Abstract
Background
Heart rate (HR) is a biomarker used to measure physiological function, health status and cardiovascular autonomic function. The purpose of this study was to determine sex- and age-specific reference values for cardiac autonomic function at rest, during maximal exercise and the recovery phase in prepubertal children.
Methods
Five hundred and twelve healthy children 7–11 years of age performed a Léger test. A heart RR-interval monitor recorded the heart data and a specific software analysed the cardiac autonomic response through HR and HR variability (HRV). It analysed HR before the test (resting HR, RHR), during the test (HRpeak) and HR recovery (HRR) in the first minute (HRR1) and the fifth minute (HRR5). The values are mean ± SD.
Results
Collectively, 91.2% of girls and 92.3% of boys were within the recommended ranges regarding RHR. The average HRpeak was 199 ± 10.83 b.p.m. and 96.8% of girls and 95.3% of boys were within the minimum threshold value recommended (180 b.p.m.). Boys showed lower values of RHR than girls (p < 0.001) and larger values of HRR 1 and HRR5 (p < 0.001).
Conclusions
This study comprehensively provides a reference set of data for the most important HR variables that can be obtained during exercise testing in prepubertal children regarding age and sex and in a field setting.
Impact
-
This is the first study to provide reference values of autonomic cardiac function at rest, during maximal exercise and during the recovery period in prepubertal children aged 7–11 years.
-
Despite the early age of participants, cardiorespiratory fitness, RHR and HRR are different according to sex.
-
Aerobic performance and HRpeak have a negative correlation with body mass index and cardiometabolic risk.
Similar content being viewed by others
Introduction
The importance of physical activity for health is well known and research has noted both physical and psychological benefits when children participate in physical activities.1 However, evidence shows that higher levels of sedentary behaviour are associated with the following poor health outcomes in children and adolescents: increased adiposity, cardiometabolic risk, poorer cardiac autonomic nervous system function and low cardiorespiratory fitness (CRF).2,3
Cardiovascular diseases are a major public health problem with a large impact on morbidity and mortality rates worldwide and evidence advises that cardiovascular diseases may at least partly originate in the earliest childhood.4 During maturation, from infancy to adulthood, changes in cardiac autonomic neural regulation induce changes in cardiac rhythm, which could be used as part of the differential diagnosis process to identify specific pathologies and those individuals at the greatest risk for complications from several diseases.5 In this regard, cardiac autonomic dysfunction is linked to several childhood diseases, such as obesity6 and diabetes.7
Exercise dysfunction precedes resting abnormalities, so exercise testing could be a tool for early detection of cardiac dysfunction in prepubertal children.8 In this regard, the cardiovascular stress response to physical exercise by measurement of simple clinical measures, such as heart rate (HR), may be a valuable additional measurement to identify subtle differences in cardiovascular health from early childhood onwards.4
It is noteworthy that autonomic nervous system balance can be ascertained by analysing several values of the cardiopulmonary exercise test, such as the rest period, HR response to dynamic exercise, HR recovery (HRR) from the exercise test and HR variability (HRV).9 However, little is known about this research topic in the school-aged population.10 In particular, children with a resting HR (RHR) ≥ 91 b.p.m. present with higher cardiovascular risk,11 while children with an RHR < 60 b.p.m. (sinus bradycardia)12 could be a consequence of different pathologies and in association with concomitant congenital heart disease.13 Moreover, bradycardia can be accompanied by exercise intolerance in older children.13 Nevertheless, regular physical activity culminates in bradycardia,14,15 being that the RHR of children endurance athletes is 11 b.p.m. lower than age-matched non-athletes.16 This phenomenon appears due to cardiac adaptations led by a vagal predominance, sinus bradycardia and increased HRV17 and is not associated with cardiovascular risk factors.
In addition, during physical exercise, there must be an HR increase led by parasympathetic withdrawal and sympathetic activation.18 This physiological phenomenon triggers an HR response, which will increase with the intensity of the activity reaching a peak (HRpeak). This value might predict cardiovascular events and mortality when attenuated due to chronotropic incompetence, which is related to the HR reserve (HRr), which is the difference between maximum age-predicted HR and HR at rest.19,20 Chronotropic incompetence has shown to be a valuable prognostic tool for patients with coronary heart disease, a predictor of all-cause mortality and has been identified in several heart diseases in the paediatric population.20 After exercise, cardiac activity is reduced by parasympathetic reactivation and sympathetic inhibition and a gradual return of the HR to resting levels is evaluated by HRR that may be valuable to distinguish trained from untrained people.18 Furthermore, in children, a better HRR is a marker of higher physical fitness and lower cardiometabolic risk and is directly associated with healthy lifestyle behaviours, such as regular physical activity and inversely associated with sedentary behaviours, such as the use of screens.21
Broadly, RHR, HRpeak, HRR and HRV are CRF indicators.18 Since CRF is a powerful biomarker of health in children and adolescents,22 CRF evaluations can provide relevant information about the lifestyle and health status of a paediatric population, provoking physiological reactions that are absent at rest.23
The cardiovascular autonomic function has been well studied for clinical purposes in children;24 however, few studies10,25,26 have investigated the impact that sex, age, CRF and weight status have on HR response to maximal exercise and following recovery. In addition, after reviewing the scientific literature, this study noticed that reference values for a cardiovascular response during rest, maximal exercise and recovery in childhood remain unknown for prepubertal Caucasian children. Thus, the main purpose of this study was to analyse cardiac autonomic function at rest, during maximum exercise and during the recovery phase to determine sex- and age-specific reference values of RHR, HRpeak and HRR in healthy Caucasian prepubertal children. The second aim was to determine the association of HR parameters with CRF and anthropometric variables.
