Abstract
Introduction
Maternal–infant equilibrium occurs when cord blood docosahexaenoic acid (DHA) is less than or equal to maternal DHA at delivery. Equilibrium may be an indicator of sufficient DHA for optimal fetal and infant neurodevelopment. The purpose of this study was to test the effect of maternal DHA supplementation on equilibrium status and fetal neurodevelopment.
Methods
Women enrolled between 12 and 20 weeks gestation and were randomized to 200 or 800 mg DHA/day until delivery. Maternal red blood cell (RBC) phospholipids were measured at enrollment, 32 weeks, delivery, and in cord blood at delivery. Fetal neurodevelopment was measured at 32 and 36 weeks gestation. Intent-to-treat analyses were conducted to test differences in equilibrium status by group. Fetal outcomes were assessed by equilibrium status and group.
Results
Three hundred women enrolled and 262 maternal–infant dyads provided blood samples at delivery. No maternal–infant dyads with maternal RBC-DHA ≤ 6.96% at delivery achieved equilibrium. The incidence of equilibrium was significantly higher in the 800 mg group. There was no effect of maternal group or equilibrium status on fetal neurodevelopment.
Conclusion
The significance of maternal–infant DHA equilibrium remains unknown. Ongoing research will test the effect of treatment group, equilibrium, and nutrient status on infant behavior and brain function.
Impact
-
Pregnant women who received a higher dose of docosahexaenoic acid (DHA) were more likely to achieve maternal–infant DHA equilibrium at delivery.
-
Equilibrium status had no effect on fetal neurodevelopment in this sample.
-
While DHA is crucial for early life neurodevelopment, the significance of achieving maternal–infant equilibrium above the lower threshold is uncertain.
-
There is a lower threshold of maternal DHA status where maternal–infant DHA equilibrium never occurs.
-
The lack of equilibrium associated with low maternal DHA status may indicate insufficient maternal status for optimal placental transfer.
Similar content being viewed by others
Introduction
Docosahexaenoic acid (DHA, 22:6n3) is a long-chain polyunsaturated fatty acid found in all cell membranes. It is considered an essential nutrient for fetal neurodevelopment, especially during the third trimester of pregnancy due to rapid brain growth, myelination, and synaptogenesis.1,2 The amount of DHA available for transfer depends upon the DHA status of the mother; because diet is the primary source of DHA, this status depends largely on the mother’s DHA intake from foods (e.g., seafood, eggs) or DHA supplements.3
Red blood cell DHA (RBC-DHA) is an indicator of DHA intake and tissue status.4 During pregnancy, there is a decrease in maternal DHA and arachidonic acid (ARA)3 as these and other essential fatty acids are preferentially transferred to the fetus via the placenta. Cord blood DHA (CB-DHA) is often higher than maternal RBC-DHA at delivery, a phenomenon termed biomagnification.5 Conversely, infant CB-DHA that is equal or less than maternal RBC-DHA is termed bioattenuation, or simply equilibrium. Some have posited that maternal–infant DHA equilibrium represents a state where the amount of DHA transferred from maternal stores has reached a level sufficient to meet fetal neurodevelopmental requirements, indicating a more optimal DHA status for both mother and newborn.6 Further, higher maternal DHA status at parturition would support continued DHA transfer to the newborn during lactation.7 Evidence to support this concept comes from the work of Luxwolda et al.6 who enrolled pregnant women with lifetime diets of low-, intermediate-, or high-fish, thereby consuming variable amounts of DHA. Using maternal and infant DHA samples collected at delivery, investigators found fetal DHA biomagnification occurred most often when maternal RBC-DHA was below 6% total fatty acids, that is, when maternal DHA status was decidedly low. The prevalence of maternal–infant DHA equilibrium increased in women with RBC-DHA levels >6%, suggesting more optimal fetal DHA accumulation.
