Abstract
Background
This study examined the relationship between prenatal maternal stress (PREMS) and non-nutritive suck (NNS) and tested its robustness across 2 demographically diverse populations.
Methods
The study involved 2 prospective birth cohorts participating in the national Environmental influences on Child Health Outcomes (ECHO) Program: Illinois Kids Development Study (IKIDS) and ECHO Puerto Rico (ECHO-PROTECT). PREMS was measured during late pregnancy via the 10-item Perceived Stress Scale (PSS-10). NNS was sampled from 1- to 8-week-olds using a custom pacifier for ~5 min.
Results
Overall, 237 mother–infant dyads completed this study. Despite several significant differences, including race/ethnicity, income, education, and PREMS levels, significant PREMS-NNS associations were found in the 2 cohorts. In adjusted linear regression models, higher PREMS, measured through PSS-10 total scores, related to fewer but longer NNS bursts per minute.
Conclusions
A significant association was observed between PREMS and NNS across two diverse cohorts. This finding is important as it may enable the earlier detection of exposure-related deficits and, as a result, earlier intervention, which potentially can optimize outcomes. More research is needed to understand how NNS affects children’s neurofunction and development.
Impact
-
In this double-cohort study, we found that higher maternal perceived stress assessed in late pregnancy was significantly associated with fewer but longer sucking bursts in 1- to 8-week-old infants.
-
This is the first study investigating the association between prenatal maternal stress (PREMS) and infant non-nutritive suck (NNS), an early indicator of central nervous system integrity.
-
Non-nutritive suck is a potential marker of increased prenatal stress in diverse populations.
-
Non-nutritive suck can potentially serve as an early indicator of exposure-related neuropsychological deficits allowing for earlier interventions and thus better prognoses.
Similar content being viewed by others
Introduction
Globally, one-third of healthy pregnant women report mild-to-moderate stress.1,2,3,4,5,6,7 Prenatal maternal stress (PREMS) is especially high in diverse female urban samples, with 78% reporting low-to-moderate PREMS and 6% reporting high PREMS.8 Common pregnancy stressors include low material resources, unfavorable employment conditions, heavy family/household responsibilities, intimate relationship strain, and pregnancy complications.9 PREMS has been shown to increase the risk for various pregnancy complications and poor birth outcomes.10,11,12,13,14,15
Additionally, PREMS is associated with children’s long-term development.16,17,18 Child outcomes linked to PREMS include neurological,19 physical, and physiological changes,20,21,22 and increased risk for attention-deficit hyperactivity disorder, conduct disorder,23,24,25,26 and cognitive and linguistic impairments.27,28 Although many studies on PREMS and neurodevelopment have focused on children, few have looked at young infants. Investigating the relationship between PREMS and young infants’ neurodevelopment is critical to moving the field forward as it may allow for the earlier identification of problems and, consequently, enable earlier interventions, which tend to have better prognoses when compared with later interventions.
Infant neurofunction assessments have been problematic and limited. Even though standardized tests, like the Bayley-III scales, can be useful in identifying children with severe levels of intellectual disability, there are several drawbacks as well, including significant examiner training, reliance on examiner’s qualitative ratings, and less than desirable precision and sensitivity. Moreover, these tests often underestimate delays and tend to poorly predict future neurofunction.29,30,31 Thus, there is a need to develop early physiological-based measures that are precise, quantitative, and related to subsequent development. Non-nutritive suck (NNS) is an early measure of neonatal brain function.32 Using a pressure transducer system to measure NNS circumvents previous assessment limitations with minimal examiner training, ease of operation, automated data collection, and more precise quantitative measures.
NNS is a suck pattern characterized by the absence of nutrient delivery. NNS has a burst-pause pattern, with each burst containing 6–12 suck cycles and a within burst frequency of 2 Hz33 (Fig. 1). NNS is controlled by brainstem interneurons and altered by sensory stimulation34,35 and experiences.36 NNS can potentially predict later neurodevelopment in various domains (e.g., motor skills, balance, intelligence, and language).37,38 This study examined the relationship between PREMS and NNS in 1- to 8-week-old infants and tested its robustness across 2 diverse cohorts. We hypothesized that higher PREMS would disrupt infant NNS. This disruption could serve as an early indicator of later neuropsychological problems.
a A picture of the non-nutritive suck (NNS) device setup, which includes a laptop connected to a 4-channel ADInstruments PowerLab 4/26 data acquisition system. This system is attached to the pressure transducer (black box) that connects to the pacifier. b An infant from ECHO-PROTECT being assessed with the NNS device. c An illustration of a report of 28 s of NNS activity generated by the NNS system. Time elapsed during the NNS test/sampling is in the x axis and is measured in seconds (s). Amplitude or strength of each suck cycle is in the y axis and is measured in cmH2O. Three NNS bursts are present with pause periods for respiration. Frequency is measured by examining the cycles per second within a burst. Black dots indicate the peak of individual NNS cycles.