Methods
Participants
A priori sample size was performed using G*Power software.27 The following parameters were selected: moderate effect size (f = 0.250), α-level of 0.05, the power level of 0.95, five groups, one measurement and critical F = 2.402. The sample size was determined to be at least 302 participants. This cross-sectional study involved a cohort of 512 healthy children (233 girls) aged 7–11 years (age = 9.18 ± 1.42 years old) and they were selected by convenience from several schools (12 schools) in southern Spain, in both urban and rural areas. Pubertal stages were determined according to the references of Tanner.28 All children were in Tanner stage 1 (prepubertal age). Inclusion criteria involved being free from physical and/or intellectual disabilities; for that purpose, the parents of the enrolled children provided a document in which they stated that their children were free of physical and intellectual disabilities. Parents voluntarily signed an informed consent for the participation of their children in this study. The study was completed following the norms of the Declaration of Helsinki (2013 version) and was approved by the Ethics Committee of the University of Jaen (SEPT.20/9.TES).
Materials and testing
Anthropometric variables
Body mass (kg) was measured using a weighing scale (Seca 899, Hamburg, Germany) and body height (m) was measured with a stadiometer (Seca 222, Hamburg, Germany). The body mass index (BMI) was calculated by dividing body mass (kg) by body height2 (in m). Waist circumference (WC) was measured at the umbilical location by using a non-elastic ergonomic circumference measuring tape (Seca 201, Germany; range 0–150 cm; accuracy: 1 mm). The waist-to-height ratio (WtHR) as a measure of cardiometabolic risk was obtained by dividing the WC (in cm) by the body height (cm) and was used as a tool to estimate the accumulation of fat in the central zone of the body; a WtHR cut-off of 0.5 is generally accepted as a universal cut-off for central obesity in children (aged ≥ 6 years).29
Cardiorespiratory fitness
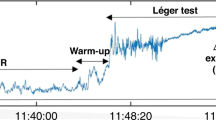
CRF was measured using the 20 Msrt (metre shuttle run test),30 which consisted of a 20-m run with increasing speed in each stage, indicating the pace with acoustic cues; velocity was increased by 0.5 km/h after every 1-min stage from a starting speed of 8.5 km/h. The best result corresponds to the highest number of stages performed, which indicates superior CRF. Furthermore, the maximum volume of oxygen uptake (VO2 max) was calculated from the speed that the participant achieved in his or her final sprint and VO2max was measured using the following equation: VO2 max = (31.025) + (3.238 × V) − (3.248 × A) + (0.1536 × A × V), where V is the speed in km/h and A is the age in years.30 Also, to obtain more information about the perceived exertion after the completion of the Léger test, the rate of perceived exertion (RPE) was recorded on a scale from 0 to 10 (0 being the lowest and 10 being the highest intensity).31
Regarding HR monitoring, a Firstbeat Bodyguard 2 of Firstbeat Technologies Ltd (Jyväskylä, Finland) was used for recording RR intervals. This monitor is a beat-to-beat HR recorder, which contains a 1-channel electrocardiogram obtained by two electrodes located on the chest. The monitor records at a sample rate of 1000 Hz and it has been validated with standard electrocardiogram equipment.32,33 With the data obtained by the RR-interval recorder, the study calculated HRV parameters through the Software Firstbeat Sports of Firstbeat Technologies Ltd (Jyväskylä, Finland). The software comprises the HRV algorithms provided by the European Society of Cardiology34 and a signal filter based on artefact correction algorithm described in Saalasti’s study.35
HRV analysis included several algorithms, all of which are based on either the frequency or time domain. The frequency domain of HRV includes the determination of the high-frequency (HF) (0.15–0.4 Hz) and low-frequency (LF) spectrum in ms2 (0.04–0.15 Hz) and provides the possibility of making a ratio LF/HF. HF may reflect parasympathetic activity, LF may indicate a combination of sympathetic and parasympathetic input and LF/HF may reflect sympathovagal balance.36 Corresponding to Bobkowski et al.,37 we deliberately omitted the HF and LF in normalised units, since there is a direct mathematical relationship between the two. On the other hand, the time domain analysis includes statistical measures that reflect parasympathetic activity, such as the root-mean-square differences of successive heart beat intervals (RMSSDs) and the mean of the standard deviation of the NN interval (normal RR) (SDNN).34,38 The study performed RR-interval data collection following the recommendations of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology34 for HRV analysis. In addition, the RR-interval data and the previously mentioned software were also applied for the calculation of HRpeak, HRR and HRr. Moreover, chronotropic index (CI) was defined as an index of maximal predicted HRr achieved; HRr is the difference between the maximum age-predicted HR and HR at rest. Particularly, the CI was accordingly defined as CI = (HRpeak − RHR)/[(220 − age) − RHR]39 and chronotropic incompetence was said to be present when values of the CI were <0.80.39 Moreover, according to previous studies,40,41,42 HR was also recorded at 1 and 5 min after the cessation of the exercise and the HRR was calculated as the difference between the HRpeak and HR at these recovery points.
