Abstract
Background
Low and high leptin levels are associated with non-alcoholic fatty liver disease (NAFLD). The LncOb rs10487505 variant has been associated with body mass index (BMI), and the C allele was reported as leptin-lowering. We evaluated the association of rs10487505 with leptin levels, liver histology, and surgery-induced weight loss in youths with NAFLD.
Methods
One-hundred five obese youths with NAFLD, of whom 19 undergoing laparoscopic sleeve gastrectomy (LSG), were analyzed for rs10487505 and leptin circulating levels.
Results
The G allele frequency was lower in youths with NAFLD than in controls (p = 0.049). No difference was found in anthropometrics, biochemistry and histology between G allele carriers and CC homozygotes, except for leptin levels (p = 0.016). Leptin correlated with body weight, BMI, BMI-z score, waist circumference, insulin resistance/sensitivity, and triglycerides (p ≤ 0.01).
A multivariable regression model including body weight and homeostasis model assessment of insulin resistance was a good predictor of plasma leptin (R2 = 0.45), and the addition of genotype to the model increased the R2 to 0.50.
Following LSG, leptin levels and body weight were more reduced in G allele carriers (p < 0.05).
Conclusions
LncOb rs10487505 variant was associated with pediatric NAFLD and high leptin levels, and with weight and leptin reduction after LSG in youths.
Impact
-
The interplay of environment, genetics and epigenetics is crucial inflating the risk of non-alcoholic fatty liver disease (NAFLD). Several long non-coding RNA (LncRNAs) are found associated with NAFLD pathogenesis.
-
Here, we evaluated the impact of the genetic variant rs10487505 in LncOb which is involved in the regulation of leptin gene expression.
-
The LncOb rs10487505 is associated with increased levels of leptin, but not with liver histology, in youths with NAFLD.
-
The LncOb rs10487505 was also associated with the significant decrease of leptin and body weight after bariatric surgery.
Similar content being viewed by others
Introduction
In light of obesity epidemic in childhood, non-alcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease and liver-related morbidity worldwide in youth.1 NAFLD encompasses fatty liver disease (FLD), i.e., hepatic steatosis with no inflammation, non-alcoholic steatohepatitis (NASH) that, in turn, embraces ballooning, necro-inflammation, and eventually fibrosis.2
Dysfunctional handling of lipids in liver is pivotal in disease pathogenesis, and genetics of lipid metabolism is involved in the onset of FLD in the first two decades of life.3 So far, single nucleotide variants in the patatin-like phospholipase domain-containing protein 3 and the transmembrane 6 superfamily member 2 genes have been associated with significantly increased risk of NAFLD in youth. Both variants affect intrahepatic lipid droplet remodelling and hepatocellular lipid secretion.1,4
Among genetic variants that may significantly influence the risk of NAFLD, also mutations in the gene encoding for the leptin receptor gene (LEPR) have been described.5,6 Leptin works by its receptor as anorexigenic hormone, but it exerts also other key endocrine, reproductive and immune functions.7 Leptin has also been implied in the intrahepatic handling of fat and progression to NASH, but mechanisms of action remain unclear.8,9 Either very low to absent hormone levels in patients with lipodystrophies,10 or, at the contrary, very high concentrations in people with obesity have been found associated with enhanced intrahepatic deposition of fat.8,9 Mutations in the leptin gene (LEP) cause hyperphagia and severe obesity and leptin replacement is effective to normalize body weight and endocrine dysfunctions.7 In the general population, leptin concentrations correlate closely with body fat mass but there is wide inter-individual variability, and 10–20% of people with obesity have leptin concentrations that are not different from those of lean people, which is to a certain extent due to genetic differences.11 Recently, Dallner et al. investigated the role of a gene variant (rs10487505) that is located 21 Kb upstream from LEP. This region has been shown to harbor a long non-coding RNA that influences the transcriptional control of leptin expression.12 The C allele of rs10487505 variant was associated with reduced LEP expression in mouse adipose tissue even though no connection with leptin mRNA transcription levels was found. In humans, the variant was associated with elevated body mass index (BMI) suggesting a protective role of the G allele against obesity.12 The variant rs10487505 was of great interest not just for its effect on leptin levels but also since it overlaps a long non-coding RNA (LncRNA) known as LncOb. LncRNAs are RNA sequences with transcript length exceeding 200 nucleotides and not translated into proteins.13 Several LncRNAs are emerging as significant regulatory molecules in NAFLD pathogenesis.14 The understanding of their regulatory role in the clinical setting is still in its infancy, but it might open the path to novel treatments.15
As a previous study reported that leptin levels significantly increased in parallel with the worsening of steatosis and NAFLD activity score (NAS) in Italian children,16 we hypothesized that LncOb rs10487505 variant might affect leptin levels, thus influencing the severity of disease in the same setting. Therefore, the aim of the present study was to investigate the potential association of LncOb rs10487505 variant with circulating leptin and liver histology derangement in a series of Italian children and adolescents with NAFLD. Moreover, we investigated the association between genotype, leptin levels and weight loss in a group of adolescents who underwent bariatric surgery.