Methods
Study population and design
Mother–infant participants were from 2 prospective birth cohorts in Illinois and Puerto Rico that began recruitment in 2013 and 2011 respectively, and became a part of the Environmental influences on Child Health Outcomes (ECHO) Program in 2019 (additional details about the ECHO program are available elsewhere39). For the current analyses, the inclusion criteria were twofold: agreement to participate in the ECHO Program, and a minimum birth GA of 37 weeks, which is considered a full-term gestation. Written informed consent for participation in each cohort was obtained during pregnancy and at the child’s first assessment. Starting in 2019, mothers who agreed to enroll in the Cohort were also invited to join the ECHO program and complete another written informed consent. Some participants consented to ECHO during pregnancy, while others consented postnatally during infancy or early childhood. Cohorts received approval and supervision from the Institutional Review Boards at the University of Illinois at Urbana-Champaign (UIUC), Northeastern University, and the University of Puerto Rico.
IKIDS cohort
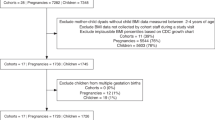
Participating mothers were recruited from 2 obstetric clinics in Champaign-Urbana, Illinois, for the Illinois Kids Development Study (IKIDS). IKIDS enrollment began in 2013 and is ongoing. The inclusion criteria were not having a child already in IKIDS, being 18–40 years of age, not having a high-risk pregnancy or carrying multiples, having English fluency, residing within a 30-min drive from the UIUC campus, and planning to remain in the area until the child’s first birthday. At enrollment (10–14 weeks’ GA), mothers provided sociodemographic/lifestyle information and medical and reproductive history. Follow-up interviews were conducted throughout pregnancy to track health/lifestyle changes. After birth, mothers were invited to participate in child assessments between 1 week and 7.5 years of age (IKIDS cohort profile.40) A total of 92 infants participated in the 1- to 5-week assessment at the research laboratory on the UIUC campus between December 2017 and November 2019. Of these infants, 75 (n = 31 females) were included in the current analysis, and the remaining infants were excluded for the following reasons: 1 was born prematurely (i.e., before 37 weeks’ gestation), 15 did not provide adequate NNS data, and 1 had a mother who did not complete the PSS-10.
ECHO-PROTECT cohort
Participating mothers were recruited before 20 weeks’ GA from 2 hospitals and 5 clinics in the Northern Karst aquifer region in Puerto Rico to be part of the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) study. Enrollment in PROTECT started in 2011 and is ongoing. The inclusion criteria were 18–40 years of age, residence in the Northern Karst aquifer region, PR, no oral contraceptives 3 months prepregnancy, no in-vitro fertilization, and no major preexisting medical conditions.41 PROTECT mothers provided basic demographic and pregnancy/lifestyle information at 14 weeks’ GA and 3 more times from 16 to 28 weeks’ GA. In 2016–2019, children born to PROTECT mothers were recruited into the Center for Research on Early Childhood Exposure and Development (CRECE) study42 and invited to participate in assessments between 2 weeks and 6 years of age (PROTECT/CRECE cohort profiles41,42). A total of 393 infants participated in the 2- to 8-week assessment at the research clinic in Manatí, Puerto Rico, from May 2017 to December 2019. Of the infants, 162 (n = 81 females) were included in the current analysis, the remaining infants were excluded because 29 were born prematurely (i.e., before 37 weeks’ gestation), 22 had mothers who declined to participate in ECHO, 7 had mothers who did not complete the PSS-10, and 3 did not provide sufficient NNS data.
PREMS
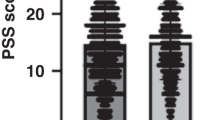
PREMS was assessed with the 10-item Perceived Stress Scale (PSS-10)43 in late pregnancy (IKIDS: 34–37 weeks’ GA, ECHO-PROTECT: 24–28 weeks’ GA). The PSS is the most widely used tool for evaluating respondents’ perception of stress in their recent lives;43 it has been validated in multiple populations and countries.44,45 Respondents are asked to rate on a 4-point Likert scale (from 0 = never to 4 = very often) how often they experienced specific feelings (e.g., being upset) and beliefs (e.g., being unable to cope with things) during the past month, (for a description of all 10 items see eTable 1 in Supplementary Materials). Responses are summed, with total scores ranging from 0 to 40. Score ranges indicating low, moderate, and high perceived stress are <14, 14–26, and >26, respectively.46
Infant NNS measures
NNS was assessed via a custom device that yields quantitative data in real-time (Fig. 1). This device includes a Soothie pacifier (Philips Avent) attached to a pressure transducer that transmits information to a data acquisition system (Power Lab, ADInstruments, Dunedin, New Zealand) (specifications and calibration methods47). Infants were offered the pacifier to suck on for ~5 min. Standardized devices, training, and administration were used across cohorts.
All NNS data were analyzed using LabChart software (ADInstruments, Dunedin, New Zealand). Trained researchers selected NNS bursts using the following criteria: bursts had >1 suck cycle with a new burst commencing when a between-cycle break was >1000 ms.34,36,48 Once an infant’s bursts were selected, they were entered into a custom NNS Burst Macro that calculated 4 variables: amplitude (units in cmH2O; strength of suck measure), number of cycles per burst (suck cycles within a burst), frequency (Hz; number of cycles per second within a burst), and burst duration (s). Next, the 2 consecutive minutes with the highest cycle count were used to calculate 2 additional variables: the number of cycles per minute and bursts per minute.
Statistical analyses
We conducted descriptive statistics on exposure (PREMS via PSS-10) and outcome (NNS) variables, and potential covariates for the IKIDS and ECHO-PROTECT samples, including maternal age, parents’ education, marital status, household income, health insurance, delivery type, parity, fetal sex, and prenatal cigarette and alcohol use. We characterized distributions of PSS and NNS parameters in addition to testing for bivariate associations between these variables and each of the potential covariates. Namely, Pearson correlation coefficients were calculated between PSS and NNS parameters. t-tests were conducted between categorical covariates and PSS and NNS parameters.
