. Diagnostics 10, 43–49 (2008).
Todorov, T., Todorova, A., Georgieva, B. & Mitev, V. A unified rapid PCR method for detection of normal and expanded trinucleotide alleles of CAG repeats in huntington chorea and CGG repeats in fragile X syndrome. Mol. Biotechnol. 45, 150–154 (2010).
Campbell, I. Chi‐squared and Fisher–Irwin tests of two‐by‐two tables with small sample recommendations. Stat. Med. 26, 3661–3675 (2007).
Richardson, J. The analysis of 2×2 contingency tables-yet again. Stat. Med. 30, 890 (2011). author reply 891-2.
Wheeler, A., Raspa, M., Hagerman, R., Mailick, M. & Riley, C. Implications of the FMR1 premutation for children, adolescents, adults, and their families. Pediatrics 139(Supplement 3), S172–S182 (2017).
Ma, Y. et al. The prevalence of CGG repeat expansion mutation in FMR1 gene in the northern Chinese women of reproductive age. BMC Med. Genet. 20, 1–5 (2019).
Sherman, S., Pletcher, B. A. & Driscoll, D. A. Fragile X syndrome: diagnostic and carrier testing. Genet. Med. 7, 584–587 (2005).
Rajendra, K., Bringman, J. J., Ward, J. & Phillips, O. P. Who should be tested for fragile X carriership? A review of 1 center’s pedigrees. Am. J. Obstet. Gynecol. 198, e51–e53 (2008).
Toledano-Alhadef, H. et al. Fragile-X carrier screening and the prevalence of premutation and full-mutation carriers in Israel. Am. J. Hum. Genet. 69, 351–360 (2001).
Fanos, J. H., Spangner, K. A. & Musci, T. J. Attitudes toward prenatal screening and testing for Fragile X. Genet. Med. 8, 129–133 (2006).
Acharya, K. & Ross, L. F. Fragile X screening: attitudes of genetic health professionals. Am. J. Med. Genet. Part A 149, 626–632 (2009).
Taber, K. A. J. et al. Clinical utility of expanded carrier screening: results-guided actionability and outcomes. Genet. Med. 21, 1041–1048 (2019).
Jang, J. H. et al. Frequency of FMR1 premutation carriers and rate of expansion to full mutation in a retrospective diagnostic FMR1 Korean sample. Clin. Genet. 85, 441–445 (2014).
Levesque, S. et al. Screening and instability of FMR1 alleles in a prospective sample of 24,449 mother–newborn pairs from the general population. Clin. Genet. 76, 511–523 (2009).
Seltzer, M. M. et al. Prevalence of CGG expansions of the FMR1 gene in a US population‐based sample. Am. J. Med. Genet. Part B: Neuropsychiatr. Genet. 159, 589–597 (2012).
Metcalfe, S. A. et al. Informed decision making and psychosocial outcomes in pregnant and nonpregnant women offered population fragile X carrier screening. Genet. Med. 19, 1346–1355 (2017).
Kraan, C. M. et al. FMR1 allele size distribution in 35,000 males and females: a comparison of developmental delay and general population cohorts. Genet. Med. 20, 1627–1634 (2018).
Berkenstadt, M., Ries‐Levavi, L., Cuckle, H., Peleg, L. & Barkai, G. Preconceptional and prenatal screening for fragile X syndrome: experience with 40 000 tests. Prenat. Diagn. 27, 991–994 (2007).
Alfaro Arenas, R., Rosell Andreo, J. & Heine Suñer, D. Islands, GftsoFitB. Fragile X syndrome screening in pregnant women and women planning pregnancy shows a remarkably high FMR1 premutation prevalence in the Balearic Islands. Am. J. Med. Genet. Part B: Neuropsychiatr. Genet. 171, 1023–1031 (2016).
Ryynänen, M. et al. Feasibility and acceptance of screening for fragile X mutations in low-risk pregnancies. Eur. J. Hum. Genet. 7, 212–216 (1999).
Nolin, S. L. et al. Fragile X AGG analysis provides new risk predictions for 45–69 repeat alleles. Am. J. Med. Genet. Part A 161, 771–778 (2013).
Capelli, L. P., Mingroni-Netto, R. C. & Vianna-Morgante, A. M. Structure and stability upon maternal transmission of common and intermediate FMR1 (Fragile X Mental Retardation 1) alleles in a sample of the Brazilian population. Genet. Mol. Biol. 28, 10–15 (2005).
Kim, M. J. et al. Fragile X carrier screening in Korean women of reproductive age. J. Med. Screen. 20, 15–20 (2013).
Fragkos, M., Bili, H., Ntelios, D., Tzimagiorgis, G. & Tarlatzis, B. Are expanded alleles of the FMR1 gene related to unexplained recurrent miscarriages? Hippokratia 22, 132 (2018).
Wang, X-H. et al. Expanded alleles of the FMR1 gene are related to unexplained recurrent miscarriages. Biosci. Rep. 37, BSR20170856 (2017).
Murray, A., Ennis, S., MacSwiney, F., Webb, J. & Morton, N. E. Reproductive and menstrual history of females with fragile X expansions. Eur. J. Hum. Genet. 8, 247–252 (2000).
Acknowledgements
The authors acknowledge all those women who participated in the study.
Funding
This study was financially supported by Higher Education Commission (HEC) under National Research Program for Universities (NRPU) project # 5886.
Author information
Authors and Affiliations
Contributions
Clinical data collection, collation, and analysis: R.S., M.Y., H.J., H.T., M.N., N.M., M.J., and S.S. Genetic testing and data analysis: R.S., M.Y., H.T., N.M., I.Z., M.Z., A.A., M.M., N.K., A.H., Z.U.R., and S.S. Manuscript writing and revision: R.S., N.K., Z.U.R., and S.S. Study supervision and coordination: Z.U.R. and S.S. The manuscript has been read and approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The approval of the present study was obtained from the Research and Ethical Committee and Advanced Studies Research Board (ASRB) of Kohat University of Science and Technology (KUST), Kohat, Khyber Pakhtunkhwa, Pakistan.
Consent for publication
Written informed consent for the publication of the details has been obtained from the participating women, who provided blood samples.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shahid, R., Yasin, M., Rehman, Z.U. et al. Maternal FMR1 alleles expansion in newborns during transmission: a prospective cohort study. Pediatr Res 93, 720–724 (2023). https://doi.org/10.1038/s41390-022-02128-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02128-2