Materials and methods
Patients, anthropometric and laboratory assessment
Bio-banked samples of 105 young patients with biopsy-proven NAFLD, stored at Bambino Gesù Children’s Hospital between January 2016 and December 2020 were used for the study; 19 patients underwent laparoscopic sleeve gastrectomy (LSG) and were re-evaluated 12 months after surgery. Fourteen of these patients were already included in our previous study.17 Patients’ data were withdrawn from electronic medical records.
Use of bio-banked samples and associated anonymized data for future studies was approved by the “Bambino Gesù” Ethics committee (protocols: 734_OPBG_2014, 1956_OPBG_2019, and 1774_OPBG_2019). Written informed consent for biobanking and future use was obtained from the child’s parent/legal guardian at the time of the enrolment.
For diagnosis of NAFLD, viral and autoimmune hepatitis, alpha1-antitrypsin deficiency, Wilson disease, and infection with hepatitis B or hepatitis C were excluded. All subjects included in the study were not under any treatment that could influence carbohydrate metabolism and/or promote steatosis (e.g., corticosteroids, tamoxifen, amiodarone, and methotrexate, contraceptives).
Regarding the adolescents undergoing LSG, clinical and surgical research protocols were reported elsewhere.17 Moreover, they did not follow nutritional advice before the surgery but adhered to a post-interventional long-term nutritional and metabolic follow-up as recommended by the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition.18 We did not recorded any difference among the adherence to dietary and exercise recommendations.
Weight, BMI, and waist circumference (WC) were measured using standard procedures and BMI-z score computed using Italian growth reference charts.19 Alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT), total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides were measured by standard laboratory methods. Glucose and insulin were measured at 0 (fasting) and 120 min following a standard 75 g oral glucose tolerance test. The homeostasis model assessment of insulin resistance (HOMA-IR),20 and the insulin sensitivity index (ISI)21 were calculated as surrogate markers of insulin resistance and sensitivity, respectively.
Liver histology
In all patients, liver biopsies were performed using an automatic core biopsy 16 or 18-gauge needle under general anaesthesia and ultrasound guidance. Diagnosis of FLD and NASH was performed by two expert pathologists (RDV & AL). Hepatic steatosis was graded: 0 = steatosis involving fewer than 5% of hepatocytes; 1 = steatosis involving up to 33% of hepatocytes; 2 = steatosis involving 33–66% of hepatocytes; and 3 = steatosis involving more than 66% of hepatocytes. Lobular inflammation was graded: 0 = none; 1 = mild and 2 = moderate. Hepatocyte ballooning was graded: 0 = no balloon cells; 1 = few balloon cells and 2 = many/prominent balloon cells. The stage of hepatic fibrosis was quantified with a five‐point scale: 0 = no fibrosis; 1 = peri-sinusoidal or periportal fibrosis [(1a) mild, zone 3, perisinusoidal; (1b) moderate, zone 3, perisinusoidal; and (1c) portal/periportal]; 2 = peri-sinusoidal and portal/periportal fibrosis: 3 = bridging fibrosis; and 4 = cirrhosis. The NAS-score was used to define NASH. Patients with NAS ≥ 5 was diagnosed as NASH and those with score ≤ 3 were diagnosed as NAFLD. Patients with NAS = 4 (N = 15) were carefully evaluated by the two pathologists to assign them either the NAFLD (N = 12) or the NASH group (N = 3). A definitive diagnosis was reached only when the two physicians agreed on the diagnosis.22
Genotyping
The rs10487505 C > G LncOb variant was genotyped by allelic discrimination using TaqMan 5′-nuclease assays (Life Technologies, Carlsbad, CA, US). Genomic DNA was isolated from venous blood using a Blood DNA Extraction Kit (Qiagen, Valencia, CA, US). Real-time PCR was performed using Applied Biosystems 7900HT Fast Real-Time PCR System (Applied Biosystems, Carlsbad, CA, US). Positive and negative controls were included on each reaction plate, to verify the reproducibility of the results. As a control group, 502 healthy European individuals from the 1000 Genomes project, for whom the genotypes of interest were available (http://www.internationalgenome.org) were considered.