We utilized multiple linear mixed models to test for associations between PSS and NNS in the combined sample of IKIDS and PROTECT. Models included random intercepts for study participant ID. We also explored associations within each cohort individually using multiple linear regression to evaluate which cohort may be driving a given association in the combined sample analysis. We also leveraged meta-analysis of the two cohort-specific estimates to compare with the combined sample analysis.49 Fully-adjusted models relied on a combination of a priori knowledge of candidate confounders and substantiated by directed acyclic graphing50,51,52 (Fig. 2). Relationships between and among exposure and outcome variables and potential confounders were depicted graphically, followed by bivariate testing to evaluate associations with exposures, outcomes, and other potential confounders. The criteria for variable inclusion in the adjusted models included occurring in the time period prior to the outcome and not in the period between exposure and outcome (i.e., not a mediator). Final linear mixed models adjusted for the following covariates: maternal age, maternal education, household income, alcohol use, infant age at assessment, and cohort. Cohort-specific linear regression models included the same covariates with the exception of cohort. Because stress is often associated with depression, we also conducted sensitivity analyses estimating associations between maternal stress and each of the six NNS parameters. Maternal depression scores in the Center for Epidemiologic Studies Depression Scale (CES-D) were measured in the PROTECT cohort at between 24 and 30 weeks during pregnancy. Maternal depression was not analyzed in the IKIDS cohort because a different instrument was used in this cohort, and with relatively small samples, it was beyond the scope of the current study to try to harmonize these data.
Finally, we wondered whether the association between PSS and each NNS variable would differ if individual PSS items were considered rather than the total score, and decided to conduct some exploratory analyses using combined-cohort models only to explore this question.
Two-tailed p-values < 0.05 were considered statistically significant. No adjustment for multiple testing was performed due to the preliminary nature of these findings. The results and interpretation focus on the magnitude and confidence intervals of the estimates. All analyses were conducted using R Studio version 4.0.3.
Results
Participants in this study did not differ from their respective larger cohorts (IKIDS or ECHO-PROTECT) on their PREMS levels (Supplementary Table 2). Table 1 reports demographic, social, and health information stratified by cohort. Total sample size for the current study was 237 (IKIDS, n = 75; ECHO-PROTECT, n = 162). Cohorts were significantly different in race/ethnicity, parents’ education, parity, household income, prenatal smoking and alcohol use, and maternal age. IKIDS mothers were largely White (82.70%), married or cohabitating (90.70%), and educated with at least a bachelor’s degree (80%). In ECHO-PROTECT, 41.4% of mothers were White, 81.5% were married or cohabitating, and 54.30% had at least a bachelor’s degree or higher. Additionally, annual household income differed between samples: most IKIDS households earned at least $50,000/year (81.40%), whereas most ECHO-PROTECT households earned less than $50,000/year (82.70%). In terms of the children, 41.3% of IKIDS infants were female compared with 50% in ECHO-PROTECT. In IKIDS, 42.7% of infants were delivered via Cesarean section compared with 47.5% of ECHO-PROTECT infants. These differences were not statistically significant.
Importantly, our sensitivity analyses revealed that maternal depression in the PROTECT cohort did not independently predict any of the NNS variables. This evidence further justifies the exclusion of maternal depression as a confounder in our analyses measuring the association between PSS scores and each NNS variable.
Table 2 reports the distribution of PSS and NNS variables in the overall combined sample and notes differences in the distributions between IKIDS and ECHO-PROTECT. The 2 cohorts differed significantly in 5 out of 10 PSS items and in total PSS scores. The median total score was 19 (interquartile range [IQR] = 9) in the overall sample, with higher scores indicating higher perceived stress. ECHO-PROTECT mothers had higher median total scores (20 [IQR = 5.25]) compared with IKIDS mothers (10 [IQR = 10.25]). Differences were observed in NNS variables between cohorts although not as striking (2 out of 6 NNS outcomes) as the PSS differences. The median burst duration was 6.25 s in ECHO-PROTECT and 4.00 s in IKIDS. Additionally, the median number of suck-cycles per minute was 63 in ECHO-PROTECT and 45.5 in IKIDS.
Pearson correlation coefficients for PSS and NNS parameters are reported for the overall sample (Supplementary Table 3A), ECHO-PROTECT only (Supplementary Table 3B), and IKIDS only (Supplementary Table 3C). The greatest positive correlation in the overall sample was observed between PSS item-4 and NNS bursts per minute (Pearson ρ = 0.32). Bivariate associations between covariates and PSS and NNS parameters are reported in Supplementary Table 4. Several sociodemographic factors were associated with total PSS scores, including maternal race, parental education, and household income. Among these factors, NNS parameters were associated with maternal race (NNS cycles and NNS cycles per bursts) and household income (NNS bursts per minute and cycles per minute).