In a previous randomized controlled trial conducted at the University of Kansas between 2009 to 2011 (Hoglund Prenatal Evaluation (HOPE); NCT01007110), 67 pregnant women were assigned to a daily DHA supplement of either 0 or 600 mg.8 At study enrollment, women randomized to the supplemented group had a median maternal RBC-DHA in phospholipids of 4.5% total fatty acids, increasing to 7.1% at delivery. Subsequent examination of these data revealed that 75% of the total maternal–infant dyads in the study failed to achieve DHA equilibrium, and equilibrium never occurred when maternal RBC-DHA at delivery was below 6.5%. This led us to question whether biomagnification, i.e., the failure to achieve maternal–infant DHA equilibrium could be an indicator of DHA insufficiency and thereby limit fetal neurodevelopment.
Heart rate variability (HRV) is an index of fetal autonomic neurodevelopment, and we have shown that it is influenced by maternal DHA status. In the HOPE trial,8 overall and short-term fetal HRV were higher in the DHA-supplemented compared to the placebo group, a positive finding for fetal autonomic neurodevelopment. We employed the universal system theory approach of evolution and development by Hoyer et al. to HRV analysis,9,10 and found the fetal autonomic brain age score (fABAS) was higher in the supplemented group.11,12
The subsequent Prenatal Autonomic Neurodevelopmental Assessment (PANDA) trial (NCT02709239) was designed to increase the incidence of maternal–infant DHA equilibrium by randomizing pregnant women to either 200 or 800 mg DHA from 12 to 20 weeks gestation to delivery. We hypothesized that the incidence of equilibrium would be higher in the group randomized to 800 mg/day and that equilibrium would result in more optimal fetal neurodevelopment indicated by higher fetal HRV and fABAS at 32 and 36 weeks gestation. Under intent-to-treat principles, we tested for equilibrium status and treatment group differences in maternal and newborn RBC-DHA, fetal HRV, and fABAS.
Methods
Trial design
This was a randomized, longitudinal, double-blind, single-center, Phase III superiority trial. The pregnancy phase of the trial was conducted at the University of Kansas Medical Center, USA between June 2016 and September 2020. The study was approved by the Human Subjects Committee (STUDY 00003792) in accordance with the Helsinki Declaration of 1975 as revised in 1983. All participants provided written informed consent. The study was overseen by a Data Safety and Monitoring Board and reviewed annually. The trial is registered at https://www.clinicaltrials.gov/ct2/show/NCT02709239.
Trial participants
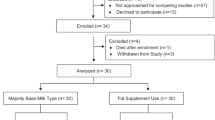
Study staff performed daily chart reviews for all patients attending the university health system OB-GYN clinic for prenatal appointments and screened for eligibility between June 2016 through February 2020. Clinic patients were not approached for enrollment if they were <18 years old and <12 or ≥20 weeks gestation, had a pre-pregnancy body mass index (BMI) ≤ 18.5 or weighed more than 250 pounds at enrollment (based on safety limit of the biomagnetometer support chair), had any known serious maternal illness likely to result in hospitalization or threaten pregnancy including cancer, lupus, hepatitis, Type I diabetes mellitus, hypertension, self-reported drug or alcohol abuse, or were carrying a fetus with known congenital cardiac arrhythmias or structural defects, or brain malformations. Eligible women were approached by study personnel if they were English speaking, 18 years of age or older, between 12 and 20 weeks gestation, and carrying a singleton pregnancy. Eligible women declined to participate in the study for a variety of reasons: did not want to take daily capsules, were unwilling to discontinue taking any prenatal DHA supplement, competing medical concerns, time constraints, lack of support from family, no interest in participating in research, but most did not give a reason for opting out or did not contact study staff to schedule enrollment appointments. After screening, 300 women were randomly assigned to the capsule allocation (Fig. 1).
