Abstract
Background
There are limited data on neutrophil function in pediatric-onset systemic lupus erythematosus (pSLE) patients. This study aimed to evaluate phagocytosis and oxidase activity of neutrophils in patients with pSLE.
Patients and methods
Eighty-seven patients with pSLE and 44 controls were enrolled. Phagocytic activity was assayed using pHrodoTMRed E. coli BioParticles Phagocytosis Kit by flow cytometry. Determination of NADPH oxidase activity was carried out by Dihyrdrorhodamine-123 (DHR-123) flow cytometry assay.
Results
Phagocytic activity of patients’ neutrophils (mean 76.59%) was lower than that in controls (91.30%) (p < 0.001). Median delta median fluorescence intensity (ΔMFI) and stimulation index (SI) in patients (ΔMFI: 0.09; SI: 2.79) were also decreased compared to controls (ΔMFI: 0.18; SI: 5.00) (p < 0.002; p < 0.001 respectively). Disease activity showed an inverse correlation with phagocytic activity. Oxidase activity was also significantly low (SI DHR < 40) in 16% of patients. No significant correlation was found between oxidative burst and disease activity.
Conclusion
Neutrophil function is impaired in patients with pSLE, as evidenced by the markedly reduced phagocytic activity. Phagocytic activity is also inversely correlated with disease activity. The oxidative activity was also reduced but not significantly.
Impact
-
Neutrophil phagocytic function is impaired in pediatric-onset systemic lupus erythematosus (pSLE).
-
There is an inverse correlation between disease activity in pSLE and phagocytic activity.
-
NADPH oxidase activity in patients with pSLE did not show significant correlation with disease activity.
Similar content being viewed by others
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disorder of unclear etiology with significant long-term morbidity and mortality, especially in children1,2. Our center has more than 25 years of experience in diagnosing and managing children with rheumatological disorders. We have previously shown that significant causes of mortality in pediatric-onset systemic lupus erythematosus (pSLE) are breakthrough infections and disease activity3,4,5.
There is some evidence that innate immunity can be significantly compromised in children with lupus6. The complement cascade and neutrophil phagocytosis (followed by production of reactive oxygen species) are two most integral components of innate immunity. Previous work done in our unit has shown that C1q deficiency is intricately linked to the pathogenesis of early-onset lupus7. In this study, we analyzed neutrophil function in children with lupus.
Neutrophils in patients with adult-onset SLE have several alterations in functional properties. These include reduced phagocytic activity, enhanced expression of adhesion molecules, raised cellular aggregation and in vivo intravascular stimulation8,9,10. Impaired function of neutrophils may also lead to severe bacterial infections8,11,12. In patients with chronic granulomatous disease, neutrophils are unable to generate reactive oxygen species upon stimulation. Patients with chronic granulomatous disease (and female carriers with monoallelic CYBB gene variants) exhibit an increased susceptibility for development of autoimmunity, especially lupus. An increased frequency of discoid lesions (2.7%) and SLE (0.5%) is seen in these carriers, but data regarding NADPH oxidase activity in patients with adult lupus are conflicting. And, there is hardly any literature on the subject in pSLE.
A paucity of data on phagocytic activity and oxidative burst activity in pSLE led us to investigate this aspect in the present study and to assess the effect of disease activity on these functions.13,14,15,16
Patients and methods
This observational study was carried out at a tertiary care center in North-West India over 18 months: July 2017 - December 2018. Eighty-seven patients with pSLE, under follow-up at the Pediatric Rheumatology Clinic of our center with disease onset before 18 years and fulfilling the Systemic Lupus International Collaborating Clinics (SLICC) Classification Criteria 2012 for SLE were enrolled17. Patients having overlap with other rheumatologic disorders were excluded.
A written, informed parental consent (for patients < 8 years) along with assent (for patients >8 years, as per our institutional guidelines) was obtained from the patient or one of the parents. The study was approved by the Departmental Review Board, Institute Thesis Committee, and Institute Ethics Committee.
Baseline information on clinical features at diagnosis, laboratory tests and treatment details were recorded in a predetermined format. Disease activity was measured by Safety of Estrogens in Lupus National Assessment Study (SELENA) - Systemic Lupus Erythematous Disease Activity Index (SLEDAI) Flare Index18. This is a composite scoring system. It includes change in the SELENA-SLEDAI score, physician global assessment (PGA), and treatment modifications. A SLEDAI score ≥3 points and ≥1-point increase in PGA (range 0–3) was considered a disease flare. Treatment modifications include change in dose of prednisolone or addition of any therapy for disease activity18.