Crude and adjusted estimates from linear regression modeling are reported in Supplementary Table 5. The magnitude of association tended to be similar across adjusted and crude models. Notably, higher (indicating more stress) total PSS scores were associated with 0.09-s longer bursts (95% confidence interval (CI), 0.01–0.17) and 0.07 fewer bursts per minute (95% CI, −0.13 to −0.02). Table 3 shows the cohort-specific analyses, which indicate that the positive association between PSS total score and burst duration in the combined-cohort analysis seems to be driven by the IKIDS sample (β = 0.18, 95% CI, 0.04–0.31; p = 0.01) participants and not ECHO-PROTECT (β = 0.01, 95% CI, −0.10 to 0.12; p = 0.80). On the other hand, the negative association between PSS scores and number of bursts, found in the combined-cohort analysis, seems to be driven by the PROTECT sample (β = −0.10, 95% CI, −0.18 to −0.03; p = 0.01) vs IKIDS (β = −0.03, 95% CI, − 0.10 to 0.04; p = 0.40), although again the β coefficient in the two samples are in the same negative direction. Moreover, whereas in the combined-cohort models neither number of cycles per minute (β = −0.23, 95% CI, −0.74 to 0.28; p = 0.37) nor number of cycles per burst (β = 0.13, 95% CI, −0.03 to 0.29; p = 0.12) was significantly associated with PSS total score, the negative association between PSS and cycles per minute was significant in the PROTECT sample alone (β = −0.76, 95% CI, −1.40 to −0.12; p = 0.02), and the association between PSS and number of cycles per burst was marginally significant in the IKIDS sample alone (β = 0.27, 95% CI, −0.01 to 0.55; p = 0.07). The results of the meta-analysis largely mirrored those of the combined-cohort analysis, showing majority effects in the same direction. The association of total PSS score and bursts per minute remained significant (β = −0.06, 95% CI, −0.11 to −0.01; p = 0.01), giving greater confidence in findings from the combined-cohort analysis. However, there were a few small differences, as shown in Table 3. Although the association of burst duration and PSS total score was no longer significant (β = 0.08, 95% CI, −0.01 to 0.16; p = 0.97), the associations of total PSS score and burst frequency (β = −0.01, 95% CI, −0.01 to 0.001; p = 0.05) and total PSS score and cycles per minute (β = −0.34, 95% CI, −0.92 to 0.13; p = 0.07) were both marginally significant.
Results from the exploratory analyses utilizing the combined cohort and individual PSS items revealed some significant associations. Higher item-2 (feeling unable to control important things) scores were associated with 0.44-s longer bursts (95% CI, 0.05–0.82) and 0.26 fewer bursts per minute (95% CI, −0.51 to −0.01). Higher item-3 (feeling nervous and stressed) scores were associated with 0.3 fewer bursts per minute (95% CI, −0.6 to 0), and higher item-6 (feeling unable to cope with all that has to be done) scores were associated with 0.04-Hz lower frequencies (95% CI, −0.07 to −0.01). Finally, higher item-9 (feeling angered because things happening are beyond one’s control) scores were associated with 0.87-cmH2O lower NNS amplitude (95% CI, −1.69 to −0.06).
Discussion
The findings indicate associations between PREMS and NNS in full-term, 1- to 8-week-old infants from 2 ECHO cohorts, IKIDS and ECHO-PROTECT, which differed significantly by geography, demographics, and PREMS levels. Across cohorts, higher PREMS, measured through PSS-10 total scores, related to fewer but longer NNS bursts. Our study is the first to characterize a link between PREMS and NNS and demonstrate its persistence across diverse populations.
Demographics across cohorts
IKIDS and ECHO-PROTECT differed on key demographic characteristics (e.g., maternal race and ethnicity, parental education, and household income). These differences highlight the need for epidemiologic analyses across diverse samples. Many epidemiologic studies have overwhelmingly focused on White, high socioeconomic status (SES) samples—limiting inferences about historically marginalized, neglected, and at-risk communities.53 We partially ameliorated this critical public health research gap by including participants in Puerto Rico, an underserved, low-income population, with high contaminant exposures and health disparities. Adequate representation of demographically diverse populations will enhance the precision and efficacy of population-level clinical interventions.54
PREMS across cohorts
PREMS differed significantly across cohorts, with ECHO-PROTECT mothers reporting higher PSS scores in total composite and individual items 1, 4, 5, 7, 8, and 9 than IKIDS mothers. Higher stress levels among Puerto Rican mothers are likely attributable to the aforementioned income and education differences and several stressful events on the island (e.g., ZIKA and Hurricane Maria). In general, Puerto Ricans experience many socioeconomic stressors, including much higher poverty rates than non-Latina Whites and Latinos as a whole.55 Our results corroborate prior findings that PREMS is especially high among underrepresented minority women8 and that poor employment conditions and resources can increase stress.9
NNS across cohorts
When compared with previous NNS studies of full-term infants in the US Northeast,48 IKIDS infants resembled older, 3-month-old infants both in the number of bursts per minute (Northeast = 4.50, IKIDS = 4.10) and the number of cycles per minute (Northeast = 49.57, IKIDS = 45.80), whereas ECHO-PROTECT infants were very different from both groups in these 2 outcomes (ECHO-PROTECT = 6.30 bursts/min; 61.40 cycles/min). The fact that ECHO-PROTECT infants were older than IKIDS infants and thus closer in age to the Northeast sample makes this finding noteworthy. More research is needed to confirm these results and explore the causes. ECHO-PROTECT is demographically distinct from the other 2 infant groups, which may partially explain the NNS differences. Additionally, compared with the other 2 groups, ECHO-PROTECT infants may have higher exposure to contaminants from the Northern Karst aquifer region in PR,56,57 which may contribute to the NNS differences.