Procedure
All the experiments were conducted in the school’s sports facilities, in the morning and at least 3 h after the last meal and children were asked to refrain from strenuous physical activity on the day prior to measurement. Prior to exercise testing, anthropometric variables were registered and RHR and HRV were measured after 10 min in a seated position and with spontaneous breathing according to Young et al.43 To stabilise the HR, children were instructed to abstain from speaking or moving during the examination. Seated short-term resting HRV measurements can be reliably achieved in the field (i.e. outside lab-controlled settings) in children.44 Subsequently, the children performed a typical warm-up. Then, the children performed familiarisation trial and followed by the Léger test. Throughout the Léger test, the HR was monitored continuously to obtain parameters, such as HRpeak at the end of the test. During exercise, the HR measurement was obtained in a subsample of 475 children (93% of the total sample), because the HR monitor lost the signal during the Léger test. The children were motivated and encouraged to reach the best score possible in the test. The HR was also recorded at the first (HRR1) and the fifth (HRR5) minute after the cessation of the exercise and HRR was calculated as the difference between HRpeak and the HR at these recovery points (HRR1 and HRR5). According to Buchheit et al.,45 at the end of the Léger test, all subjects immediately sat passively on a chair placed adjacent to the sports court. The time duration between the end of exercise and sitting was <5 s. Therefore, pre- and postexercise measures of HR were performed in the seated position for at least 10 min (subjects were instructed to sit still, breath normally and not engage in conversation) according to Bentley et al.46
Statistical analysis
Data were analysed using SPSS, v.22.0 for Windows (SPSS Inc., Chicago) and the R statistical program with the Generalised Additive Model for Location, Scale and Shape (GAMLSS) package. Tests of normal distribution and homogeneity (Kolmogorov–Smirnov and Levene’s test, respectively) were conducted on all data prior the analyses. Descriptive data are reported in terms of means and SDs. Differences in the anthropometric characteristics and cardiometabolic risk factors were analysed using t tests and analysis of variance adjusted by the Bonferroni test. Differences in the HR parameters between boys and girls were analysed using the Mann–Whitney U test, whereas the differences between the values computed from the 7- to 11-year-old group were analysed using the Kruskal–Wallis test and post hoc analysis by the Mann–Whitney U test was performed to determine which measures were different between groups adjusted by the Bonferroni test (α = 0.05/5 = 0.01). In addition, to verify the relationship between HR parameters, CRF and anthropometric variables, partial correlation analysis and simple linear regression analysis (adjusted by age and sex) were used. The magnitude of correlation between measurement variables was designated as: <0.1 (trivial), 0.1–0.3 (small), 0.3–0.5 (moderate), 0.5–0.7 (large), 0.7–0.9 (very large) and 0.9–1.0 (almost perfect).47 The percentile curves were calculated as a function of age stratified by sex using the mbda, mu, sigma, power exponential method, assuming a Box–Cox power exponential distribution, which is a generalised model of the lambda, mu, sigma method. This method has been implemented in the GAMLSS package in R software. The significance level was set at P < 0.05.
Results
Table 1 illustrates anthropometric characteristics and cardiometabolic risk factor results regarding sex and age group. No significant differences between boys and girls were found in any variable; in addition, the WtHR did not indicate changes regarding age.
Table 2 indicates Léger test performance and HR at rest, during the Léger test and during the recovery period after exercise testing relating to sex and age group. Boys showed lower values of RHR than girls (p < 0.001). In addition, boys exhibited higher performance than girls in the Léger test (p < 0.001); higher values of VO2 max (p < 0.001), HRr (p < 0.001), LF (p < 0.01) and LF/HF (p < 0.05) at rest; and larger values of HRR1 and HRR5 (p < 0.001).
More precisely, these differences between sexes were analysed by post hoc analysis and revealed in which age groups they were found. In this regard, Fig. 1 indicates the interaction between sex and age groups in the variables: RHR, HRpeak, HRR1 and HRR5. Boys displayed a lower RHR than girls at age 7 (p = 0.004), age 9 (p < 0.05) and age 10 years (p < 0.001). Regarding HRR, boys showed a higher HRR1 at age 8 (p = 0.001) and age 10 years (p < 0.001) than girls. With regard to HRR5, boys showed higher values at age 7 (p < 0.05) and age 8 years (p < 0.05) than girls. A within-group analysis noted that only in the boys’ group was there significant differences in RHR and HRR1 regarding age. There were significant differences between ages 7 and 8 (p = 0.028), 9 and 10 (p = 0.002), 8 and 10 (p = 0.001) and 8 and 11 years (p = 0.032) in RHR. In addition, there were significant differences in boys between 10 and 11 (p = 0.002), 8 and 11 (p = 0.008) and between 9 and 10 years of age (p = 0.028) in HRR1. Furthermore, 92.4% of girls and 87.4% of boys displayed a CI > 0.80 (p = 0.088).
The scatter plot for HRR5 vs. HRr was shown in Fig. 2. A significantly positive association was seen between the two variables, which were similar in both sexes. The R2 for the model was 0.379 and 0.382 for boys and girls, respectively.
Pearson’s correlation analysis showed significant correlations between VO2 max and RHR (r = −0.114; p = 0.015), HRpeak (r = 0.298; p < 0.001), HRr (r = 0.320; p < 0.001) and CI (r = 0.318; p < 0.001). RHR showed significant correlations with HRpeak (r = 0.279; p < 0.001), HRr (r = − 0.709; p < 0.001), HRR1 (r = −0.343; p < 0.001) and HRR5 (r = −0.388; p < 0.001). In addition, HRpeak was related to HRr (r = 0.480; p < 0.001), CI (r = 0.986; p < 0.001) and HRR5 (r = 0.363; p < 0.001). Furthermore, HRr displayed significant correlation with CI (r = 0.580; p < 0.001), HRR1 (r = 0.395; p < 0.001) and HRR5 (r = 0.598; p < 0.001). HRR1 and HRR5 displayed significant correlation with CI (r = 0.154; p = 0.002 and r = 0.399; p < 0.001, respectively).