Forty-four children were also enrolled as controls. Control group comprised age and sex-matched healthy children attending pediatric outpatient department for vaccination or health check-up.
Demographic features
Of 87 patients with pSLE, 63 (72.4%) were female. The mean age at onset of disease was 8.52 ± 2.95 years, while mean age at recruitment in the study was 12.34 ± 5.67 years. Duration of illness at time of enrollment was 3.04 ± 3.45 years. Family history of autoimmune disease was present in 8 (9.2%) patients. Major organ involvement was seen in 44 (50.6%) patients (Table 1). Two patients (2.3%) had monogenic lupus due to C1q deficiency, while 2 (2.3%) had selective IgA deficiency. Both children with C1q deficiency had had disease onset below 5 years of age. Functional activity of the classical complement pathway (as determined by a commercial ELISA) was markedly reduced, C1q levels were low, and complement C3 and C4 were normal in these 2 patients. The values were as follows in both patients - CH50 (0%; 0.1%), C3 (1610; 2500 mg/L), C4 (420; 500 mg/L) and C1q (0.37; 0.24 mg/L). This subset has been published previously from our center7.
Laboratory protocols
All laboratory tests were carried out at the Pediatric Immunology Laboratory, a designated Indian Council of Medical Research Centre for Advanced Research for Primary Immunodeficiency Diseases.
Phagocytosis assay
Phagocytic activity was assayed using pHrodoTMRed E. coli bioparticles Phagocytosis Kit (Cat no A10025; Thermo Fisher Scientific), by flow cytometry on heparinized peripheral blood. This kit uses unique and patented pHrhodo dyes that fluoresce when pH of the surrounding medium becomes acidic upon ingestion by phagocytic cells, thereby eliminating the steps of quenching required for other flow-based tests. Phagocytic function of neutrophils and monocytes was measured by adding pHrodoTM E. Coli bioparticles to heparinized whole blood samples of patients and controls. These bioparticles were incubated with 100 µL of patient and control blood samples in four tubes; two tubes were incubated for 15 minutes at 37 °C and the other two tubes were incubated on ice. Sample tubes incubated at 37 °C were then transferred to ice. Lysing solution (buffer A–Thermo Fisher ScientificTM) was added to all tubes for lysing red cells, followed by incubation with buffer B (Thermo Fisher ScientificTM). Tubes were then washed with washing buffer and re-suspended for acquisition on a flow cytometer (Navios, Beckman Coulter). Neutrophils and monocytes were gated on Forward Scatter (FSc) and Side Scatter (SSc).
Ingestion of bacteria was determined by measuring pHrhodo fluorescence at 585 nm using 488 nm excitation. Data were analyzed using Kaluza Flow Cytometry Analysis Software Version 2.1. Percentage of neutrophils showing phagocytosis and their median fluorescence intensity were determined. These included the percentage of neutrophils showing phagocytosis and different median fluorescence intensity (MFI) values of phagocytosis in samples, both on-ice and at 37 °C temperature. Difference in the median fluorescence intensity of samples incubated at 37 °C and on ice (ΔMFI) and stimulation index (SI) for phagocytosis i.e., ratio of median fluorescence intensity at 37 °C and median fluorescence intensity on ice were calculated for each sample. SI was calculated by dividing MFI of phagocytosis at 37 °C by MFI of phagocytosis on ice for test and control samples. ΔMFI was calculated by subtracting MFI of phagocytosis on ice from MFI of phagocytosis at 37 °C for test and control samples.