Enzyme linked immunosorbent (ELISA) assays
Leptin plasma levels were measured, after 8 h overnight fast, by commercially available ELISA kit (Human Leptin Quantikine ELISA Kit; Catalog number DLP00; RRID: AB_2783014; R&D Systems, Minneapolis, Minnesota, US; detection range: 15.6–1000 pg/mL; sensitivity: 7.8 pg/mL) according to manufacturer’s instructions.
Statistical analysis
Data are given as mean ± standard deviation (SD), or median and interquartile range (IQR), or numbers and percentage. Normality of data distribution was tested using the Shapiro test. Continuous data were compared using the Mann–Whitney U-test and the ANOVA; categorical data using the chi-squared test. Correlation coefficient were obtained by using the Spearman’s test. Variables that were significantly correlated with levels of leptin were enclosed in multivariable linear regression models to identify predictors of leptin values. Models were run with a stepwise procedure, and age and sex were included in all the models. Statistical significance was defined as two-sided P value < 0.05. Statistical analysis was performed using IBM SPSS statistic for Windows, version 20.0.
Results
Cross-sectional study of LncOb rs10487505, liver histology and leptin levels
In the NAFLD studied population, frequency distribution of LncOb rs10487505 was in Hardy–Weinberg equilibrium. In particular, the minor allele frequency (MAF) G in these pediatric subjects was lower (MAF 0.42, p = 0.049) than in 502 healthy Europeans included in the 1000 Genomes dataset (MAF 0.49).23 Indeed, 67 (63.8%) children/adolescents were G allele carriers and 38 (36.2%) CC homozygotes. The G allele distribution was not significantly different (p = 0.31) between patients with FLD (n = 35, 33.3%, MAF 0.37) and NASH (n = 70, 66.7%, MAF 0.44).
No difference was found in anthropometrics and biochemistry between G allele carriers and CC homozygotes (Table 1).
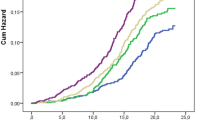
We found that circulating leptin levels ranged from 3.56 to 70.10 ng/mL in whole 105 subjects with NAFLD. Noteworthy, as shown in Fig. 1a, mean levels of leptin were significantly (p = 0.016) lower in patients with CC genotype (17.92 ± 13.0 ng/mL) than in G allele carriers (25.08 ± 15.2 ng/mL). No statistically significant (p = 0.752) difference was found between youths with FLD and NASH (Fig. 1b). Likely, as shown in Table 2, the genotype was not associated with steatosis (p = 0.79), lobular inflammation (p = 0.20), ballooning (p = 0.33), and fibrosis (p = 0.13).
Values of leptin were correlated to measures of obesity. Significant correlations were found with: body weight (coeff. 0.455, p < 0.0001), BMI (coeff. 0.538, p < 0.0001), BMI-z score (coeff. 0.537, p < 0.0001), WC (coeff. 0.365, p < 0.0001), HOMA-IR (coeff. 0.425, p < 0.0001) and ISI (coeff. −0.4.51, p < 0.0001), and triglyceride levels (coeff. 0.235, p = 0.01).
These variables were entered in multivariable models to identify predictors of leptin. As shown in Table 3, body weight and HOMA-IR were good predictors of circulating leptin (R2 = 0.45). The addition of genotype to the model increased the R2 to 0.50. Age and sex did not significantly contribute to the model.