Considering the parameters related to cardiovascular risk, the WtHR was associated with HRpeak (r = −0.220; p < 0.001) and CI (r = −0.205; p < 0.001). Likewise, BMI presented significant correlations with VO2 max (r = −0.409; p < 0.001), HRpeak (r = −0.219; p < 0.001), CI (r = −0.207; p < 0.001) and HRr (r = −0.202; p < 0.001).
HRV at rest did not show correlation with WtHR, BMI and VO2 max, only LF and LF/HF displayed a correlation with HRr (r = 0.520; p < 0.001 and r = 0.235; p < 0.001, respectively) and LF with HRR1 (r = 0.227; p < 0.001) and HRR5 (r = 0.299; p < 0.001).
Simple linear regression analysis revealed that HRr, CI and LF were the variables that showed association with all HR parameters (Table 3).
Finally, the 0.4th, 2nd, 10th, 25th, 50th, 75th, 90th, 98th and 99.6th percentile curves were computed for RHR, HRpeak, HRR1 and HRR5 according to sex and age (Fig. 3).
Discussion
The main purpose of this study was to analyse cardiac autonomic function at rest, during maximal exercise and during a recovery phase to determine sex- and age-specific reference values of RHR, HRpeak and HRR in healthy Caucasian prepubertal children. To our knowledge, this is the first study to provide reference values of autonomic cardiac function in children 7–11 years of age. The main findings of this study showed that: (1) There were significant differences between boys and girls in RHR and HRR; boys display a lower RHR and higher HRR1 and HRR5 than girls. These values were associated with the highest performance in the Léger test by boys. (2) Regarding age, only RHR and HRR were influenced by age in boys. (3) BMI and WtHR were negatively correlated with, CRF, HRpeak and CI.
Cardiac autonomic function at rest
In relation to RHR, in the current study, sex and aerobic capacity influence RHR, thereby a high RHR was associated with females and low aerobic capacity. The significant sex difference in RHR could be explained by the better values of CRF shown by the boys. Sex differences in RHR have been found in previous studies and suggest that girls display higher RHR values than boys.48,49 In this regard, Rabbia et al.50 indicated that the RHR was higher in girls and independently associated with somatic growth indices, physical activity and sociocultural level. Other authors suggest that the regulation of RHR was different in the two sexes,49 through the sympathetic modulation of the sinus node.48
Although in general, obese children had a reduction in the parasympathetic activity of the autonomic nervous system when compared to non-obese children,51 in the current study RHR showed no correlation with BMI and WtHR. Regarding weight status, the findings of the current study aligned with those of Kwok et al.,49 who observed a weak association between RHR and obesity. Previous studies indicated that the association between RHR and cardiometabolic risk was more likely related to fatness49 and blood pressure11 rather than body size. This could be because a growth in body fat induces an increase in the activity of the sympathetic nervous system.52
Regarding aerobic capacity, although we found a weak association between aerobic capacity and RHR, previous studies have reported strong correlations in children and adolescents.48,53 The changes in RHR could be due to the training status,54 which has not been controlled in this study, although previous studies found no changes in RHR after 10 weeks and 10 months of training in prepubertal children.55,56 In this sense, it seems that intense activities were necessary to observe more favourable vagal indexes in prepubescent children.57
Although it may be difficult to define an optimal RHR for a given individual, it seemed desirable to maintain the RHR in the recommended range of 80–130 b.p.m. at 7 years of age and 70–120 b.p.m. from 8 to 11 years of age.58 In this study, 91.2% of girls and 92.3% of boys were within these recommendation ranges.
Cardiac autonomic function at maximum exercise
Regarding HRpeak, in the current study, several factors, such as BMI, WtHR, aerobic capacity, RHR, CI, HRR and LF, influenced this parameter. It is noteworthy to suggest that a high HRpeak was associated with high aerobic capacity and high HRR. Likewise, Gelbar et al.59 observed through regression analysis that RHR, physical fitness, body mass and fat per cent were predictors of maximal HR, whereas age was not. Furthermore, no sex difference was found in HRpeak, in accordance with previous studies.60,61
Another important finding of the current study was that BMI and WtHR were negatively correlated with HRpeak and CI. Following these results, Nikolaidis et al.62 observed a lower HRpeak among overweight and obese children. Likewise, a previous study reported that children with a higher WHtR had a significantly lower HR at the end of the 6-min walk and that WHtR was the most strongly correlated factor with distance achieved on the 6-min walk test in children with obesity, as a measure of exercise tolerance.63 This result may be explained by the fact that obese children avoid moderate or strenuous exercise, because of the higher degree of effort required.64 Although in the current study no significant correlation was found between RPE and BMI, another possible explanation points to the fact that obese children showed a more sedentary lifestyle and excess adiposity, which negatively influences fitness and maximum HR.63,65 Nevertheless, the results of the sympathetic component of the autonomic nervous system were contradictory, with some studies indicating a reduction of sympathetic activity and other studies displaying an increment, as well as other studies that found no variations in this system in obese children compared to non-obese children.51
Furthermore, in the current study, it was interesting to note that HRpeak showed a weak association with age. This finding was in agreement with the study by Van Brussel et al.,66 who showed that the average HRpeak remained relatively stable, around 195–197 (bicycle) to 200 b.p.m. (treadmill), in children and adolescents. Considering the narrow range of HRpeak in youth, Gelbart et al.59 proposed using 197 b.p.m. as the mean HRpeak in children and adolescents, with 180 b.p.m. the minimum threshold value. The current findings aligned with those aforementioned, thereby the average HRpeak was 198 ± 10.83 b.p.m. and 96.8% of girls and 95.3% of boys were within this minimum threshold value.