Randomization and masking
After obtaining informed consent, women were randomized to receive four capsules containing algal oil that provided a total of either 200 or 800 mg DHA daily (Life’s DHA™-S oil, DSM Nutritional Products LLC, Switzerland). The study statistician provided the computer-generated randomization schedule to the Investigational Pharmacy at the University of Kansas Health System. Only the pharmacy knew the subject allocation; all members of the study team and participants were blinded to the capsule assignment throughout the duration of the study. Study personnel delivered the first bottle at enrollment, and thereafter, capsules were mailed monthly to participants with a return envelope to the Investigational Pharmacy. Participants were instructed to place the previously mailed bottle with any unused capsules in a prepaid package addressed to the Investigational Pharmacy and to begin taking capsules from the new bottle upon receipt. The returned capsules were counted and discarded by the Investigational Pharmacy. The capsules were packaged identically in opaque bottles and the same color, size, and flavor to prevent study personnel and participants from guessing their group allocation. Women were instructed to stop taking capsules at delivery.
Following enrollment, study personnel contacted participants monthly until delivery by phone, text, or email to record any adverse events, check compliance and address concerns. Those participants who wanted to discontinue taking capsules provided written consent whether to allow their data and medical record to be used or not.
Blood collection and analysis
Maternal blood samples were collected at enrollment, 32 weeks gestation, and at delivery. Umbilical cord blood was collected at delivery. Samples were collected in EDTA tubes (BD Vacutainer, Franklin Lakes, NH), placed on wet ice, and processed within 24 h, in the co-investigator (SEC) laboratory. Samples were centrifuged (3000g, 10 min, 4 °C) to separate the plasma, buffy coat, and RBCs, and stored at −80 °C in barcoded vials until analysis. The analytical method for determining RBC phospholipid fatty acids is described in detail in Gustafson et al.,8 updated by using an Agilent 6890N gas chromatograph using Agilent OpenLab CDS ChemStation Edition c.01.09. DHA is reported here as RBC phospholipid weight percent of total fatty acids (wt% TFA). Maternal–infant DHA equilibrium was coded YES if infant CB-DHA was less than or equal to maternal RBC-DHA at delivery. If infant CB-DHA was greater than maternal RBC-DHA, the outcome was coded NO.
Diet History Questionnaire (DHQ-II) and prior DHA supplement intake
At enrollment and 32 weeks gestation, women completed The National Cancer Institute Diet History Questionnaire II (DHQ-II), a food frequency and portion size questionnaire.13 The database associated with the DHQ-II is based on the National Health and Nutrition Examination Surveys (NHANES) data collection from 2001 to 2006. At enrollment, participants completed version 1 (past year, with portion size) to determine pre-pregnancy diet. At 32 weeks, participants completed version 3 (past month, with portion size) to determine diet during pregnancy. At each visit, participants received detailed survey instructions and they had the opportunity to ask questions. The survey was completed through the NCI online portal via the participants unique username and password. Diet*Calc analysis software was used to extract nutrient and food group intake estimates.14 Prior supplemental DHA intake was also assessed at enrollment.
Magnetocardiogram
Fetal magnetocardiograms (MCG) were recorded at 32 and 36 weeks gestation. Women were instructed to eat within 2 h of their study visit. An ultrasound was recorded prior to the MCG recording to determine the fetal activity state and body position. After the ultrasound, women entered a magnetically shielded room and were seated comfortably in a slightly reclining position, their abdomen making slight contact with the sensor array of an 83-channel dedicated fetal biomagnetometer (CTF MEG, Coquitlam, BC, Canada). A 1200 Hz sampling rate with a recording filter of 0–75 Hz was used for the 30-min MCG recording. Deidentified raw data were stored securely on University servers.
Raw data were digitally filtered from 2 to 40 Hz offline using a bidirectional fourth-order Butterworth filter. The multivariate data were presented to an Infomax ICA algorithm in EEGLAB toolbox (version 4.311) to separate individual maternal and fetal components from their spatially distinct electrophysiological sources. The individual components that comprised the fetal MCG were identified and summed to reconstruct the signal in channel space.
