Abstract
Background
Effects of probiotics on intestinal microbiota and feeding tolerance remain unclear in extremely low-birth-weight (ELBW) infants.
Methods
ELBW infants were randomly assigned to receive probiotics or no intervention. Stool samples were collected prior to, 2 and 4 weeks after initiation, and 2 weeks after probiotics cessation for infants in the probiotics group, and at matched postnatal age time points for infants in the control group.
Results
Of the 102 infants assessed for eligibility, sixty-two were included. Infants who received probiotics reached full enteral feeds sooner (Mean difference (MD) −1.8; 95% CI:−3.7 to −0.01 day), had a tendency toward lower incidence of hematochezia before hospital discharge (22.6% vs 3.2%; P = 0.053), and were less likely to require extensively hydrolyzed- or amino acids-based formulas to alleviate signs of cow’s milk protein intolerance in the first 6 months of life (19.4% vs 51.6%; P = 0.008). Infants on probiotics were more likely to receive wide-spectrum antibiotics (64.5% vs 32.2%; P = 0.01). Multi-strain probiotics resulted in significant increase in fecal Bifidobacterium (P < 0.001) and Lactobacillus (P = 0.005), and marked reduction in fecal candida abundance (P = 0.04).
Conclusion
Probiotics sustained intestinal Bifidobacterium and reduced time to achieve full enteral feeds in extremely preterm infants. Probiotics might improve tolerance for cow’s milk protein supplements.
Clinical trial registration
This trial has been registered at www.clinicaltrials.gov (identifier NCT03422562).
Impact
-
Probiotics may help extremely preterm infants achieve full enteral feeds sooner.
-
Probiotics may improve tolerance for cow’s milk protein supplements.
-
Multi-strain probiotics can sustain intestinal Bifidobacterium and Lactobacillus until hospital discharge.
Similar content being viewed by others
Introduction
Extremely preterm infants are at high risk for intestinal microbiome perturbations due to high rates of Cesarean birth, prolonged stay in neonatal intensive care unit (NICU), and exposure to multiple antibiotics.1 This state is characterized by overgrowth of pathogenic bacteria such as Enterobacter and Pseudomonas species at the expense of commensal and beneficial bacteria such as Bifidobacterium species.2 Current evidence suggests that disruption and delayed microbiome maturation trajectory play an important role in the development of necrotizing enterocolitis (NEC)3,4 and the pathogenesis of cow’s milk protein intolerance.5,6,7 NEC is a leading cause of mortality and morbidity in preterm infants, particularly extremely low birth weight (ELBW; less than 1000 g) infants. Cow’s milk protein intolerance may result in frequent interruption of feeding plans and often prolonged time on parenteral nutrition during hospital stay.8
Probiotics are frequently proposed as beneficial supplements for preterm infants. Nonetheless, the evidence for benefits have been largely generated from small randomized controlled trials (RCTs).9 A recent Cochrane systematic review indicated that probiotic supplementation in very low birth weight infants (VLBW; less than 1500 g) might reduce the risk of NEC, death, and serious infection.9 However, only a few trials provided data for ELBW infants, and these trials did not show any benefit.9 There are several gaps in current knowledge about the effect of probiotics on intestinal microbiota, the fate after administration of a specific probiotic strain to a given infant, and the degree of influence on the pre-existing and developing microbiome. Therefore, it is essential that gut microbial data are obtained and correlated with clinical data for the probiotic used, particularly in ELBW infants.
Although many NICUs across the world have implemented routine probiotic supplementation, the low certainty of the evidence, limited benefits in ELBW infants who are at the highest risk for NEC, and the fear from probiotic infection10,11 have resulted in significant skepticism. There are several gaps in current knowledge about the effect of probiotics on intestinal microbiota, the fate after administration of a specific probiotic strain to a given infant, and the degree of influence on the pre-existing and developing microbiome. Therefore, it is essential that gut microbial data are obtained and correlated with clinical data for the probiotic used, particularly in ELBW infants. A recent network meta-analysis of 45 RCTs suggested a combined use of Lactobacillus, Bifidobacterium, and prebiotics to achieve optimal effects on preterm infants’ health.12 However, due to a lack of cumulative effects between Bifidobacterium, Lactobacillus and prebiotics, and insufficient data for extremely preterm infants, the authors indicated the need for more RCTs to identify the benefits and optimal dosage in ELBW infants.