Oxidative burst assay
Determination of NADPH oxidase activity was carried out by Dihyrdrorhodamine-123 (DHR-123) flow cytometry assay. For this purpose, we took 100 μL of blood in 3 polypropylene test tubes. These were labeled as (A) cells alone, (B) unstimulated and, (C) stimulated. We then added 0.75 µL (7.5 µg) of DHR-123 dye to tubes (B) and (C). The tubes were then vortexed and incubated at 37 °C in a shaking-water bath for 5 minutes. After incubation, 2 µL (200 ng) of PMA (1:10 diluted) was added to tube (C) and vortexed well. Tubes were then incubated at 37 °C in shaking water bath for 15 minutes. Following incubation, red cells were lysed by using a lysis buffer. All tubes were washed with phosphate-buffered saline (PBS), and pellet was re-suspended in 300 μL of PBS. Samples were acquired on a flow cytometer within 15–30 min of completion of the test procedure. Oxidase activity was analyzed by estimating the median fluorescence intensity of PMA stimulated neutrophils compared to unstimulated neutrophils and the percentage of neutrophils showing oxidase activity. Neutrophils were gated on FSc and SSc and MFI of stimulated and unstimulated neutrophils were computed using the Kaluza software Version 2.1. SI and ΔMFI were calculated.
The calculation of oxidase or phagocytic activity based merely on the percentage of neutrophils or monocytes showing activity is fallacious as it does not quantity the intensity of this activity. Calculation of SI and ∆MFI provides a more objective measure of intensities of these activities. There could be instances wherein percentage of neutrophils and monocytes showing NADPH oxidase activity or phagocytosis may be normal yet the amount of this activity may be significantly impaired. This can be detected by using SI or ∆MFI in addition to the percentage positivity.
Statistical analysis
Parameters showing normal distribution were depicted as mean and standard deviation, and variability between cases and controls was tested with Student’s T test. Variables with skewed distribution were expressed as median and interquartile ranges (IQR), and variation between groups was measured by Mann-Whitney U test. A p-value of <0.05 was regarded as significant. Statistical analysis was accomplished using SPSS statistical software version 20.0 (SPSS Inc., Chicago, IL).
Results
Based on SELENA-SLEDAI Flare Index, patients were categorized into two groups: pSLE with active disease (n = 31; 35.6%) and pSLE with inactive disease (n = 56; 64.4%). Of 31 patients with active disease, three also had evidence of a concurrent infection. There was no patient with infection in inactive disease group. Patients with active disease had significantly lower hemoglobin, total leukocyte count, absolute lymphocyte count, and platelet count than patients with inactive disease (Table 1).
Phagocytic assay
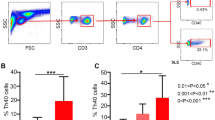
All three analytical parameters (i.e., neutrophils showing % phagocytic activity, ΔMFI and SI values) were lower in children with pSLE than controls (Fig. 1, Supplementary Table). Mean phagocytic activity in active and inactive pSLE were 65.45 ± 23.65%, 85.74 ± 11.59%, respectively (p < 0.001). Median ΔMFI phagocytosis in active and inactive pSLE were 0.06 (0.03–0.12) and 0.12 (0.08–0.20) respectively (p < 0.001). Median (IQR) SI phagocytosis inactive disease and inactive pSLE were 2.40 (1.65–3.55) and 3 (2.00–4.28) respectively (p = 0.16) (Fig. 1). The dose of prednisolone had no significant correlation with percentage phagocytic activity, ΔMFI phagocytosis, and SI phagocytosis. The phagocytic activity of neutrophils in the two C1q deficient patients was similar to the other patients in the cohort.
a–d: 1a: phRhodo E. coli BioParticles based phagocytic assay showing decreased phagocytic activity in a pSLE patient when compared to healthy control; 1b: percentage phagocytic activity; 1c: Delta median fluorescence intensity (ΔMFI) phagocytosis; 1d: stimulation index (SI) phagocytosis in patients with active disease, inactive disease, and healthy controls [DHR: dihyrdrorhodamine; ΔMFI: Delta median fluorescence intensity; SI: Stimulation index; **p ≤ 0.001; and ***p < 0.0001. Bar indicating the standard error of the mean (SEM)].