Fetal HRV analysis
The reconstructed fetal MCG from the single channel that best represented the cardiac signal was exported as an ASCII text file and imported into Kubios HRV software15 (version 3.4.2; Kuopio, Finland) where the R-peaks were marked and ectopic beats corrected using an automatic artifact correction algorithm followed by visual inspection. The software generates multiple metrics of HRV in time, frequency, and nonlinear domains. Many metrics across domains are highly correlated and some have limited value in fetal assessment; therefore, this report will be limited to fetal heart rate in beats per minute (HR; bpm), the standard deviation of normal-to-normal RR intervals in milliseconds (SDNN; ms) as an index of overall HRV, the root mean square of successive differences in milliseconds (RMSSD; ms), a measure of short-term HRV, approximate entropy (ApEN), an index of HRV complexity or irregularity, and detrended fluctuation analysis (DFA), a measure of the fractal correlation within the data at different time scales. DFA1 measures short-term and DFA2 long-term correlations. The R–R intervals were exported for use in the fABAS analysis.
fABAS analysis
The fABAS describes the change of essential developmental indices obtained from HRV characteristics over the gestational age period from 20 weeks up to 38 weeks in typically developing, healthy fetuses.16 It is a comprehensive measure of fetal brain maturation linked to autonomic control, one that considers universal principles of complex system behavior during important maturational periods in the second and third trimesters. Most of the changing features of fetal heart rate patterns are explained by measures related to increasing fluctuation amplitude, complexity, and pattern formation, thereby serving as an index of fetal neurodevelopment.9,10 The resulting score is reported as an approximate gestational age of maturation in weeks.
For the present study, only behavioral states classified as active sleep (2F) were used in the analysis of the fABAS. Fetal activity state was classified according to standard criteria adapted from Nijhuis17,18,19,20,21 by consensus decision of three independent collaborators. In each recording, the HRV indices of all identified 2F sections of at least 5 min duration with 1-minute shift were averaged. Measures of HRV used to obtain the fABAS were calculated from the RR interval series. First, all R–R interval series were automatically corrected using established criteria.22 Any outliers identified as artifacts were corrected by interpolation up to three artifactual beat intervals and deleted if more than three subsequent values failed. In all individual analyzed recordings, the rate of interpolated artifacts was below 2%.
The HRV parameters AMP20, skewness, gMSE3, pNN5, and VLF/LF of the linear regression model were previously explored as fetal maturation age predicting parameters set by stepwise feature selection.9 The age-dependent changes of indices correlate with fundamental developmental/evolutional characteristics, such as increasing fluctuation amplitude (AMP20) and complexity of sympathovagal control (gMSE3), as well as the increasing dominance of sympathetically determined pattern heart rate accelerations vs. decelerations (skewness) and baseline stabilization (VLF/LF). The HRV parameter calculations and interpretations are as follows: AMP20: The 20–95 inter-quantile distance of detrended normal-to-normal (NN) interval series, a measure of the fluctuation range of heartbeat intervals. Skewness: Skewness of NN interval series: Asymmetry between vagally and sympathetically mediated pattern, decline of dominating heart rate decelerations, and increase of dominating heart rate accelerations. MSE3: Generalized mutual information at coarse graining level 3 of NN intervals (related to multi scale entropy), complexity of sympathovagal modulations. pNN5: Percentage of differences between adjacent NN intervals exceeding 5 ms, short, mainly vagal modulations. VLF/LF: ratio between very low (0.02–0.08 Hz) and low (0.08–0.2 Hz) frequency band power, baseline fluctuation. In the present work, the fABAS was refitted to a data set of 636 recordings of the normal fetal development group (Jena database) that contained at least one 2F section.