The aim of our RCT was to examine the effects of the combined use of Bifidobacterium, Lactobacillus, and prebiotics on intestinal microbiota and to study the association between probiotics-altered gut microbiota and feeding outcomes in ELBW infants.
Methods
Study design and participants
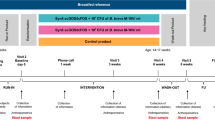
This study was an open-label, parallel, RCT conducted between October 2017 and December 2019 in the level III neonatal intensive care unit (NICU) of Foothills Medical Centre in Calgary, Alberta, Canada. The trial was approved by the Conjoint Research Ethics Board of the University of Calgary and registered at www.clinicaltrials.gov (identifier NCT03422562). Written informed consent was obtained from parents prenatally or within 24 h after birth.
Infants born less than 290/7 weeks gestation and weighing ≤1000 g were eligible for the study. Infants were excluded if they had major congenital anomalies, antenatal concern related to the fetal gastrointestinal tract, hypoxic-ischemic injury, and intestinal perforation before enrollment.
The primary outcome of the study was the changes in fecal microbiota after probiotic supplementation. The secondary outcomes included: (i) incidence of feeding intolerance defined by interruption in enteral feeding, unrelated to a clinical procedure or diagnosis of NEC, that lasted for equal to or more than 24 h, and (ii) time required to reach full enteral feeds defined by reaching 120 mL/kg per day. Our definition of feeding intolerance did not differentiate between causes related to immaturity of gastrointestinal functions and cow’s milk protein intolerance/allergy (CMPA). Therefore we elected to report the incidence of CMPA separately. CMPA defined by occurrence of recurrent vomiting, significant abdominal distension, or presence of frank blood in stool in the absence of clinical or radiological evidence of NEC that improved after elimination of cow’s milk protein products from mother and infant’s diets. A registered dietitian counseled lactating mothers to avoid products that contain cow’s milk protein. Extensively hydrolyzed protein (Nutramigen® or Pregestimil®) or amino acid-based formula (Neocate®) were used to fortify expressed human milk or as a sole formula in exclusively formula-fed infants with suspected CMPA.
Randomization
A biostatistician, who was independent of the study investigators, conducted the randomization sequence using SAS statistical software (SAS Institute Inc., Cary, NC). Randomization was stratified to <750 g and 750–1000 g. Allocation was 1:1 with random block size of 4. The sequence code was kept in sequential numbered sealed opaque envelopes. Infants from multiple births who met the inclusion criteria were included as one unit and randomized to the same arm of the study. Once the informed consent was obtained from a parent, the research assistant or a study investigator opened the numbered sealed envelope to reveal the study group.
Study intervention
The probiotic supplement used was Florababy (Appendix 1). Infants in the study group received one probiotic sachet per day once the enteral feeding started, and after informed consent was obtained. Probiotic sachets were mixed with mother’s own milk (MOM) or donor human milk (DHM) by the infant’s registered nurse at the bedside and were administered via naso- or oro-gastric feeding tube. Probiotics were held if the infant was placed nil per os, and restarted when the most responsible physician recommended resuming feeding. The study group received probiotics until 37 weeks corrected gestational age (CGA) or hospital discharge, whichever occurred earlier. Probiotics (Florababy®) are used routinely for preterm infants weighing ≥1000 g in our NICU. Given the ongoing concern about the commercially available probiotics preparation and potential contamination,13 we designed the study in a way that infants in the control group receive no placebo to minimize the exposure to the manufactured products. We intended to evaluate the product as a whole before expanding its use for ELBW infants. All infants in the study received oral immune therapy by administering 0.1–0.2 mL of MOM every 4 h as soon as it was available. Actual feeds of MOM or DHM were initiated within 12 h of life based on standardized feeding tables specific to birth weight.
Fecal sample collection
Nurses collected stool samples at four time points in NICUs: prior to, 2 weeks and 4 weeks after probiotic administration was commenced, and 2 weeks after cessation. Stool samples for the control group were collected at matched postnatal age time points. Infants discharged home prior to the 4th collection time point had their stool sample collected and stored in a home fridge by their parents. A courier transported home-collected samples in a cold chain from the participant’s home to Alberta Precision Laboratories. Microbial analyses are summarized in Appendix 2. Species-specific primer was developed to identify that Bifidobacterium and Lactobacillus in the probiotic group were the same as in the probiotics sachets.