NNS and PREMS associations by cohort
When examining the adjusted linear models for associations between PSS total scores and NNS outcomes in IKIDS only, there was a significant positive association with burst duration and a marginally significant positive association with cycles per burst. These findings indicate that higher PREMS in IKIDS was associated with longer bursts that had more suck cycles within the burst. While not significant, the associations of PSS total score with burst duration and PSS total score with cycles per burst in ECHO-PROTECT were in the same positive direction as IKIDS.
When examining the adjusted linear models for associations between PSS total scores and NNS outcomes in ECHO-PROTECT only, there was a significant negative association with number of bursts and number of cycles. These findings show that higher PREMS was associated with fewer bursts and fewer sucks per minute. The IKIDS models for these two variables were not significant; however, the association was in the same direction for number of bursts but not for number of cycles. The fact that the meta-analysis also revealed a significant negative association between PSS total scores and bursts per minute suggests that differences between cohorts in this context may be largely driven by sample size. Additionally, differences may also be driven by residual unmeasured confounding.
As exploratory analyses, we examined the relationship between individual PSS items and the different NNS outcomes in adjusted regression models with the two cohorts combined. Findings indicated that higher total and item-2 (feeling unable to control important things) scores were associated with fewer but longer bursts per minute. In addition, higher item-3 (feeling nervous and stressed) scores were associated with fewer bursts per minute. Higher item-6 (feeling unable to cope with all that has to be done) scores were linked with slower sucks (i.e., lower frequencies). Lastly, higher item-9 (feeling angered because things happening are beyond one’s control) scores were associated with weaker sucks (i.e., lower amplitudes). Altogether, these findings indicate that mothers who reported feeling more nervous and stressed, less able to control or cope with things, and angrier by their lack of control had infants with lower and weaker sucking activity (i.e., fewer bursts per minute, slower frequencies, and lower amplitude), but the activity lasted longer (i.e., longer burst duration). Possibly, infants of more stressed mothers are sucking more slowly and for longer intervals as a self-soothing mechanism,58,59,60 but this notion needs further testing. Alternatively, infants may suck for longer durations to compensate for the fewer bursts and slower and weaker sucks. Interestingly, total and item-2 (feeling unable to control one’s life) scores were related to the same NNS variables (burst duration and number), whereas item-3 (feeling nervous/stressed), item-2 (unable to cope), and item-9 (angry by lack of control) scores were related to different NNS variables (bursts per minute, frequency, and amplitude). Therefore, certain aspects of stress may be differentially associated with NNS variables.
Mechanisms underlying these findings remain incompletely understood. PREMS was previously found to alter fetal cardiorespiratory function.61 Additionally, PREMS has been associated with reduced fetal movement,62,63 which seems to mirror the lower NNS activity in our study. Importantly, the associations between PREMS, fetal heart rate, and activity level are exacerbated by lower SES.64 Moreover, the newborns’ biochemical/physiological profiles were found to mimic their mother’s prenatal profile, with stressed or depressed mothers delivering infants with stressed or depressed profiles (e.g., elevated cortisol and lower dopamine and serotonin).65 Finally, recent studies with the IKIDS cohort demonstrate that PREMS negatively impacts neurofunction (i.e., physical reasoning and information processing speed) in 4- and 8-month-olds.66,67 These findings suggest that maternal mood and stress powerfully impacts infants’ central nervous system starting prenatally and through the first months of life.
Implications
NNS may serve as a quantifiable marker of PREMS in neonates, allowing for the earlier detection of risk for PREMS-related complications, including impairments on children’s health and neuropsychological function.16,17,18,19,20,21,22,23,24,25,26,27,28,68 NNS is an important early measure because it has been used as an index of newborns’ brain integrity37,69,70 and has been linked to later neurobehavioral functions (e.g., motor skills, balance, intelligence, and language).37,38 Overall findings from the current study suggest that when the mother is stressed during pregnancy her infant’s NNS is characterized by fewer bursting attempts on the pacifier but when bursts were attempted, the activity occurred for a longer duration of time. What this specifically means for subsequent neurodevelopment remains unknown as this research represents some of the first attempts to measure infant’s NNS quantitatively and examine its association with prenatal exposures. It is clear, however, that higher PREMS is associated with disruptions in infant’s NNS, and future studies—examining the association between quantitative NNS measures in the neonatal period with development across domains later in infancy and childhood—will shed light as to what specific changes in NNS patterns mean. Indeed, this is the goal of one of our follow-up studies: to examine the associations between neonatal NNS patterns and visual attention, memory, and speed of processing at 7 months of age. Our findings also highlight the importance of intervention programs that reduce PREMS to avoid disruptions and promote optimal fetal and infant development.71,72
Strengths and limitations
The strengths of the current study include analyses of 2 diverse samples—one from an underserved, low-SES, at-risk population—yielding more generalizable findings and addressing some critical public health research gaps. Additionally, the study has a robust prospective cohort design.
A limitation of this study is that we did not examine other variables that could impact maternal stress, such as anxiety, adverse-life events, and social support as we do not have these data. However, subsequent studies should include these factors in their analyses if possible. Another limitation is the lack of longitudinal NNS assessments, which would allow us to examine the association of NNS with growth and neurodevelopment over time. Follow-up studies are needed to continue the current work and address these limitations across patient populations.