Primary and secondary outcomes
The primary outcome was the rate of maternal–infant equilibrium at delivery and its effect on fetal HRV and fABAS scores at 32 and 36 weeks gestation. Secondary outcomes included maternal RBC-DHA, total RBC n-6 and n-3, and RBC n6:n:3 ratios at enrollment, 32 weeks gestation, and delivery and CB-DHA at delivery. Serious adverse events (death, life-threatening event, hospitalization, or prolonged hospitalization, persistent or significant disability/incapacity or congenital anomaly/birth defect) and adverse events (clinical signs and symptoms possibly related to DHA safety) were recorded for women and neonates.
Statistical analysis
All analyses were conducted under intention-to-treat principles; participants were included in statistical analyses according to the treatment group for which they were randomly assigned at study enrollment. Power computations indicated that 125 completed participants per group were needed to detect a 15% increase in the incidence of DHA equilibrium and an increase of 0.8 fABAS score with 88% power.
Continuous variables were evaluated for approximately normal distributions and log-transformed prior to analysis if distributions were skewed. Two-sample t-tests (for continuous variables) and chi-square tests for association (for categorical variables) were used to test differences in maternal demographic, dietary, and blood-based fatty acid characteristics between groups, as well as group differences in infant characteristics at delivery and cord-blood RBC-DHA. Logistic regression (for binary outcomes) and linear regression (for continuous outcomes) models were used to estimate univariate and adjusted associations between group and equilibrium status or infant cord blood RBC-DHA. Maternal RBC-DHA (enrollment and delivery time points), fABAS scores, and HRV (32 and 36 weeks gestational age) were modeled by random-intercept mixed-effects ANOVA models using factors of group, time, and group-by-time interactions, when statistically significant. Variances were modeled using an unstructured covariance matrix after evaluating covariance patterns and AIC and −2 log likelihood model fit characteristics. The Kenward–Roger approximation was used to estimate the degrees of freedom. Maternal RBC-DHA at 32 weeks GA was not included in mixed-effects models, as the 32 week GA and delivery time points showed no difference in the RBC-DHA level with a post hoc one-way within-subjects ANOVA pairwise comparison (mean difference = 0.11, p = 0.9). Potential maternal confounders, including dietary DHA intake, RBC n6:n3 fatty acid ratio, weight,23 smoking, and household income for maternal models; infant sex and maternal weight for fetal or infant models were identified a priori and included in multivariate models if inclusion changed the estimated outcome parameter by >10%, if estimated coefficients were statistically significant, or if the covariate was identified as necessary for inclusion per study protocol. Final maternal models were adjusted for dietary DHA intake, maternal RBC n6:n3 fatty acid ratio, and maternal weight; final fABAS models were adjusted for maternal weight. Statistical significance for all analyses was defined as p < 0.05, and statistical analyses were performed using SAS software, version 9.4 (SAS Institute, Inc., Cary, North Carolina) or R, version 3.6.1.
Bayesian quantities are calculated for the safety data using a Bayesian hierarchical model by body system using the model by Berry and Berry.24 We utilized OpenBUGS version 3.2.3 rev 1012 for all Bayesian analyses. All analyses were fitted using 1000 burn-in draws of Markov chain Monte Carlo, followed by 100,000 draws for inference. From this model we were able to determine by category the posterior probability (pp) 800 mg had a better safety profile than 200 mg for that category.
Results
Participants
Of the 2559 women screened for eligibility 2259 were excluded, leaving 300 participants (Fig. 1). The baseline and delivery characteristics of the study population are shown in Table 1. Of the 300 women allocated to treatment, 278 were followed until delivery (135 female, 142 male, 1 newborn with documented date of birth but no sex provided). Both treatment groups maintained excellent retention through delivery (90% in the 200 mg group; 87% in the 800 mg group). However, the COVID-19 pandemic shutdown prevented us from seeing participants for 32 and 36 weeks study visits between March 2020 and May 2020, resulting in 22 missed visits at 32 weeks, and 27 missed visits at 36 weeks. Reasons for other missing data are outlined in Fig. 1. At enrollment, the groups did not differ by age, education, SES, BMI, GA, or RBC-DHA. There was no group difference in maternal pregnancy weight gain from enrollment to delivery, gestational age at delivery, birth weight, length, or head circumference.