Longitudinal study of weight loss, leptin reduction and FLD reversal after LSG
In Table 4 anthropometrics and biochemistry of 19 patients at baseline and 12 months after LSG are reported. Most of anthropometrical and metabolic parameters (e.g., BMI, WC, total/HDL/LDL cholesterol, triglycerides, AST, ALT, insulin values, HOMA-IR and ISI) were significantly improved following surgery. Data reported in Table 4 also show that median values of leptin was significantly reduced (p < 0.0001) and histological features significantly improved (p < 0.05) after 12 months.
Table 5 reported baseline and follow-up data of patients depending on the LncOb genotype, highlighting differential outcomes. While there was no difference between genotypes at baseline, steatosis, lobular inflammation and NAS persisted more severe in CC homozygous than in G allele carriers after surgery. Indeed, the improvement of these histological features at follow-up was significant only in G allele carriers group (p < 0.001 at the intra-group comparison). Likewise, the genotype greatly influenced leptin levels and weight loss. In particular, the reduction of leptin levels after surgery was more evident in G allele carriers than in CC homozygotes (Fig. 2a–b). As shown in the Fig. 2c–d, also G carriers exhibited a significant reduction of weight loss.
a The graphs report the circulating levels of leptin at baseline and after 12 months from surgery according to the genotype; b The histogram shows the percentage of reduction of leptin levels at follow-up according to LncOb rs10487505 variant. c The graphs report the body weight at baseline and after 12 months from surgery according to the genotype; d The histogram shows the percentage of reduction of weight at follow-up according to LncOb rs10487505 variant.
Discussion
In the present study, we investigated the association of LncOb rs10487505 variant with hepato-metabolic derangements in youths with NAFLD. We compared a cohort of Italian youths with NAFLD with subjects from European populations of the 1000 Genomes Project Consortium, which even if not including healthy children/adolescents, is often used as global reference for studies on genetic variants in humans.23 Our findings revealed that G allele frequency is lower in our cohort suggesting that this minor allele could be protective for the risk of NAFLD. Nevertheless, the lack of young controls and the small size of population limited the strength of our results. In fact, we found that the G allele frequency was 0.38 in patients with FLD, and 0.44 in subjects with NASH, thus overturning our initial hypothesis. Moreover, from the analyses no difference emerged in NAFLD-related anthropometrics, biochemistry, and histological features stratifying the patients for LncOb rs10487505 variant. On the contrary, the G allele was associated with higher leptin levels in our sample. Studies demonstrated that high leptin levels may be associated with both FLD and NASH, but we found that severity of liver damage was not correlated with either leptin concentration or the genetic of LncOb variant.9,10,16 It is conceivable that the small sample size could affect our results. However, other convincing reasons could explain why the G allele is associated with higher leptin levels but not with NASH. This uncoupling, as suggested by Ramírez-Vélez et al., could be ascribable to a major role of leptin levels in the onset of NAFLD rather than in NAFLD progression, where insulin resistance seems to represent the core.24
We believe, that when obesity challenges the leptin homeostatic system, CC homozygotes could have a very tight control of the leptin levels that remain low, while G carriers could respond with an increased release of the hormone. Therefore, at the extreme of a spectrum, individuals with obesity and low leptin might present a phenotype of obesity responsive to leptin treatment, while people with obesity and high leptin are expected to have hyperleptinaemia and leptin resistance both accounting for a pathological state.25,26 The regulation of the leptin homeostatic system, however, is likely more complex, and various factors may modulate the circulating levels of the hormone. In our series, genetic of the LncOb variant, body weight and insulin resistance may interact, thus influencing the leptin production and release. The inclusion of the genetic variant in the models with the body weight and the HOMA-IR ameliorated the performance of the model explaining the variability of leptin levels but to a limited extent. Indeed, by a practical standpoint, body weight and insulin resistance had the largest weight on the changes of leptin levels. The regulatory effect of body weight and HOMA-IR on leptin levels could be exerted via proximal LEP gene promoter and/or enhancer sites that are both required for proper leptin expression. In fact, while the LncOb exclusively binds the proximal promoter, excess body weight and insulin resistance have been found modulating LEP expression through DNA methylation of promoters in children with obesity.12,26,27 However, the validity and regulatory elements of this mechanistic model has to be confirmed.