Conclusions
We identified associations between PREMS and 1- to 8-week-old infant NNS in 2 cohorts with distinct demographics and PREMS levels. Specifically, higher PREMS resulted in fewer but longer bursts. This is the first evidence of a PREMS-NNS association. This finding is important as it may enable the earlier detection of exposure-related deficits and, as a result, earlier intervention, which potentially can optimize outcomes. More research is needed to further investigate this association and its potential to serve as an early clinical indicator.
References
Woody, C. A., Ferrari, A. J., Siskind, D. J., Whiteford, H. A. & Harris, M. G. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J. Affect Disord. 219, 86–92 (2017).
Dennis, C. L., Falah-Hassani, K. & Shiri, R. Prevalence of antenatal and postnatal anxiety: systematic review and meta-analysis. Br. J. Psychiatry 210, 315–323 (2017).
Loomans, E. M. et al. Psychosocial stress during pregnancy is related to adverse birth outcomes: results from a large multi-ethnic community-based birth cohort. Eur. J. Public Health 23, 485–491 (2013).
Phelan, A. L., DiBenedetto, M. R., Paul, I. M., Zhu, J. & Kjerulff, K. H. Psychosocial stress during first pregnancy predicts infant health outcomes in the first postnatal year. Matern. Child Health J. 19, 2587–2597 (2015).
Yuksel, F., Akin, S. & Durna, Z. Prenatal distress in Turkish pregnant women and factors associated with maternal prenatal distress. J. Clin. Nurs. 23, 54–64 (2014).
Hou, Q. et al. The associations between maternal lifestyles and antenatal stress and anxiety in Chinese pregnant women: a cross-sectional study. Sci. Rep. 8, 10771 (2018).
Tang, X., Lu, Z., Hu, D. & Zhong, X. Influencing factors for prenatal stress, anxiety and depression in early pregnancy among women in Chongqing, China. J. Affect Disord. 253, 292–302 (2019).
Woods, S. M., Melville, J. L., Guo, Y., Fan, M. Y. & Gavin, A. Psychosocial stress during pregnancy. Am. J. Obstet. Gynecol. 202, 61 e61–61.e67 (2010).
Dunkel Schetter, C. & Tanner, L. Anxiety, depression and stress in pregnancy: implications for mothers, children, research, and practice. Curr. Opin. Psychiatry 25, 141–148 (2012).
Fenster, L. et al. Psychologic stress in the workplace and spontaneous abortion. Am. J. Epidemiol. 142, 1176–1183 (1995).
Hansen, D., Lou, H. C. & Olsen, J. Serious life events and congenital malformations: a national study with complete follow-up. Lancet 356, 875–880 (2000).
Lou, H. C. et al. Prenatal stressors of human life affect fetal brain development. Dev. Med. Child Neurol. 36, 826–832 (1994).
Neugebauer, R. et al. Association of stressful life events with chromosomally normal spontaneous abortion. Am. J. Epidemiol. 143, 588–596 (1996).
Nimby, G. T., Lundberg, L., Sveger, T. & McNeil, T. F. Maternal distress and congenital malformations: do mothers of malformed fetuses have more problems? J. Psychiatr. Res. 33, 291–301 (1999).
Paarlberg, K. M. et al. Psychosocial factors and pregnancy outcome: a review with emphasis on methodological issues. J. Psychosom. Res. 39, 563–595 (1995).
Glover, V. Annual Research Review: Prenatal stress and the origins of psychopathology: an evolutionary perspective. J. Child Psychol. Psychiatry 52, 356–367 (2011).
Talge, N. M., Neal, C. & Glover, V. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J. Child Psychol. Psychiatry 48, 245–261 (2007).
Van den Bergh, B. R., Mulder, E. J., Mennes, M. & Glover, V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: links and possible mechanisms. A review. Neurosci. Biobehav. Rev. 29, 237–258 (2005).
Buss, C., Davis, E. P., Muftuler, L. T., Head, K. & Sandman, C. A. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6-9-year-old children. Psychoneuroendocrinology 35, 141–153 (2010).
Glover, V., O’Connor, T. G., Heron, J., Golding, J. & team, A. S. Antenatal maternal anxiety is linked with atypical handedness in the child. Early Hum. Dev. 79, 107–118 (2004).
Obel, C., Hedegaard, M., Henriksen, T. B., Secher, N. J. & Olsen, J. Psychological factors in pregnancy and mixed-handedness in the offspring. Dev. Med. Child Neurol. 45, 557–561 (2003).
King, S. et al. Prenatal maternal stress from a natural disaster predicts dermatoglyphic asymmetry in humans. Dev. Psychopathol. 21, 343–353 (2009).
Kleinhaus, K. et al. Prenatal stress and affective disorders in a population birth cohort. Bipolar Disord. 15, 92–99 (2013).
O’Connor, T. G., Heron, J., Golding, J., Glover, V. & Team, A. S. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J. Child Psychol. Psychiatry 44, 1025–1036 (2003).
Rice, F. et al. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychol. Med. 40, 335–345 (2010).
Rodriguez, A. & Bohlin, G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? J. Child Psychol. Psychiatry 46, 246–254 (2005).
Laplante, D. P., Brunet, A., Schmitz, N., Ciampi, A. & King, S. Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 1/2-year-old children. J. Am. Acad. Child Adolesc. Psychiatry 47, 1063–1072 (2008).