Safety data
Specific adverse and serious adverse events for mothers are shown in Supplementary Table 1 and for neonates in Supplementary Table 2. Participants assigned to the higher dose had fewer maternal adverse specifically related to sinusitis (pp = 0.94), heartburn/acid reflux (pp = 0.92), and preterm contractions (0.97), whereas the lower dose had fewer maternal adverse events related to other urogenital (pp = 0.07). For maternal serious adverse events the overall numbers were about the same but favoring high dose for all categories (pp > 0.5) except preeclampsia which slightly favored low dose but not strongly (pp = 0.17). For infants the adverse events were about the same favoring high dose in all categories (pp > 0.5) except for intrauterine growth restriction favoring low dose (pp = 0.24). Participants assigned to the higher dose had fewer infant serious adverse events overall and stronger signals for neurological symptoms (pp = 0.93) and other infant category (pp = 0.99).
Adherence to capsule allocation
Thirteen women opted out of capsule consumption during the study but attended study visits and were included in the analysis, owing to intent-to-treat principles. The average capsule intake was 24 capsules/week in the 200 mg group and 23.7 capsules/week in the 800 mg group. Compliance did not differ by group.
Diet History Questionnaire (DHQ-II)
The mean daily intake of dietary DHA was 67 ± 64 mg during pre-pregnancy (1 year prior to enrollment) and 56 ± 63 mg during the third trimester of pregnancy (approximately 31–32 weeks gestational age) resulting in a significant decrease between the two time points: (mean difference = −10.85 mg/day, p = 0.01). Dietary DHA intake did not differ between groups at either timepoint (enrollment: p = 0.73; 32 weeks: p = 0.45). A total of 206 participants (69%) reported taking a DHA supplement prior to enrollment, with a mean intake of 104 mg/day.
Maternal and infant RBC-DHA
Sample collection at each timepoint is outlined in Fig. 1. Missing maternal samples were due to participants being a difficult draw or limited availability of phlebotomy staff. At delivery, samples were not collected if a participant was removed for medical reasons, lost to follow-up, or withdrew from the study (Fig. 1). The summary results for RBC fatty acids are shown in Table 2. Mean maternal RBC-DHA (6.94 ± 1.77%) did not differ by group allocation at enrollment (p = 0.87). The repeated measures mixed-effects model (Table 3) showed significant main effects for group (p < 0.0001) and visit (p < 0.0001) and a group-by-time interaction for maternal RBC-DHA (p < 0.0001), indicating that treatment-associated increases in maternal RBC-DHA were greatest over time. There was a significant increase in maternal RBC-DHA between enrollment and at 32 weeks GA (F1,1057 = 274.37, p < 0.0001) with no significant change between the 32 week and post-partum samples (F1,1057 = 0.01, p = 0.9). Newborn CB-DHA was significantly higher in the 800 mg group compared to the 200 mg group (p < 0.0001) (Table 2).
Maternal–infant RBC-DHA equilibrium
The attainment of equilibrium was significantly affected by group assignment. Of the 262 maternal–newborn dyads who provided both maternal and cord blood samples at delivery, maternal–infant DHA equilibrium was achieved in 113 (43.1%); of those, 31 (23.7%) were in the 200 mg group and 82 (62.6%) were in the 800 mg group (logistic regression adjusted for dietary DHA and RBC n6:n3 ratio OR = 2.26, 95% CI = 1.12, 4.55, p = 0.02). No maternal–infant dyads with maternal post-partum RBC-DHA ≤ 6.96% achieved equilibrium (n = 26; 9.9%) (Fig. 2). However, most maternal–infant dyads who did not achieve equilibrium (123 of 149) were well above this threshold, with maternal post-partum RBC-DHA as high as 14.7% and newborn RBC-DHA as high as 16.1%.