In the LSG cohort, G allele carriers, differently from CC homozygotes, experienced a strong reduction of leptin levels and weight loss, and amelioration of liver histology following the surgery. This finding seems to controvert results from the Swedish Obesity Study (SOS). Indeed, while the SOS reported that the G allele was associated with weight regain after 12 years from the surgical procedure, we found that G allele carriers displayed greater body weight loss in the first 12 months after surgery. On the contrary, no association was found with early weight loss in the SOS.28 Different age and surgical procedure might be relevant to explain such different results. Normalization of weight loss and insulin resistance in youths might be pivotal inducing epigenetic changes in the LEP promoter modulating hyperleptinemia, and restoring leptin homeostasis.27,29 In summary, our data indicate that bariatric surgery outcomes, when the procedure is performed at young age, are better in G allele carriers.
It appears clear that the LncOb variant participates to the leptin homeostatic control very early in life. Indeed, the most pronounced association between the LncOb variant and the BMI was found in early childhood as compared to adults in one study,12 and particularly in children at the age of 18 months in a birth cohort study of 8 years-old children.30 Moreover, Wilhelm et al. reported that serum leptin levels were significantly higher before than after bariatric surgery, when the leptin promoter appeared to be hypomethylated.31 Since nuclear LncRNAs exert their functions by modifying chromatin structure, thereby influencing gene transcription, precise mechanisms of action of the LncOb has to be investigated.32
Our findings are in keeping with the notion that genetics that controls leptin homeostasis may be one of the factors potentially contributing to inter-individual variability in response to treatments for obesity.33 Therefore, early surgery-induced weight loss might produce more beneficial metabolic effects when performed in a life-window of still great organ plasticity.
In conclusion, the presence LncOb rs10487505 variant was associated with increased levels of leptin in youths with NAFLD. More importantly, the G allele was associated with a strong reduction of leptin levels and body weight after LSG in adolescents. In the light of our results the role of LncOb rs10487505 variant in NAFLD deserves further investigation.
Data availability
The dataset analyzed during the current study is not publicly available but is available from the corresponding author on reasonable request.
References
Nobili, V. et al. NAFLD in children: new genes, new diagnostic modalities and new drugs. Nat. Rev. Gastroenterol. Hepatol. 16, 517–530 (2019).
Brunt, E. M. et al. NAFLD: Reporting Histologic Findings in Clinical Practice. Hepatology 73, 2028–2038 (2021).
Bedogni, G. et al. Association of Bright Liver With the PNPLA3 I148M Gene Variant in 1-Year-Old Toddlers. J. Clin. Endocrinol. Metab. 104, 2163–2170 (2019).
Valenti, L. et al. I148M patatin-like phospholipase domain-containing 3 gene variant and severity of pediatric nonalcoholic fatty liver disease. Hepatology 52, 1274–1280 (2010).
Zain, S. M. et al. Impact of leptin receptor gene variants on risk of non-alcoholic fatty liver disease and its interaction with adiponutrin gene. J. Gastroenterol. Hepatol. 28, 873–879 (2013).
Pan, X. et al. The LEPR K109R and Q223R Might Contribute to the Risk of NAFLD: a meta-analysis. Curr. Mol. Med. 18, 91–99 (2018).
Montague, C. T. et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387, 903–908 (1997).
Panera, N. et al. Recent advances in understanding the role of adipocytokines during non-alcoholic fatty liver disease pathogenesis and their link with hepatokines. Expert. Rev. Gastroenterol. Hepatol. 10, 393–403 (2016).
Polyzos, S. A. et al. Circulating leptin in non-alcoholic fatty liver disease: a systematic review and meta-analysis. Diabetologia 59, 30–43 (2016).
Hackl, M. T. et al. Brain leptin reduces liver lipids by increasing hepatic triglyceride secretion and lowering lipogenesis. Nat. Commun. 10, 2717 (2019).
Narkiewicz, K. et al. Heritability of plasma leptin levels: a twin study. J. Hypertens. 17, 27–31 (1999).
Dallner, O. S. et al. Dysregulation of a long noncoding RNA reduces leptin leading to a leptin-responsive form of obesity. Nat. Med. 25, 507–516 (2019).
Dahariya, S. et al. Long non-coding RNA: classification, biogenesis and functions in blood cells. Mol. Immunol. 112, 82–92 (2019).