Finally, data regarding the chronotropic response during exercise in children and adolescents were limited.39 In the current study, CI and HRr indicated a significant increase with the age of both boys and girls, mainly between age 7 and 11 years. However, interpretation of this parameter demanded appropriate consideration of the age of the children and the type of exercise test used.39 In the current study, the average values of CI achieved by both boys and girls >0.80 could indicate a healthy HR response to exercise. Nevertheless, Scheidt et al.39 found values of CI < 0.80 in healthy children compared with children with congenital heart diseases, so it was advisable not to use the threshold of 0.8 for the identification of chronotropic incompetence using treadmill exercise testing in children.
Cardiac autonomic function in recovery after exercise
Considering HRR, several factors, such as sex, BMI, HRr, CI, LF and LF/HF, influence this parameter. It is noteworthy to indicate that HRR1 was also more sensitive to other variables (such as aerobic capacity, RMSSDs and SDNN) than HRR5.
Our study corroborates the findings of Singh et al.,67 who indicated that HRR1 was higher in boys. Likewise, Lintu et al.68 observed that girls (6–8 years of age) had a slower HR decrease within 2 min of the maximal exercise than boys. These findings were contrary to those observed by Guilkey et al.,41 who found no significant differences in HRR following maximal and submaximal exercise between boys and girls, respectively, pointing out that parasympathetic modulation was similar between boys and girls at rest and during recovery from exercise.
No significant relationships were found between HRR and WtHR, and there was a trivial relationship between HRR and BMI. These findings were consistent with those found by Easley et al.,40 who note that obesity alone did not seem to significantly impact HRR. Likewise, a recent study noted that there were no differences in autonomic function during recovery (HRR1) from maximal exercise in lean and obese 8–12-year-old children.69 Although in the present study the trivial relationship between HRR and BMI could be related to the inverse relationship found between BMI and HRpeak.
Additionally, HRR was associated with some cardiovascular fitness indices, such as VO2 max.70 Recently, Scheidt et al.39 demonstrated a moderate correlation between HRR1 and VO2 max (r = −0.35) in children and adolescents. In this regard, the present study has been unable to demonstrate strong relationships between VO2 max and HRR. Likewise, Fernando et al.71 demonstrated that there was no relation between aerobic fitness and HRR1 after a 3 min modified Harvard step test in children 7–11 years of age.
HR variability
Another finding of this study was that HRV differs between boys and girls; only in the frequency domain (LF and LF/HF) did boys display higher values than girls. This finding was in agreement in part with the findings from Jarrin et al.,72 who showed significantly greater HRV values for all time and frequency domain variables in boys in comparison to girls. Cysarz et al.73 indicated that 5–10-year-old boys displayed especially higher values than girls in LF. These differences could be explained in part by differences in the lower HR in boys.74 However, more recently, literature has emerged that offers contradictory findings regarding the influence of sex on HRV.37
Concerning age, a review by Eyre and colleagues indicated that HRV modifications during childhood were consistent with a clear and progressive increase in cardiac parasympathetic activity relative to sympathetic activity.75 In the current study, HRV remained constant in different age subgroups and no significant correlations between age and HRV parameters derived from time or frequency domains were found. These results may be explained by the fact that, while standard HRV may remain constant in different age subgroups, HRV normalisation with respect to HR decreases with age along with a reduction in average HR.24
In addition, there was no correlation between HRV, WtHR and BMI. The present findings seem to be consistent with other research that has not found a clear relationship between weight status, cardiometabolic risk and HRV.69 However, a recent study indicated that higher cardiometabolic risk was associated with smaller HRV in 6–8-year-old children, mainly indicating lower parasympathetic activity.76
On the other hand, taking into account CRF, the findings of the current study further supported the idea that there was no strong association between CRF and HRV.26 In an intervention study, 7 weeks of high intermittent exercise training improved aerobic fitness. However, this training did not produce significant changes in HRV in prepubertal children.77 Several cases reported by Da Silva et al.78 also supported the hypothesis that physical training did not improve HRV in healthy children. Although, recently, Pereira et al.79 observed that performance in Yo-Yo intermittent recovery test level 1 was largely related to resting HRV (r = 0.72; p < 0.05). Likewise, a recent study noted that lower PA and CRF were associated with poorer cardiac autonomic nervous system function in 6–9-year-old children.25 Therefore, future studies are recommended to clarify the role of CRF on HRV in prepubertal children.
Several limitations of this study must be highlighted. The primary limitation of this study was the use of a cross-sectional design; autonomic cardiac function during maximal exercise and recovery in growing children should be measured in a long-term longitudinal study. Moreover, the HR response needed to consider the mode of exercise test (i.e. treadmill vs. bicycle). However, the strengths of this study were that many children were studied throughout a large geographical area, both rural and urban schools were included and we jointly analysed several variables of the autonomic cardiac function, such as RHR, HRpeak, HRR and HRV.
Practically, reference percentile values from this study provide needed comparative data for teachers, coaches and physicians who wish to use the simple and low-cost Léger test to monitor the HR response in elementary school children. This would allow the development of healthy physical activity programmes based on measurable values of HR. Moreover, the extreme percentiles can be used as a ‘warning signal’ where it would be necessary to conduct additional tests in order to identify possible disorders.