Newborn cord blood RBC DHA is seen on the Y-axis, maternal postpartum RBC DHA on the X-axis. The diagonal line denotes a 1:1 relationship between maternal–infant DHA. Those dyads achieving equilibrium, i.e., maternal DHA ≥ infant DHA (coded yes) are below the diagonal line. Conversely, those dyads where infant DHA was > maternal DHA (coded no) are above the diagonal line.
Maternal RBC-DHA at delivery was significantly higher in the dyads who achieved equilibrium (mean difference in RBC-DHA = 2.91%, 95% CI = 3.46, 2.37%, p ≤ 0.0001) (Table 4). CB-DHA did not differ between those who did and did not reach equilibrium with their mothers (mean difference = 0.21%, 95% CI = −0.32, 0.75, p = 0.44). While there was a lower maternal post-partum RBC-DHA threshold where maternal–infant equilibrium was never achieved (6.96%), there was no upper limit that guaranteed maternal–infant equilibrium.
Fetal heart rate variability (HRV)
In repeated measures mixed-effects models, there were no main effects of maternal treatment group or equilibrium on fetal metrics related to HR, overall variability, short-term variability, complexity, or fluctuation analysis. For the treatment group analysis (Table 3) there was a significant effect of time on fetal SDNN (p = 0.008), RMSSD (p = 0.002), ApEn (p = 0.002), and DFA1 (p = 0.002). There was no main effect of time on fetal HR or DFA2 (fractal long-term correlations).
When analyzed by equilibrium status (Table 4), the outcomes were nearly identical with a significant effect of time on fetal HRV: SDNN (p = 0.008), RMSSD (p = 0.003), ApEn (p = 0.003), and DFA1 (p = 0.003), but no effect of equilibrium status on fetal HR or DFA2.
Fetal Autonomic Brain Age Score (fABAS)
Group assignment was not associated with fABAS scores at 32 or 36 weeks (32 weeks: mean difference = 0.23, 95% CI = −0.29, 0.74; 36 weeks: mean difference = 0.29, 95% CI = −0.33, 0.91). In repeated measures mixed-effects models, there were no main effects of maternal treatment group (Table 3), incidence of maternal–infant RBC-DHA equilibrium (Table 4) on the fABAS scores, nor any interaction effects with time. There was an expected main effect of time, with higher scores at the 36 week visit (p < 0.0001).
Discussion
Supplementing pregnant women with 200 or 800 mg of DHA during pregnancy significantly increased the incidence of maternal–infant DHA equilibrium in a dose-dependent manner, with a higher incidence of equilibrium in the 800 mg group. DHA equilibrium between women and their newborn infant never occurred below 6.96% maternal RBC-DHA at delivery. Above the threshold of 6.96%, there was a roughly equal chance of equilibrium or not (43.1% Yes, 46.9% No), with fetal biomagnification occurring at maternal RBC-DHA as high as 14.7%. Moreover, the achievement of maternal-infant DHA equilibrium was not related to fetal neurodevelopment as assessed by HRV and fABAS.
Other investigators have estimated a maternal–infant DHA equilibrium threshold of ~6% maternal DHA at delivery, concluding that fetal DHA biomagnification is a potential indicator of maternal DHA insufficiency.6,7 It is notable that in both the current (PANDA) and the previous (HOPE) trial, equilibrium never occurred below 6%, in line with the report of Luxwolda et al.6 While maternal–infant equilibrium is not a meaningful biomarker for maternal DHA insufficiency when maternal RBC-DHA is above 6%, maternal RBC-DHA ≤ 6% is a reasonable threshold for low maternal DHA status, potentially increasing the risk of insufficient DHA for fetal uptake. This risk may be greater for women with obesity,25 preeclampsia,26 Type 1, II,27,28 and gestational diabetes mellitus29 as these conditions have been shown to have a negative effect on placental transfer of polyunsaturated fatty acids.