DiStefano, J. K. & Gerhard, G. S. Long Noncoding RNAs and Human Liver Disease. Annu. Rev. Pathol. 17, 1–21 (2021).
Hanson, A., Wilhelmsen, D. & DiStefano, J. K. The Role of Long Non-Coding RNAs (lncRNAs) in the Development and Progression of Fibrosis Associated with Nonalcoholic Fatty Liver Disease (NAFLD). Noncoding RNA 4, 18 (2018).
Nobili, V. et al. Leptin, free leptin index, insulin resistance and liver fibrosis in children with non-alcoholic fatty liver disease. Eur. J. Endocrinol. 155, 735–743 (2006).
Manco, M. et al. The Benefit of Sleeve Gastrectomy in Obese Adolescents on Nonalcoholic Steatohepatitis and Hepatic Fibrosis. J. Pediatr. 180, 31–37.e2 (2017).
Nobili, V. et al. Indications and limitations of bariatric intervention in severely obese children and adolescents with and without nonalcoholic steatohepatitis: ESPGHAN Hepatology Committee Position Statement. J. Pediatr. Gastroenterol. Nutr. 60, 550–561 (2015).
Cacciari, E. et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 29, 581–593 (2006).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
Matsuda, M. & DeFronzo, R. A. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470 (1999).
Kleiner, D. E. et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41, 1313–1321 (2005).
1000 Genomes Project Consortium. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Ramírez-Vélez, R. et al. Serum leptin as a mediator of the influence of insulin resistance on hepatic steatosis in youths with excess adiposity. Nutr. Metab. Cardiovasc. Dis. 31, 1308–1316 (2021).
Morris, A. Subtyping obesity. Nat. Rev. Endocrinol. 15, 316 (2019).
Münzberg, H. & Heymsfield, S. B. New Insights into the Regulation of Leptin Gene Expression. Cell. Metab. 29, 1013–1014 (2019).
García-Cardona, M. C. et al. DNA methylation of leptin and adiponectin promoters in children is reduced by the combined presence of obesity and insulin resistance. Int. J. Obes. (Lond.). 38, 1457–1465 (2014).
Sarzynski, M. A. et al. Associations of markers in 11 obesity candidate genes with maximal weight loss and weight regain in the SOS bariatric surgery cases. Int. J. Obes. (Lond.). 35, 676–683 (2011).
Wróblewski, A. et al. Molecular Insight into the Interaction between Epigenetics and Leptin in Metabolic Disorders. Nutrients 11, 1872 (2019).
Helgeland, Ø. et al. Genome-wide association study reveals dynamic role of genetic variation in infant and early childhood growth. Nat. Commun. 10, 4448 (2019).
Wilhelm, J. et al. Promoter Methylation of LEP and LEPR before and after bariatric surgery: a cross-sectional study. Obes. Facts. 14, 1–7 (2021).
Fernandes, J. C. R., Acuña, S. M., Aoki, J. I., Floeter-Winter, L. M. & Muxel, S. M. Long Non-Coding RNAs in the Regulation of Gene Expression: Physiology and Disease. Noncoding RNA 5, 17 (2019).
Dent, R., McPherson, R. & Harper, M. E. Factors affecting weight loss variability in obesity. Metabolism 113, 154388 (2020).
Funding
This work was supported by a grant from Italian Ministry of Health 5 × 1000_2016 & 5 × 1000_2019 to Anna Alisi and 5 × 1000_2019 to Melania Manco.
Author information
Authors and Affiliations
Contributions
Conceptualization, funding acquisition, and drafting M.M., and A.A.; patients study and follow-up; data curation, A.M., R.C., R.D.V., and F.D.P.; genetics and biochemistry A.C., M.R.B.; histology R.D.V. and F.P.; data analysis, M.M., A.C., and A.A.; review for important intellectual content, all authors; data accountability, A.A. All authors have read and agreed to the published version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Written informed consent was given by participants’ parents/legal guardians.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Manco, M., Crudele, A., Mosca, A. et al. LncOb rs10487505 variant is associated with leptin levels in pediatric non-alcoholic fatty liver disease. Pediatr Res 92, 1737–1743 (2022). https://doi.org/10.1038/s41390-022-02032-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02032-9