Conclusion
This study comprehensively provides a reference set of data for the most important HR variables that can be obtained during exercise testing in prepubertal children regarding age and sex and in a field setting. In this regard, despite the young age of participants, RHR and HRR are different according to sex. These results are supported by the highest performance in the Léger test of boys vs. girls. In addition, this study concludes that aerobic performance and HRpeak have a negative correlation with BMI and cardiometabolic risk.
References
Ahn, S. & Fedewa, A. L. A meta-analysis of the relationship between children’s physical activity and mental Health. J. Pediatr. Psychol. 36, 385–397 (2011).
Martínez-Gómez, D. et al. Sedentary behavior, adiposity, and cardiovascular risk factors in adolescents. The AFINOS study. Rev. Esp. Cardiol. 63, 277–285 (2010).
Salmon, J., Tremblay, M. S., Marshall, S. J. & Hume, C. Health risks, correlates, and interventions to reduce sedentary behavior in young people. Am. J. Prev. Med. 41, 197–206 (2011).
Bongers-Karmaoui, M. N., Jaddoe, V. W. V., Roest, A. A. W. & Gaillard, R. The cardiovascular stress response as early life marker of cardiovascular health: applications in population-based pediatric studies—a narrative review. Pediatr. Cardiol. 41, 1739–1755 (2020).
Billman, G. E., Sacha, J., Werner, B., Jelen, P. J. & Gąsior, J. S. Editorial: heart rate variability and other autonomic markers in children and adolescents. Front. Physiol. 11, 1265 (2019).
Evans, C. A., Selvadurai, H., Baur, L. A. & Waters, K. A. Effects of obstructive sleep apnea and obesity on exercise function in children. Sleep 37, 1103–1110 (2014).
Metwalley, K. A., Hamed, S. A. & Farghaly, H. S. Cardiac autonomic function in children with type 1 diabetes. Eur. J. Pediatr. 177, 805–813 (2018).
Schuster, I. et al. Cardiac function during exercise in obese prepubertal boys: effect of degree of obesity. Obesity 17, 1878–1883 (2009).
Freeman, J. V., Dewey, F. E., Hadley, D. M., Myers, J. & Froelicher, V. F. Autonomic nervous system interaction with the cardiovascular system during exercise. Prog. Cardiovasc. Dis. 48, 342–362 (2006).
Plaza-Florido, A. et al. Heart rate is a better predictor of cardiorespiratory fitness than heart rate variability in overweight/obese children: the Activebrains project. Front. Physiol. 10, 510 (2019).
Fernandes, R. A. et al. Resting heart rate is associated with blood pressure in male children and adolescents. J. Pediatr. 158, 634–637 (2011).
Clausen, H., Theophilos, T., Jackno, K. & Babl, F. E. Paediatric arrhythmias in the emergency department. Emerg. Med. J. 29, 732–737 (2012).
Baruteau, A. E., Perry, J. C., Sanatani, S., Horie, M. & Dubin, A. M. Evaluation and management of bradycardia in neonates and children. Eur. J. Pediatr. 175, 151–161 (2016).
Alom, M. M. et al. Physical training induced resting bradycardia and its association with cardiac autonomic nervous activities. Mymensingh Med. J. 20, 665–670 (2011).
Obert, P. et al. Effect of aerobic training and detraining on left ventricular dimensions and diastolic function in prepubertal boys and girls. Int. J. Sports Med. 22, 90–96 (2001).
Rowland, T. W. in The Young Athlete (eds Helge, H. & Oded, B.-O.) Ch. 4, 39–49 (Oxford, Blackwell, 2008).
Triposkiadis, F. et al. Cardiac adaptation to intensive training in prepubertal swimmers. Eur. J. Clin. Invest. 32, 16–23 (2002).
Cataldo, A. et al. Assessment of autonomic function as marker of training status: the role of heart rate recovery after exercise. Eur. J. Sport Stud. 2, 89–97 (2014).
Brubaker, P. H. & Kitzman, D. W. Chronotropic incompetence: causes, consequences, and management. Circulation 123, 1010–1020 (2011).
Baba, R., Iwagaki, S., Tauchi, N. & Tsurusawa, M. Is the chronotropic index applicable to children and adolescents? Circ. J. 69, 471–474 (2005).
Simhaee, D. et al. Recovery heart rate: an indicator of cardiovascular risk among middle school children. Pediatr. Cardiol. 34, 1431–1437 (2013).
Ortega, F. B., Ruiz, J. R., Castillo, M. J. & Sjöström, M. Physical fitness in childhood and adolescence: a powerful marker of health. Int. J. Obes. 32, 1–11 (2008).
Akdur, H. et al. The evaluation of cardiovascular response to exercise in healthy Turkish children. Turk. J. Pediatr. 51, 472–477 (2009).
Gasior, J. S. et al. Normative values for heart rate variability parameters in school-aged children: simple approach considering differences in average heart rate. Front. Physiol. 24, 1495 (2018).
Veijalainen, A. et al. Associations of physical activity, sedentary time, and cardiorespiratory fitness with heart rate variability in 6- to 9-year-old children: the PANIC study. Eur. J. Appl. Physiol. 119, 2487–2498 (2019).
Oliveira, R. S., Barker, A. R., Wilkinson, K. M., Abbott, R. A. & Williams, C. A. Is cardiac autonomic function associated with cardiorespiratory fitness and physical activity in children and adolescents? A systematic review of cross-sectional studies. Int. J. Cardiol. 236, 113–122 (2017).