Oxidative burst assay
Neutrophils showing oxidase activity were lower in patients with pSLE than controls (Fig. 2). There was a distinct trend towards lower oxidative burst activity in patients with pSLE. Fourteen patients (16.09%) had significantly reduced SI values (<40) on DHR compared to controls. On inter-group comparison between pSLE and control groups, however, the mean difference in ΔMFI and SI values were not significantly different between patients and controls (Supplementary Table). In addition, two girls showed a typical mosaic pattern with double peaks on DHR flow cytometry (Fig. 2). Oxidase activity on neutrophils in active and inactive pSLE was 83.12 ± 17.85% and 84.76 ± 17.15%, respectively (p = 0.85). Median ΔMFI on DHR assay in patients with active and inactive pSLE was 133881.4 (81487.2–161305.2) and 129858.6 (55578.67–155194.29), respectively (p = 0.87). Median SI DHR assay in active and inactive pSLE was 84.20 (57.19–138.75) and 106.62 (20.78–158.38), respectively (p = 0.65) (Fig. 2). Dose of prednisolone did not show any significant correlation with ΔMFI and SI values.
a–e: 2a: Dihyrdrorhodamine (DHR) flow cytometry plots of a pSLE patient showing reduced oxidative burst assay; 2b: DHR flow of a pSLE patient with double peak (mosaic pattern) on oxidative burst assay as compared to control; 2C: Percentage oxidase activity; 2d: ΔMFI dihyrdrorhodamine (DHR); 2e: SI DHR in patients with active disease, inactive disease, and healthy controls [DHR: dihyrdrorhodamine; ΔMFI: Delta median fluorescence intensity; SI: Stimulation index; *p ≤ 0.05. Bar indicating the standard error of the mean (SEM)].
Correlation of SLEDAI score and neutrophil functions
Disease activity in patients with pSLE was measured by SLEDAI scoring. Phagocytic activity (Fig. 3) and oxidative burst activity were analyzed for correlation with SLEDAI scores. Both oxidative burst and phagocytic activity showed an inverse correlation with SLEDAI scores (Table 2).
We also analyzed the neutrophil phagocytic functions and oxidase activity excluding 3 patients with disease activity accompanied with ongoing infection. However, disease activity showed impaired phagocytosis independent of ongoing infection in patients with disease activity (Supplementary Figure). At bedside, it is difficult to differentiate between infection or disease activity in children with lupus.
Discussion
Patients with pSLE have significant long-term morbidity, and mortality3,4,19. Previous work done at our center has shown that pediatric lupus is associated with mortality rates as high as 20%3,20. We have further demonstrated that significant causes of mortality in pSLE are disease activity and breakthrough infections.
Major and often life-threatening organ involvement in patients with pSLE is responsible for the rapid clinical deterioration that is often seen in these patients3. Renal, neuropsychiatric, and myocardial involvement is usually incriminated in this deterioration4,19,21,22.
However, it is possible that another significant contributory factor could be an underlying and hitherto unrecognized immune defect in these patients. We have previously demonstrated in our cohort of pSLE that children with disease onset below five years have early complement deficiencies, especially C1q deficiency7. However, not much is known about the role of other aspects of innate immunity in pSLE. This lacuna in existing literature led us to conduct the present study.
We herein report the largest and most comprehensive analysis of innate immune function in patients with pSLE. We have assayed phagocytosis and NADPH oxidase activity by DHR in 87 patients with pSLE. There is a paucity of published literature on the subject. Most of the previous studies had small sample size and had carried out much less detailed laboratory work-up of neutrophilic function (Table 3)13,14,15,16,23.
Our results showed that phagocytic activity (as assessed by pHrodoTM E. Coli bioparticles phagocytosis assay) was significantly reduced in pSLE patients in comparison to controls (p < 0.001). Phagocytic activity of neutrophils showed an inverse correlation with the SLEDAI score and was lower in patients with active lupus. Sampath et al have performed pHrodo labeled E. Coli beads based phagocytic assay in neutrophils of patients with pSLE. They have also shown that patients with pSLE had lower phagocytic ability than controls, and disease activity negatively correlated with phagocytosis16. However, their study comprised only six patients. Moreover, precise details of laboratory techniques were not available. Wu et al. studied the function of neutrophils in 34 patients concerning peroxidase production, chemotaxis, and phagocytosis in 34 pSLE patients. While phagocytic ability against Salmonella-specific LPS was significantly decreased in patients with pSLE (mean 74.91%) compared to controls (mean 94.32%), phagocytic ability against Staphylococcus aureus and E. coli in patients with active and inactive SLE when compared to healthy controls were similar15. Our study has conclusively demonstrated reduced phagocytic activity in pediatric lupus patients with active disease using a technically superior and more sensitive method, namely the use of patented pHRhodo bioparticles, which fluoresce only in the acidic environment of phagolysosomes. Ballantine et al have also shown that the phagocytic function of monocytes/macrophages is reduced after the addition of serum of pSLE patients compared to controls14. However, the study sample was small (n = 8), and controls were not age-matched. To summarize we have shown impaired neutrophil phagocytosis in patients with lupus in comparison to healthy controls. However, there may be other confounding variables like antibodies and complements which may also impact the phagocytic activity.