Mennes, M., Stiers, P., Lagae, L. & Van den Bergh, B. Long-term cognitive sequelae of antenatal maternal anxiety: involvement of the orbitofrontal cortex. Neurosci. Biobehav. Rev. 30, 1078–1086 (2006).
Anderson, P. J. et al. Underestimation of developmental delay by the new Bayley-III Scale. Arch. Pediatr. Adolesc. Med. 164, 352–356 (2010).
Anderson, P. J. & Burnett, A. Assessing developmental delay in early childhood—concerns with the Bayley-III scales. Clin. Neuropsychol. 31, 371–381 (2017).
Spencer-Smith, M. M., Spittle, A. J., Lee, K. J., Doyle, L. W. & Anderson, P. J. Bayley-III cognitive and language scales in preterm children. Pediatrics 135, e1258–e1265 (2015).
Slattery, J., Morgan, A. & Douglas, J. Early sucking and swallowing problems as predictors of neurodevelopmental outcome in children with neonatal brain injury: a systematic review. Dev. Med. Child Neurol. 54, 796–806 (2012).
Wolff, P. H. The serial organization of sucking in the young infant. Pediatrics 42, 943–956 (1968).
Barlow, S. M., Burch, M., Venkatesan, L., Harold, M. & Zimmerman, E. Frequency modulation and spatiotemporal stability of the sCPG in preterm infants with RDS. Int. J. Pediatr. 2012, 581538 (2012).
Zimmerman, E. & DeSousa, C. Social visual stimuli increase infants suck response: a preliminary study. PLoS ONE 13, e0207230 (2018).
Estep, M., Barlow, S. M., Vantipalli, R., Finan, D. & Lee, J. Non-nutritive suck parameter in preterm infants with RDS. J. Neonatal Nurs. 14, 28–34 (2008).
Wolthuis-Stigter, M. I. et al. Sucking behaviour in infants born preterm and developmental outcomes at primary school age. Dev. Med. Child Neurol. 59, 871–877 (2017).
Wolthuis-Stigter, M. I. et al. The association between sucking behavior in preterm infants and neurodevelopmental outcomes at 2 years of age. J. Pediatr. 166, 26–30 (2015).
LeWinn, K. Z., Caretta, E., Davis, A., Anderson, A. L., Oken E. & Program Collaborators for Environmental Influences on Child Health O. SPR perspectives: environmental influences on Child Health Outcomes (ECHO) Program: overcoming challenges to generate engaged, multidisciplinary science. Pediatr. Res. https://doi.org/10.1038/s41390-021-01598-0 (2021).
Eick, S. M. et al. Associations of maternal stress, prenatal exposure to per- and polyfluoroalkyl substances (PFAS), and demographic risk factors with birth outcomes and offspring neurodevelopment: an overview of the ECHO.CA.IL Prospective Birth Cohorts. Int. J. Environ. Res. Public Health. 18, 742 (2021).
Ferguson, K. K. et al. Demographic risk factors for adverse birth outcomes in Puerto Rico in the PROTECT cohort. PLoS ONE 14, e0217770 (2019).
Manjourides, J. et al. Cohort profile: center for research on early childhood exposure and development in Puerto Rico. BMJ Open 10, e036389 (2020).
Cohen, S. Perceived Stress Scale. https://www.mindgarden.com/documents/PerceivedStressScale.pdf (1994).
Cohen, S. & Janicki-Deverts, D. Who’s stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. J. Appl Soc. Psychol. 42, 1320–1334 (2012).
Lee, E. H. Review of the psychometric evidence of the perceived stress scale (vol 6, pg 121, 2012). Asian Nurs. Res. 7, 160–160 (2013).
MD APP. Perceived Stress Scale Calculator. https://www.mdapp.co/perceived-stress-scale-pss-calculator-389/ (2020).
Zimmerman, E. et al. Associations of gestational phthalate exposure and non-nutritive suck among infants from the Puerto Rico Testsite for Exploring Contamination Threats (PROTECT) birth cohort study. Environ. Int. 152, 106480 (2021).
Martens, A., Hines, M. & Zimmerman, E. Changes in non-nutritive suck between 3 and 12 months. Early Hum. Dev. 149, 105141 (2020).
Willer, C. J., Li, Y. & Abecasis, G. R. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 26, 2190–2191 (2010).
Hernan, M. A., Hernandez-Diaz, S., Werler, M. M. & Mitchell, A. A. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am. J. Epidemiol. 155, 176–184 (2002).
Shrier, I. & Platt, R. W. Reducing bias through directed acyclic graphs. BMC Med. Res. Methodol. 8, 70 (2008).
Williams, T. C., Bach, C. C., Matthiesen, N. B., Henriksen, T. B. & Gagliardi, L. Directed acyclic graphs: a tool for causal studies in paediatrics. Pediatr. Res. 84, 487–493 (2018).
Lin, S. & Kelsey, J. Use of race and ethnicity in epidemiologic research: concepts, methodological issues, and suggestions for research. Epidemiol. Rev. 22, 187–202 (2000).
Oh, S. S. et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 12, e1001918 (2015).
Motel, S. & Patten, E. Hispanics of Puerto Rican Origin in the Unites States, 2010 (2012).
Cantonwine, D. E. et al. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: distribution, temporal variability, and predictors. Environ. Int. 62, 1–11 (2014).
Ferguson, K. K. et al. Environmental phthalate exposure and preterm birth in the PROTECT birth cohort. Environ. Int. 132, 105099 (2019).