Faul, F., Erdfelder, E., Lang, A. & Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191 (2007).
Tanner, jM. Growth at adolescence. Eugen. Rev. 49, 37–38 (1957).
Chung, I. H., Park, S., Park, M. J. & Yoo, E. G. Waist-to-height ratio as an index for cardiometabolic risk in adolescents: results from the 1998−2008 KNHANES. Yonsei Med. J. 57, 658–663 (2016).
Léger, L. A., Mercier, D., Gadoury, C. & Lambert, J. The multistage 20 metre shuttle run test for aerobic fitness. J. Sports Sci. 6, 93–101 (1988).
Borg, G. A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 14, 377–381 (1982).
Parak, J. et al. Evaluation of the beat-to-beat detection accuracy of pulse on wearable optical heart rate monitor. In 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) 2015-November, 8099–8102 (IEEE, 2015).
Parak, J. & Korhonen, I. Accuracy of Firstbeat Bodyguard 2 Beat-to-Beat Heart Rate Monitor. White Paper 1–3 (2013).
European Society of Cardiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93, 1043–1065 (1996).
Sami, S., Mikko, S. & Antti, K. Advanced methods for processing bioelectrical signals artefact correction for heart beat iinterval data. Adv. Methods Process. Bioelectrical Signals 1–10 (2004). https://www.firstbeat.com/app/uploads/2015/10/saalasti_et_al_probisi_2004_congress.pdf
Routledge, F. S., Campbell, T. S., McFetridge-Durdle, J. A. & Bacon, S. L. Improvements in heart rate variability with exercise therapy. Can. J. Cardiol. 26, 303–312 (2010).
Bobkowski, W. et al. Measures of heart rate variability in 24-h ECGs depend on age but not gender of healthy children. Front. Physiol. 8, 311 (2017).
Cachadiña, E. S. et al. Heart rate variability is lower in patients with intermittent claudication: a preliminary study. Arch. Med. Deporte 35, 218–221 (2018).
von Scheidt, F. et al. Heart rate response during treadmill exercise test in children and adolescents with congenital heart disease. Front. Pediatr. 7, 65 (2019).
Easley, E. A. et al. Recovery responses to maximal exercise in healthy-weight children and children with obesity. Res. Q. Exerc. Sport 89, 38–46 (2018).
Guilkey, J. P., Overstreet, M. & Mahon, A. D. Heart rate recovery and parasympathetic modulation in boys and girls following maximal and submaximal exercise. Eur. J. Appl. Physiol. 115, 2125–2133 (2015).
Hager, A. Normal values for cardiopulmonary exercise testing in children. Eur. J. Prev. Cardiol. 18, 675 (2011).
Young, F. L. S. & Leicht, A. S. Short-term stability of resting heart rate variability: influence of position and gender. Appl. Physiol. Nutr. Metab. 36, 210–218 (2011).
Speer, K. E., Semple, S., Naumovski, N. & McKune, A. J. Measuring heart rate variability using commercially available devices in healthy children: a validity and reliability study. Eur. J. Investig. Health Psychol. Educ. 10, 390–404 (2020).
Buchheit, M. et al. Supramaximal training and postexercise parasympathetic reactivation in adolescents. Med. Sci. Sport. Exerc. 40, 362–371 (2008).
Bentley, R. F. et al. Heart rate variability and recovery following maximal exercise in endurance athletes and physically active individuals. Appl. Physiol. Nutr. Metab. 45, 1138–1144 (2020).
Hopkins, W. G., Marshall, S. W., Batterham, A. M. & Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 41, 3–13 (2009).
Sarganas, G., Schaffrath Rosario, A. & Neuhauser, H. K. Resting heart rate percentiles and associated factors in children and adolescents. J. Pediatr. 187, 174–181.e3 (2017).
Kwok, S. Y. et al. Resting heart rate in children and adolescents: association with blood pressure, exercise and obesity. Arch. Dis. Child. 98, 287–291 (2013).
Rabbia, F. et al. Assessing resting heart rate in adolescents: determinants and correlates. J. Hum. Hypertens. 16, 327–332 (2002).
Souza, N. Mde et al. Heart rate variability in obese children. J. Hum. Growth Dev. 22, 328–333 (2012).
Davy, K. P. & Hall, J. E. Obesity and hypertension: two epidemics or one?. Am. J. Physiol. Integr. Comp. Physiol. 286, R803–R813 (2004).
Ørntoft, C. et al. Physical fitness and body composition in 10-12-year-old Danish children in relation to leisure-time club-based sporting activities. Biomed. Res. Int. 27, 9807569 (2018).
Pieles, G. E. & Stuart, A. G. The adolescent athlete’s heart; A miniature adult or grown‐up child? Clin. Cardiol. 43, 852–862 (2020).
Larsen, M. N. et al. Cardiovascular adaptations after 10 months of intense school‐based physical training for 8‐ to 10‐year‐old children. Scand. J. Med. Sci. Sports 28, 33–41 (2018).
Krustrup, P. et al. Structural and functional cardiac adaptations to a 10‐week school‐based football intervention for 9–10‐year‐old children. Scand. J. Med. Sci. Sports 24, 4–9 (2014).
Buchheit, M., Platat, C., Oujaa, M. & Simon, C. Habitual physical activity, physical fitness and heart rate variability in preadolescents. Int. J. Sports Med. 28, 204–210 (2007).