The second major aspect of our study was the assay of oxidative burst activity as measured by the DHR test. There was a significantly lower percentage of oxidase-positive neutrophils in pSLE patients compared to healthy controls. Although there was a trend towards a decrease in ΔMFI and SI on DHR in pSLE patients, this did not achieve statistical significance. However, 14 (16.09%) patients had significantly reduced SI values as compared to controls.
Marini et al. measured superoxide production by spectrophotometric method and showed lower superoxide production in pSLE when compared to controls. However, there was no difference in patients with and without disease activity13. It may be noted that the sample size of the study was comparatively small (n = 23). Further, the investigators did not include age-matched controls and had compared their results with adult controls. It is, therefore, difficult to interpret their results in the context of pSLE.
Wu et al. have studied peroxidase production by fluorescence microscopy method and showed no difference in peroxidase production between patients with pSLE and controls. Further, there was no effect of lupus disease activity on peroxidase production15. However, it may be noted that fluorescence microcopy, at best, provides only a semi-quantitative estimate of peroxidase production. Flow cytometry-based tests of oxidase activity (such as the Dihydrorhodamine) are a more sensitive and accurate method for measuring oxidase function. We had carried out the latter test in our study. In a more recent study, El-Ghoneimy et al. enrolled 50 patients with pSLE. The investigators showed no significant difference in NADPH oxidase activity between patients and controls23. It was also demonstrated that DHR test values correlated negatively with SLEDAI score and lupus disease activity23.
Our study has shown a distinct trend towards decreased oxidative burst activity in patients with pSLE compared to healthy controls. Sixteen per cent of patients with pSLE had significantly reduced SI values on DHR compared to controls. However, the differences did not reach statistical significance probably because the sample size was not large enough. Oxidative burst activity in active and inactive pSLE was comparable. Although SLEDAI scores had shown a negative correlation with oxidative burst activity, these were not statistically significant.
In our study, both phagocytic activity and oxidative burst did not exhibit any notable correlation with the dose of corticosteroids. Our findings align with those reported by Wu et al, who also showed no remarkable correlation between cumulative steroid dose and phagocytosis against salmonella-specific lipopolysaccharide in their cohort of pSLE15. Two patients in our study showed a typical mosaic pattern with a characteristic double peak on DHR flow cytometry assay, thereby indicating two distinct populations of neutrophils with present and absent oxidase activity.
To summarize, this study on 87 patients with pSLE has shown significantly reduced phagocytic activity of neutrophils. A distinct trend towards decreased oxidative burst activity in patients with pSLE was present. This is the largest study on innate immune function in patients with pSLE. We have carried out a detailed and comprehensive assay of neutrophil functions in patients with pSLE. Our results will help in understanding the role of neutrophil functions in pathogenesis and disease activity of patients with pSLE.
Strengths of the study
-
1.
Patient enrollment was very systematic, and inclusion criteria were strictly followed. We have enrolled the patients who fulfilled the SLICC Criteria. Patients with lupus who had an overlap of other rheumatological disorders were excluded. The study was carried out in a center with more than 25 years of experience diagnosing and managing children with lupus.
-
2.
Our study group comprised 87 patients. This is the most significant number of pSLE ever enrolled for a study of innate immunity in pSLE.
-
3.
All tests were carried out in an accredited laboratory, a designated Indian Council of Medical Research Centre for Advanced Research in Primary Immunodeficiency Diseases. All flow cytometry tests were performed on standardized protocols under an experienced laboratory consultant with over 12 years of experience. All assays were paired with controls.
-
4.
Phagocytic activity was assessed with pHrhodo dyes. These fluoresce only in the acidic environment of phagolysosome and are thus the most sensitive and specific markers for phagocytosis.
-
5.
There was an unequivocal demonstration of reduced phagocytic activity in pSLE patients compared to controls. This was also statistically significant.
-
6.