Statistical analyses
Assuming an alpha value of 0.05 and a power of 0.80, a sample size of 60 was calculated to detect a 0.70 effect size for a two-tailed Wilcoxon–Mann–Whitney test when comparing relative abundance of fecal probiotics microbial species between the 2 groups after a conservative assumption of a background logistic distribution.
Descriptive statistics including means, medians, and standard deviations (SD) were used to describe the study population. Continuous variables were analyzed using independent, two-sample t tests or Wilcoxon rank-sum test as appropriate. Categorical variables were evaluated using χ2 and Fisher’s exact tests. All statistical tests were two-tailed, based on intention-to-treat principle, and the level of significance was set at 0.05. For continuous outcomes with outliers, we used robust regression analysis to examine the effect of probiotics on these outcomes. Changes in outcome measures over time (T1, T2, T3, and T4), the main effect of randomization groups, and randomization group (probiotics) by time (T) interactions were investigated on an intention-to-treat basis using linear mixed-effects regression models (LMA) accounting for correlations arising from repeated measures. LMA models allow using all data available from each participant under the assumption of missing at random. Whether changes in outcomes over time differed by randomization groups were evaluated by examining the interaction effects of group (probiotics) by time (probiotics × time point). R version 4.0.5 (R Development Core Team, Vienna, Austria) R and STATA16 (Stata Corporation, College Station, TX) were used.
Results
Study population
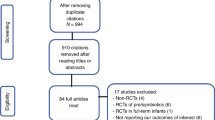
One hundred and two infants were assessed for eligibility. Forty of them were excluded for parental refusal to participate (n = 38) and not meeting the inclusion criteria (n = 2). A total of 62 infants were included in the trial. Three participants randomized into the probiotics group developed spontaneous intestinal perforation (SIP) before the use of probiotics but after the first stool sample collection, therefore they did not receive the probiotic treatment. Of note, there were no changes to the statistical significance when these infants were excluded therefore we kept them for the analysis. Two infants in probiotics group died due to severe lung disease that led to respiratory failure. Death of these two infants occurred after time point 3 at 42 and 46 days of life. Figure 1 shows the participants flow diagram.
Clinical outcomes
Maternal and neonatal baseline characteristics are summarized in Table 1. Clinical outcomes of the study are summarized in Table 2. Major neonatal morbidities including culture-proven sepsis were similar between the two groups. The organisms identified by blood culture in the control group were Coagulase-negative Staphylococcus (n = 2) and Escherichia coli (n = 1). The organisms identified in the probiotics group were Coagulase-negative Staphylococcus (n = 2), E. coli (n = 3) and Staphylococcus aureus (n = 3). There was no sepsis with any of the probiotics strains. Total antimicrobial days of therapy (DOT) was higher in the probiotics group. Twenty (64.5%) infants in the probiotics group received wide-spectrum antibiotics compared to 10 (32.2%) in the control group. Proportion of infants that received each antimicrobial drug is presented in supplementary Table S3.
Duration of parenteral nutrition, total duration of central venous access, and length of hospital stay were similar between the two groups, however, outliers were common for duration of parenteral nutrition, duration of central venous access, and time to full enteral feeds. Using robust regression methods revealed similar durations for parenteral nutrition (MD 0.4; 95% CI: −4.6 to 3.8 day) and central vascular catheter days (MD 24.4; 95%CI: −59.0 to 10.2 days per 1000 patient-days) but less time to full enteral feeds in the probiotics group (MD −1.8; 95% CI: −3.7 to −0.01 day).
More infants in the control group required elimination of cow’s milk protein-based fortifiers from mother and infant’s diet due to suspected CMPA between birth and 6 months CGA (Table 2). The post-discharge use of extensively hydrolyzed protein- or amino acids-based formula was directed by pediatricians who were neither affiliated with our NICU nor aware of the study.
Intestinal microbiome
Species richness and diversity
Before hospital discharge, 216 fecal samples were collected and subjected to 16S rRNA gene sequencing. Number of fecal samples at each time point, and postnatal age when the samples are collected are summarized in Supplementary Table S4.