Field, T. et al. Nonnutritive sucking during tube feedings: effects on preterm neonates in an intensive care unit. Pediatrics 70, 381–384 (1982).
Boyle, E. M. et al. Sucrose and non-nutritive sucking for the relief of pain in screening for retinopathy of prematurity: a randomised controlled trial. Arch. Dis. Child Fetal Neonatal Ed. 91, F166–F168 (2006).
Foster, J. P., Psaila, K. & Patterson, T. Non-nutritive sucking for increasing physiologic stability and nutrition in preterm infants. Cochrane Database Syst. Rev. 10, CD001071 (2016).
DiPietro, J. A., Hodgson, D. M., Costigan, K. A. & Johnson, T. R. Fetal antecedents of infant temperament. Child Dev. 67, 2568–2583 (1996).
DiPietro, J. A., Costigan, K. A. & Gurewitsch, E. D. Fetal response to induced maternal stress. Early Hum. Dev. 74, 125–138 (2003).
Kinsella, M. T. & Monk, C. Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clin. Obstet. Gynecol. 52, 425–440 (2009).
DiPietro, J. A., Costigan, K. A., Shupe, A. K., Pressman, E. K. & Johnson, T. R. Fetal neurobehavioral development: associations with socioeconomic class and fetal sex. Dev. Psychobiol. 33, 79–91 (1998).
Field, T. Prenatal depression risk factors, developmental effects and interventions: a review. J. Pregnancy Child Health. 4, 301 (2017).
Merced-Nieves, F. M., Dzwilewski, K. L. C., Aguiar, A., Lin, J. & Schantz, S. L. Associations of prenatal maternal stress with measures of cognition in 7.5-month-old infants. Dev. Psychobiol. 63, 960–972 (2020).
Merced-Nieves, F. M. et al. Association of prenatal maternal perceived stress with a sexually dimorphic measure of cognition in 4.5-month-old infants. Neurotoxicol. Teratol. 77, 106850 (2020).
Bergman, K., Sarkar, P., O’Connor, T. G., Modi, N. & Glover, V. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J. Am. Acad. Child Adolesc. Psychiatry 46, 1454–1463 (2007).
Adams-Chapman, I., Bann, C. M., Vaucher, Y. E., Stoll, B. J. & Eksni, C. Association between feeding difficulties and language delay in preterm infants using Bayley scales of infant development. J. Pediatr. 163, 680 (2013).
Wolthuis-Stigter, M. I. et al. The association between sucking behavior in preterm infants and neurodevelopmental outcomes at 2 years of age. J. Pediatr. 166, 26 (2015).
Glover, V. Maternal depression, anxiety and stress during pregnancy and child outcome; what needs to be done. Best. Pr. Res Clin. Obstet. Gynaecol. 28, 25–35 (2014).
Heinrichs, M. et al. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J. Clin. Endocrinol. Metab. 86, 4798–4804 (2001).
Acknowledgements
We wish to thank our ECHO colleagues; the medical, nursing, and program staff; and the children and families participating in the ECHO cohorts. We also acknowledge the contribution of the following ECHO program collaborators: ECHO Components—Coordinating Center: Duke Clinical Research Institute, Durham, North Carolina: Smith PB, Newby KL, Benjamin DK.
Funding
Research reported in this publication was supported by the Environmental influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health, under award numbers U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), U24OD023319 (PRO Core), and U2COD023375 (OIF). Dr. Aung’s work was supported by NIH award number P30ES030284. Research on the ECHO-PROTECT cohort was supported by the Children’s Environmental Health and Disease Prevention Research Center (NNIEHS P42ES017198, P50ES026049, and UH3OD023251), the Environmental Protection Agency (EPA) grant R836155, the Environmental Influences on Child Health Outcomes (ECHO) Program (NIH OD023251), and the National Institute on Minority Health and Health Disparities, award number U54MD007600. Research on the IKIDS cohort was supported by the Children’s Environmental Health and Disease Prevention Research Center (NIEHS P01 ES022848 and USEPA RD83543401) and the ECHO Program (NIH OD023272).
Author information
Authors and Affiliations
Consortia
Contributions
E.Z. and A.A. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: E.Z. and A.A. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: E.Z. and A.A. Critical revision of the manuscript for important intellectual content: E.Z., A.A., S.D.G., and M.T.A. Review of the original draft: all authors. Statistical analysis: M.T.A. and S.D.G. Obtained funding: E.Z., A.A., S.L.S., A.N.A., J.F.C., and J.D.M. Administrative, technical, or material support: E.Z., A.A., S.L.S., and A.N.A. Supervision: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Written informed consent was obtained from participants during pregnancy and at the child’s first assessment.
Disclaimer
The content and views expressed here are the sole responsibility of the authors and do not necessarily represent the official views of the EPA, NIEHS, NIH, or National Institute on Minority Health and Health Disparities, or Duke University (which manages Drs. Zimmerman and Aguiar’s NIH award number U2COD023375).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zimmerman, E., Aguiar, A., Aung, M.T. et al. Examining the association between prenatal maternal stress and infant non-nutritive suck. Pediatr Res 93, 1285–1293 (2023). https://doi.org/10.1038/s41390-021-01894-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01894-9
This article is cited by
-
Impact of intrapartum oxytocin administration on neonatal sucking behavior and breastfeeding
Scientific Reports (2024)