Two patients had a typical mosaic pattern of double peaks on DHR flow-based assays. This is a pointer towards a putative carrier state for X-linked chronic granulomatous disease. This may suggest that some patients with pSLE may have a defect in an oxidative burst of neutrophils similar to the defect seen in patients with chronic granulomatous disease. Patients with pSLE who have such mosaic patterns on flow cytometry may benefit from long-term prophylactic antimicrobials.
Limitations of the study
-
1.
DHR was performed using PMA as stimulant. We were unable to repeat the test using other stimulants [such as N-formyl-methionyl-leucyl-phenylalanine (fMLP), E Coli, Candida] because of ethical reasons. Repeating these tests would have mandated more venepunctures in children.
-
2.
As pediatric lupus is not a common disease, the number of patients of pSLE at any given center is always limited. A larger sample size using a multi-centric cohort would undoubtedly be the way forward. However, this was beyond the mandate of the present study.
-
3.
We have shown impaired neutrophil phagocytosis in patients with lupus in comparison to healthy controls. However, there may be other confounding variables like antibodies and complements that may also impact the phagocytic activity.
-
4.
In our study we did not use specific markers for gating neutrophils as there was a clear separation of lymphocyte, monocyte and neutrophil populations based on FSc and SSc in all cases and controls. However, addition of specific markers for gating would have increase the specificity of gated populations.
Conclusions
The present study indicates that neutrophil function is impaired in patients with pSLE and correlates with disease activity. Our results show that the E. coli based phagocytic function of neutrophils was notably decreased in patients with pSLE in comparison to healthy controls. Phagocytic function of neutrophils was appreciably lower in pSLE patients with active disease and those with breakthrough infections. Oxidative burst activity (SI values in DHR) was reduced in 16% of patients with pSLE compared to healthy controls, and there was a distinct trend towards decreased oxidative burst activity. These defects in innate immunity may be contributing to the high morbidity and mortality of pSLE. It is hoped that our results will help in better delineation of the role of neutrophils in pathogenesis and disease activity of children with lupus. However, it is conjectural whether patients having significantly impaired phagocytosis would have more severe disease course.
Data availability
The data necessary for the findings of this paper are included in the manuscript, online supplementary data, and in our records. These data can be shared on request.
References
Descloux, E. et al. Influence of age at disease onset in the outcome of paediatric systemic lupus erythematosus. Rheumatol. Oxf. Engl. 48, 779–784 (2009).
Singh, S. et al. Clinical and immunological profile of SLE: some unusual features. Indian Pediatr. 34, 979–986 (1997).
Abujam, B., Gupta, A., Suri, D., Rawat, A. & Singh, S. Trends and predictors of mortality in childhood onset lupus in a single North-Indian centre over 23 years: a retrospective study. Clin. Exp. Rheumatol. 34, 554–559 (2016).
Singh, S. et al. Childhood lupus nephritis in a developing country-24 years’ single-center experience from North India. Lupus 24, 641–647 (2015).
Srivastava, P. et al. Outcome of lupus nephritis in childhood onset SLE in North and Central India: single-centre experience over 25 years. Lupus 25, 547–557 (2016).
Herrada, A. A. et al. Innate immune cells’ contribution to systemic Lupus Erythematosus. Front Immunol. 10, 772 (2019).
Bhattad, S. et al. Early complement component deficiency in a single-centre cohort of pediatric onset lupus. J. Clin. Immunol. 35, 777–785 (2015).
Kaplan, M. J. Role of neutrophils in systemic autoimmune diseases. Arthritis Res Ther. 15, 219 (2013).
Deng, G.-M., Guo, X., Fang, X., Qiao, W. & Fei, X. The role of neutrophils in organ tissue damage in SLE. J. Immunol. 198, 55.5–55.5 (2017).
Moulton, V. R. et al. Pathogenesis of human systemic lupus erythematosus: a cellular perspective. Trends Mol. Med. 23, 615–635 (2017).
Kaplan, M. J. Neutrophils in the pathogenesis and manifestations of SLE. Nat. Rev. Rheumatol. 7, 691–699 (2011).
Landry, M. Phagocyte function and cell-mediated immunity in systemic lupus erythematosus. Arch. Dermatol. 113, 147–154 (1977).
Marini, R., Condino-Neto, A., Appenzeller, S., Morcillo, A. M. & Costallat, L. T. L. Superoxide release in juvenile systemic lupus erythematosus. Rheumatol. Int. 32, 1977–1983 (2012).