Figure 2 shows the changes in relative abundance of genera over the study period. Bifidobacterium was dominant in the probiotics group with high relative abundance during supplementation (T2–T3) and at 2 weeks after cessation of probiotics (T4) (Supplementary Fig. S1). Lactobacillus was detected in a fewer number of infants in both the probiotics and control groups with a higher relative abundance during supplementation (T2–T3) in the probiotics group compared with control group (Supplementary Fig. S1). The effects of probiotic supplementation, postnatal age (time points), and the interaction between probiotics and time points are summarized in Supplementary Table S5. Postnatal age has significant effect on Bifidobacterium, Staphylococcus and Enterobacteria, particularly after 2–3 weeks of age (after T2). Significant interactions are noted between probiotics and time point for Bifidobacterium and Lactobacillus only.
A significant increase was observed in species richness and diversity over time in both groups (Supplementary Fig. S2). There were no significant effect of probiotics on Shannon or Chao1 indices as indicated by the lack of interactions between probiotics and time points (probiotics x time point) when the linear mixed-effect models used to adjust for gestational age, Cesarean birth, early use of antibiotics, and type of feed (Fig. 3). Of note, both early use of antibiotics and type of feed, particularly formula, have significant influence on alpha diversity indices. There was significant difference in beta diversity after probiotic supplementation (Supplementary Fig. S2). The effect of probiotics on beta diversity continued after probiotics cessation.
Fungal species
Probiotic supplementation resulted in a transient increase in fungal alpha diversity (Shannon) at time point 4 (Supplementary Fig. S3A). These effects disappeared after probiotics cessation. In contrast, fungal beta diversity (beta dispersion) decreased during probiotic supplementation (Supplementary Fig. S3B). Beta dispersion continued to be lower in the probiotic group after probiotics cessation. Figure 2b shows the relative abundance of fungal phyla and genera in control and probiotics groups. Probiotic supplementation resulted in a significant decrease in heterogeneity of fungal microbiome with marked reduction in candida sp abundance (P = 0.04).
Discussion
Probiotic supplementation for ELBW infants in our study led to less time required to establish full enteral feeds and a lower requirement for extensively hydrolyzed- or amino acids-based formulas to alleviate signs of cow’s milk protein intolerance. Probiotic supplementation also increased Bifidobacterium and sustained Lactobacillus. Postnatal age, early empiric antibiotic use after birth, and type of milk feeding appear to have more effect on microbial diversity than probiotics.
Feed intolerance is commonly encountered in ELBW infants and often leads to disruption of the enteral feeding plan and delayed time to attain full enteral feeds.8 Our finding of lower incidence of feeding intolerance with probiotic supplementation is consistent with that in the literature. In a systematic review and meta-analysis by Athalye-Jape et al., probiotics reduced the time to full enteral feeds in preterm infants with a mean difference of −1.54 days (95% CI −2.75, −0.32).14 A more recent prospective study nested within a RCT investigating single (Bifidobacterium breve) versus multi-strain (Bifidobacterium breve, Bifidobacterium infantis, Bifidobacterium longum) probiotic supplementation showed significant reduction in the time to full feeds and any hemorrhagic gastric residuals in extremely preterm infants who received single- or multi-strain probiotics compared with infants who received placebo.15 Improved gastric emptying and gut motility may explain the benefits of probiotics (Limosilactobacillus formerly Lactobacillus Reuteri) on feed tolerance, particularly in formula-fed preterm infants.16,17 It is important to note that comparison between studies reporting feeding intolerance, particularly in relation to probiotic supplementation, is challenging given the lack of consensus on the definition.18
Infants who received probiotics in our study had lower rates of cow’s milk protein intolerance, or suspected CMPA, and were less likely to require extensively hydrolyzed protein fortification during the first 6 months of life. In our study, the main reason to change the infants’ feeds to extensively hydrolyzed or amino acid-based formula was the presence of blood in the stool in the absence of NEC or other causes such as anal fissure. Improvement of hematochezia with cow’s milk protein elimination was used by health care providers to make the diagnosis of CMPA. Diagnosis of CMPA in preterm infants, particularly during NICU stay, remains challenging and is largely based on improvement of gastrointestinal signs after exclusion of cow’s milk protein from mother and infant’s diets.19,20 Cow’s milk protein challenge; the gold standard for the diagnosis of CMPA, is rarely undertaken in preterm infants.20 Data on probiotics for prevention of CMPA in preterm infants is scarce. A systematic review and meta-analysis reported that probiotic supplementation to mothers and