Advanced Life Support Group. Advanced Paediatric Life Support: A Practical Approach to Emergencies (APLS) (Advanced Life Support Group, 2016).
Gelbart, M., Ziv-Baran, T., Williams, C. A., Yarom, Y. & Dubnov-Raz, G. Prediction of maximal heart rate in children and adolescents. Clin. J. Sport Med. 27, 139–144 (2017).
Fomin, Å. et al. Sex differences in response to maximal exercise stress test in trained adolescents. BMC Pediatr. 12, 127 (2012).
Verschuren, O., Maltais, D. B. & Takken, T. The 220-age equation does not predict maximum heart rate in children and adolescents. Dev. Med. Child Neurol. 53, 861–864 (2011).
Nikolaidis, P. et al. The effect of body mass index on acute cardiometabolic responses to graded exercise testing in children: a narrative review. Sports 6, 103 (2018).
Axley, J. D. & Werk, L. N. Relationship between abdominal adiposity and exercise tolerance in children with obesity. Pediatr. Phys. Ther. 28, 386–391 (2016).
Reybrouck, T., Weymans, M., Vinckx, J., Stijns, H. & Vanderschueren‐Lodeweyckx, M. Cardiorespiratory function during exercise in obese children. Acta Paediatr. 76, 342–348 (1987).
Participating, N. & Several, N. Exercise intolerance. ERS Handb. Paediatr. Respir. Med. 65, 65–69 (2013).
Van Brussel, M., Bongers, B. C., Hulzebos, E. H. J., Burghard, M. & Takken, T. A systematic approach to interpreting the cardiopulmonary exercise test in pediatrics. Pediatr. Exerc. Sci. 31, 194–203 (2019).
Singh, T. P., Rhodes, J. & Gauvreau, K. Determinants of heart rate recovery following exercise in children. Med. Sci. Sports Exerc. 40, 601–605 (2008).
Lintu, N. et al. Cardiovascular fitness and haemodynamic responses to maximal cycle ergometer exercise test in children 6-8 years of age. J. Sports Sci. 32, 652–659 (2014).
Guilkey, J. P., Dykstra, B., Erichsen, J. & Mahon, A. D. Heart rate response and variability following maximal exercise in overweight children. Pediatr. Exerc. Sci. 29, 341–349 (2017).
Dimkpa, U. Post-exercise heart rate recovery: an index of cardiovascular fitness. J. Exerc. Physiol. 12, 10–22 (2009).
Fernando, R. J., Ravichandran, K. & Vaz, M. Aerobic fitness, heart rate recovery and heart rate recovery time in indian school children. Indian J. Physiol. Pharmacol. 59, 407–413 (2015).
Jarrin, D. C. et al. Short-term heart rate variability in a population-based sample of 10-year-old children. Pediatr. Cardiol. 36, 41–48 (2015).
Cysarz, D. et al. Unexpected course of nonlinear cardiac interbeat interval dynamics during childhood and adolescence. PLoS ONE 6, e19400 (2011).
Gasior, J. S. et al. Interaction between heart rate variability and heart rate in pediatric population. Front. Physiol. 18, 385 (2015).
Eyre, E. L. J., Duncan, M. J., Birch, S. L. & Fisher, J. P. The influence of age and weight status on cardiac autonomic control in healthy children: a review. Auton. Neurosci. Basic Clin. 186, 8–21 (2014).
Leppänen, M. H. et al. Associations of cardiometabolic risk factors with heart rate variability in 6- to 8-year-old children: the PANIC Study. Pediatr. Diabetes 21, 251–258 (2020).
Gamelin, F. X. et al. Effect of high intensity intermittent training on heart rate variability in prepubescent children. Eur. J. Appl. Physiol. 105, 731–738 (2009).
Da Silva, C. C., Pereira, L. M., Cardoso, J. R., Moore, J. P. & Nakamura, F. Y. The effect of physical training on heart rate variability in healthy children: a systematic review with meta-analysis. Pediatr. Exerc. Sci. 26, 147–158 (2014).
Pereira, L. A. et al. Relationship between resting heart rate variability and intermittent endurance performance in novice soccer players. Res. Q. Exerc. Sport 90, 355–361 (2019).
Acknowledgements
This project would not have been possible without the participation of the numerous schools of Andalusia, to which we are very grateful. We also want to have a special mention and thank you to the parents and teachers of the children who have been involved in this study. We deeply appreciate their trust and support of this project.
Funding
This study does not have any external funding. This research received no external funding.
Author information
Authors and Affiliations
Contributions
P.A.L.-R., J.A.-V. and J.A.P.-M. designed the research; J.A.-V., J.S.-S., M.M.-R. and P.J.C.-G. conducted the research; P.J.C.-G, J.A.-V., M.M.-R. and J.S.-S. provided essential reagents or provided essential materials; P.A.L.-R., E.S.-C. and J.A.-V. analysed data or performed statistical analysis; P.A.L.-R., P.J.C.-G. and J.A.-V. wrote the paper; P.A.L-R., J.A.-V. and J.J.S.-S. had primary responsibility for the final content. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Parents voluntarily signed an informed consent for the participation of their children in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Latorre-Román, P.A., Floody, P.D., Martínez-Redondo, M. et al. Comprehensive cardiac evaluation to maximal exercise in a contemporary population of prepubertal children. Pediatr Res 92, 526–535 (2022). https://doi.org/10.1038/s41390-021-01809-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01809-8
This article is cited by
-
Influence of physical fitness and weight status on autonomic cardiac modulation in children
Pediatric Research (2023)