Ballantine, L. et al. Increased soluble phagocytic receptors sMer, sTyro3 and sAxl and reduced phagocytosis in juvenile-onset systemic lupus erythematosus. Pediatr. Rheumatol. Online J. 13, 10 (2015).
Wu, S.-A. et al. Impaired phagocytosis and susceptibility to infection in pediatric-onset systemic lupus erythematosus. Lupus 22, 279–288 (2013).
Sampath, S. Juvenile systemic lupus erythematosus serum reduces phagocytic activity of healthy polymorphonuclear cells. Rheumatology 53, i148–i149 (2014).
Petri, M. et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 64, 2677–2686 (2012).
Petri, M. et al. Combined oral contraceptives in women with systemic lupus erythematosus. N. Engl. J. Med. 353, 2550–2558 (2005).
Singh, S., Gupta, M. K., Ahluwalia, J., Singh, P. & Malhi, P. Neuropsychiatric manifestations and antiphospholipid antibodies in pediatric onset lupus: 14 years of experience from a tertiary center of North India. Rheumatol. Int. 29, 1455–1461 (2009).
Singh, S., Devidayal, null, Kumar, L. & Joshi, K. Mortality patterns in childhood lupus-10 years’ experience in a developing country. Clin. Rheumatol. 21, 462–465 (2002).
Singh, S., Devidayal, null, Minz, R., Nada, R. & Joshi, K. Childhood lupus nephritis: 12 years experience from North India. Rheumatol. Int. 26, 604–607 (2006).
Harrison, M. J., Zühlke, L. J., Lewandowski, L. B. & Scott, C. Pediatric systemic lupus erythematosus patients in South Africa have high prevalence and severity of cardiac and vascular manifestations. Pediatr. Rheumatol. Online J. 17, 76 (2019).
El-Ghoneimy, D. H., Hesham, M., Hasan, R., Tarif, M. & Gouda, S. The behavior of neutrophil extracellular traps and NADPH oxidative activity in pediatric systemic lupus erythematosus: relation to disease activity and lupus nephritis. Clin. Rheumatol. 38, 2585–2593 (2019).
Acknowledgements
The authors thankfully acknowledge Postgraduate Institute of Medical Education and Research, Chandigarh, India for funding vide Grant No (PGI/MERC/2017/351715). The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation. This study was approved by the Institute Ethics Committee, Institute Thesis Committee and Departmental Review Board of Pediatrics at the Advanced Pediatrics Centre, Postgraduate Institute of Medical Education and Research, Chandigarh, India. We deeply thank patients and their parents for their cooperation.
Funding
We thankfully acknowledge Postgraduate Institute of Medical Education and Research, Chandigarh, India for granting Institutional Thesis Grant (PGI/MERC/2017/351715) for this work.
Author information
Authors and Affiliations
Contributions
R.K.P.: inception of idea, design of the research, performance of laboratory tests, data collection, patient management, data interpretation, writing of first draft, review of literature, editing of manuscript and critical revision of manuscript at all stages. A.R.: inception of idea, design of the research, standardization of flow cytometry tests, data interpretation, editing of manuscript, critical revision at all stages, and final approval of the manuscript. R.K.P., A.R., J.S., and K.A. standardized and performed the flow cytometry tests. R.K.P., A.R., J.S., K.A., A.G., B.S., M.S., G.K., and S.S. performed the research. R.K.P., A.R., K.A., and S.S. wrote the paper. A.R., A.G., B.S., and S.S. critically supervised the manuscript. All the authors read and approve the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the Departmental Review Board, Institute Thesis Committee, and Institute Ethics Committee.
Patient consent
A written, informed parental consent (for patients 8 years, as per our institutional guidelines) was obtained from the patient or one of the parents.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Pilania, R.K., Rawat, A., Shandilya, J. et al. Pediatric systemic lupus erythematosus: phagocytic defect and oxidase activity of neutrophils. Pediatr Res 92, 1535–1542 (2022). https://doi.org/10.1038/s41390-022-02055-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02055-2
This article is cited by
-
Disease activity is associated with changes in the innate immune function in patients with systemic lupus erythematosus
Clinical Rheumatology (2024)
-
ECI Biocommentary: Rakesh Kumar Pilania
Pediatric Research (2022)