term-born infants in the first 6–12 months of life is effective in reducing food hypersensitivity.21 However, postnatal only supplementation failed to show similar benefit.21 A sub-study of the ProPrems (probiotics to reduce incidence of late-onset sepsis in very preterm infants) trial revealed a similar incidence of food allergy including CMPA between infants who received probiotics (B. infantis, B. bifidum, and Streptococcus thermophilus) versus placebo.22 Evidence suggests that certain commensal bacterial species (clostridia) and their metabolites, particularly short-chain fatty acids (butyrate), may positively modulate intestinal permeability and immune tolerance mechanisms.23,24 Available data suggest that intestinal dysbiosis might be associated with CMPA in infants with IgE- and non-IgE-mediated CMPA.7,25 Canani et al. indicated higher microbial diversity and significant decrease in Bifidobacterium with IgE-mediated CMPA.26 Of note, extensively hydrolyzed casein formula containing Lactobacillus rhamnosus in the former study resulted in significant enrichment in fecal butyrate-producing bacteria.26 In another study by Canani et al. in infants affected by non-IgE-mediated CMPA, microbial dysbiosis was characterized with an enrichment in Bacteroides and Alistipes.7 Identification of specific bacteria that may contribute to the development of cow’s milk protein intolerance or allergy could open the door for future prevention or treatment of these conditions.
Exposure to widespread antibiotics was more common in infants who received probiotics although the rate of sepsis was similar between the two groups. Esaiassen et al. reported similar findings in extremely preterm infants receiving probiotics. However, the infants in their control group were more mature.27
Probiotic supplementation resulted in significant increase in the relative abundance of Bifidobacterium. A recent cohort study by Alcone-Giner et al. revealed a similar association between probiotics (Bifidobacterium and Lactobacillus) supplementation and increased Bifidobacterium abundance that is comparable to natural gut colonization of full-term infants.28 It is noteworthy to mention that Bifidobacterium became dominant within 2 weeks of probiotic supplementation. The rapid increase of Bifidobacterium is not surprising given that all preterm infants in our study were on breastmilk during the first 4 weeks of life; which provides the necessary human milk oligosaccharides for the growth of Bifidobacterium.29 Nevertheless, the increase was more pronounced in infants who received probiotics. Bifidobacterium continued to be dominant after stopping probiotics in the study group. Yousuf et al. indicated that Bifidobacterium could persist at high abundance up to 5 months after stopping probiotic supplementation.30
The difference in the Lactobacillus abundance observed after 4 weeks of probiotic supplementation was driven by the decline in the control group while maintaining stable, albeit very low, relative abundance in the probiotics group. The lack of association between probiotics exposure and the abundance of Lactobacillus is described in several studies in preterm infants and could be multifactorial.30,31,32 Stool analysis may underestimate Lactobacillus abundance as Lactobacillus colonizes the small intestine and attaches to colonic mucosa.33 Watkins et al. reported late increases in relative Lactobacillus abundance after 31 weeks CGA using another product (Infloran®) that contains 1 billion CFU of bifidobacteria and 1 billion CFU of Lactobacillus.32 Whether a higher Lactobacillus dose can enhance genus colonization or confer clinical benefit is yet to be determined.
Probiotic supplementation in our study did not affect alpha diversity measures. In contrast, beta diversity analysis showed a marked shift in bacterial community during and two weeks after probiotics cessation. Other studies in preterm infants described similar findings. Yousuf et al. found no effect of probiotic supplementation on alpha diversity in a small cohort of preterm infants.30 In their study, microbial communities from probiotic-exposed infants at term equivalent age were found to cluster more with full-term compared with those from unexposed participants.30 Of note, postnatal age as reflected by the time point of stool collection, early use of empiric antibiotics, and feeding formula had important impact on alpha diversity. These findings are consistent with other studies in preterm infants.30,34
Supplementation with multi-strain probiotics modified intestinal mycobiome with a marked anti-Candida effect. This agrees with previous studies that primarily involved older preterm infants.35,36 A systematic review and meta-analysis in preterm infants indicated that probiotics can reduce the risk of Candida colonization in preterm neonates in NICUs and may prevent invasive fungal sepsis.35 Similar effects are also reported in a single-strain probiotics. In a RCT in VLBW infants, Manzoni et al revealed, that oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species.36 Our findings add to the growing body of evidence on the role of probiotics in stabilizing not only the bacterial but also the intestinal fungal microbiome of preterm infants.
Strengths of our study include the randomized controlled design, longitudinal collection of stool samples, and the inclusion of ELBW infants who are at the highest risk for intestinal dysbiosis and complications. Nonetheless, our study has some limitations that include the lack of a placebo control to account for the prebiotic effect of maltodextrin and ascorbic acid, and the limited ability to explore the effects of probiotics on common morbidities such as NEC due to small sample size. Furthermore, the diagnosis of cow’s milk protein intolerance or allergy is made based on clinical signs and health care providers may have used different criteria to define these conditions.
Conclusion
Multi-strain probiotic supplementation for ELBW infants during hospitalization in NICU may result in improved feed tolerance and lower the incidence of suspected cow’s milk protein intolerance. Although the signs of CMPA, particularly the hematochezia, improved shortly after utilizing the cow’s milk protein elimination diet in the NICU, a challenge with cow’s milk protein-based diet before discharge would have improved the accuracy of our CMPA diagnosis. Probiotic supplementation results in a significant increase in fecal Bifidobacterium and, to less extent, Lactobacillus. Further research is needed to identify whether higher doses or different ratios between Bifidobacterium and Lactobacillus can induce favorable microbiome composition and explore functional roles of probiotic supplementation on the intestinal immune development.
References
Underwood, M. A., Mukhopadhyay, S., Lakshminrusimha, S. & Bevins, C. L. Neonatal intestinal dysbiosis. J. Perinatol. 40, 1597–1608 (2020).
Tauchi, H. et al. Gut microbiota development of preterm infants hospitalised in intensive care units. Benef. Microbes 10, 641–651 (2019).
Fundora, J. B., Guha, P., Shores, D. R., Pammi, M. & Maheshwari, A. Intestinal dysbiosis and necrotizing enterocolitis: assessment for causality using Bradford Hill criteria. Pediatr. Res. 87, 235–248 (2020).
Pammi, M. et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 5, 31 (2017).
Yuan, Z. et al. Feeding intolerance alters the gut microbiota of preterm infants. PLoS ONE 14, e0210609 (2019).
Chandrasekharan, B. et al. Interactions between commensal bacteria and enteric neurons, via FPR1 induction of ROS, increase gastrointestinal motility in mice. Gastroenterology 157, 179–192 e172 (2019).
Berni Canani, R. et al. Gut microbiota composition and butyrate production in children affected by non-IgE-mediated cow’s milk allergy. Sci. Rep. 8, 12500 (2018).
Cordova, J. et al. Manifestations of cow’s-milk protein intolerance in preterm infants. J. Pediatr. Gastroenterol. Nutr. 62, 140–144 (2016).
Sharif, S., Meader, N., Oddie, S. J., Rojas-Reyes, M. X. & McGuire, W. Probiotics to prevent necrotising enterocolitis in very preterm or very low birth weight infants. Cochrane Database Syst. Rev. 10, CD005496 (2020).
Zbinden, A., Zbinden, R., Berger, C. & Arlettaz, R. Case series of Bifidobacterium longum bacteremia in three preterm infants on probiotic therapy. Neonatology 107, 56–59 (2015).
Jenke, A., Ruf, E. M., Hoppe, T., Heldmann, M. & Wirth, S. Bifidobacterium septicaemia in an extremely low-birthweight infant under probiotic therapy. Arch. Dis. Child. Fetal Neonatal Ed. 97, F217–F218 (2012).
Chi, C. et al. Effects of probiotics in preterm infants: a network meta-analysis. Pediatrics 147. https://doi.org/10.1542/peds.2020-0706 (2021).
Poindexter, B., Committee on Fetus & Newborn. Use of probiotics in preterm infants. Pediatrics 147. https://doi.org/10.1542/peds.2021-051485 (2021).
Athalye-Jape, G., Deshpande, G., Rao, S. & Patole, S. Benefits of probiotics on enteral nutrition in preterm neonates: a systematic review. Am. J. Clin. Nutr. 100, 1508–1519 (2014).
Gayatri, A. J. et al. Composition of coloured gastric residuals in extremely preterm infants-a nested prospective observational study. Nutrients 12. https://doi.org/10.3390/nu12092585 (2020).
Indrio, F. et al. The effects of probiotics on feeding tolerance, bowel habits, and gastrointestinal motility in preterm newborns. J. Pediatr. 152, 801–806 (2008).
Indrio, F. et al. Effects of probiotic and prebiotic on gastrointestinal motility in newborns. J. Physiol. Pharmacol. 60, 27–31 (2009).
Weeks, C. L., Marino, L. V. & Johnson, M. J. A systematic review of the definitions and prevalence of feeding intolerance in preterm infants. Clin. Nutr. 40, 5576–5586 (2021).
Koletzko, S. et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J. Pediatr. Gastroenterol. Nutr. 55, 221–229 (2012).
Lenfestey, M. W., de la Cruz, D. & Neu, J. Food protein-induced enterocolitis instead of necrotizing enterocolitis? A neonatal intensive care unit case series. J. Pediatrics 200, 270–273 (2018).
Zhang, G. Q. et al. Probiotics for prevention of atopy and food hypersensitivity in early childhood: A PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine 95, e2562 (2016).
Plummer, E. L. et al. Postnatal probiotics and allergic disease in very preterm infants: sub-study to the ProPrems randomized trial. Allergy 75, 127–136 (2020).
Feehley, T. et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat. Med. 25, 448–453 (2019).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Ling, Z. et al. Altered fecal microbiota composition associated with food allergy in infants. Appl. Environ. Microbiol. 80, 2546–2554 (2014).
Berni Canani, R. et al. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 10, 742–750 (2016).
Esaiassen, E. et al. Effects of probiotic supplementation on the gut microbiota and antibiotic resistome development in preterm infants. Front. Pediatr. 6, 347 (2018).
Alcon-Giner, C. et al. Microbiota supplementation with Bifidobacterium and Lactobacillus modifies the preterm infant gut microbiota and metabolome: an observational study. Cell Rep. Med. 1, 100077 (2020).
Lawson, M. A. E. et al. Breast milk-derived human milk oligosaccharides promote Bifidobacterium interactions within a single ecosystem. ISME J. 14, 635–648 (2020).
Yousuf, E. I. et al. Persistence of suspected probiotic organisms in preterm infant gut microbiota weeks after probiotic supplementation in the NICU. Front. Microbiol. 11, 574137 (2020).
Abdulkadir, B. et al. Routine use of probiotics in preterm infants: longitudinal impact on the microbiome and metabolome. Neonatology 109, 239–247 (2016).
Watkins, C. et al. Dose-interval study of a dual probiotic in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 104, F159–F164 (2019).
Alander, M. et al. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl. Environ. Microbiol. 65, 351–354 (1999).
Kok, C. R. et al. Stool microbiome, pH and short/branched chain fatty acids in infants receiving extensively hydrolyzed formula, amino acid formula, or human milk through two months of age. BMC Microbiol. 20, 337 (2020).
Hu, H. J., Zhang, G. Q., Zhang, Q., Shakya, S. & Li, Z. Y. Probiotics prevent candida colonization and invasive fungal sepsis in preterm neonates: a systematic review and meta-analysis of randomized controlled trials. Pediatrics Neonatol. 58, 103–110 (2017).
Manzoni, P. et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin. Infect. Dis. 42, 1735–1742 (2006).
Acknowledgements
We thank the parents of the infants participating in this study and the nurses at our NICU for helping collect fecal samples.
Author information
Authors and Affiliations
Contributions
B.A. conceptualized, designed, supervised the study, and drafted the initial protocol and the manuscript. J.S. supervised the recruitment, collected the data, performed the microbial analysis, and critically reviewed the manuscript. A.S. interpreted the data and critically reviewed the manuscript. S.M. and T.F. conducted the statistical analyses and critically reviewed the manuscript. D.D. supervised the drug supplementation and distribution, and critically reviewed the manuscript. M.A. reviewed the protocol, supervised the microbial analyses, interpreted the data, and critically reviewed the manuscript. H.A. designed the study, and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Consent from parents was obtained.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Alshaikh, B., Samara, J., Moossavi, S. et al. Multi-strain probiotics for extremely preterm infants: a randomized controlled trial. Pediatr Res 92, 1663–1670 (2022). https://doi.org/10.1038/s41390-022-02004-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02004-z
This article is cited by
-
Bifidobacterium infantis as a probiotic in preterm infants: a systematic review and meta-analysis
Pediatric Research (2023)