The dosing strategy resulted in a significant increase in maternal DHA from enrollment to 32 weeks gestation in both treatment groups. From 32 weeks to delivery, there was no change in maternal RBC-DHA, evidence that maternal DHA status plateaued 12–20 weeks after women were randomized to capsules. At delivery, maternal RBC-DHA and CB-DHA were higher in the 800 mg group. At baseline, women entering the PANDA study had higher RBC-DHA (~7%) than their predecessors entering the HOPE trial (~4%). Since DHA intake from foods has not changed significantly and the dietary intake results reported here are consistent with mean usual dietary intake of DHA of 62.2 mg/day in United States women of childbearing-age,30 we attribute higher baseline status to the increased use of prenatal DHA supplements. Sixty-nine percent of the women enrolling in PANDA reported taking a prenatal DHA supplement with a mean intake of 104 mg/day, while in previous trials 12% reported taking a prenatal DHA supplement,8,31 with a mean intake of 25 mg/day. Another important difference between the current and past clinical trials was that neither we nor our sponsor considered it ethical to use a true placebo and instead used a low dose roughly equivalent to that included with prenatal vitamins or commercially available DHA supplements. We used a dose higher than the previous 600 mg with the intention of increasing the incidence of equilibrium. While we achieved this aim, the dosing strategy also had the side result of preventing DHA insufficiency, as only 26 participants (9.9%) fell below the 6% threshold we hereby define as a marker of DHA insufficiency. If this were a placebo-controlled trial or observational trial without DHA supplementation, it is likely that there would have been more cases of DHA insufficiency, since the mean maternal RBC-DHA at enrollment was 6.94%, and maternal dietary DHA intake was low and decreased significantly during pregnancy.
Because maternal–infant DHA equilibrium never occurred below 6% maternal RBC-DHA at delivery and this finding is consistent across studies, maternal RBC-DHA ≤ 6% may serve as an indicator of low maternal status. It stands to reason that women with low DHA status would benefit most from supplementation. This is evident in the results of two recent large-scale trials where women with low fatty acid status at enrollment (≤6% RBC-DHA) benefited more from DHA supplementation, resulting in lower rates of early preterm birth.32,33 Specifically, the rate of early preterm birth was reduced by half if women with low DHA status (≤6% total fatty acids) were assigned to 1000 mg DHA/day versus 200 mg/day. In contrast, for women with high DHA status (>6%) the incidence of early preterm birth was lower and there was no benefit of dose.32 Higher DHA status may also provide a direct benefit to women as studies have shown an association between low status and maternal depression.34,35
Neither treatment group nor maternal–infant DHA equilibrium status influenced fetal neurodevelopment as indexed by HRV metrics or fABAS scores at 32 and 36 weeks gestational age. As expected, fetal maturation between the two time points was responsible for the significant effect of time on HRV metrics and fABAS scores. Previously, the fABAS was sensitive to group differences in the placebo-controlled HOPE trial where fABAS scores and fetal HRV were higher in the supplemented group, especially in metrics quantifying fluctuation amplitude and complexity.12,12 However, maternal and infant DHA status were markedly lower at delivery in the HOPE trial than the current study (maternal: placebo 4.99%, supplemented 7.09%; infant: placebo 6.18%, supplemented 7.75%). The inability to detect a meaningful difference in fetal neurodevelopment related to treatment group or equilibrium status in the current trial are likely due to the higher maternal DHA status at enrollment (6.94%) and our decision to test the superiority of two doses (200 vs. 800 mg) instead of controlling with placebo.
There were limitations to the study. The COVID-19 pandemic and resulting shutdown caused the majority of and higher than predicted attrition, preventing us from reaching our power analysis target of 125 participants per group for the fABAS. However, given the lack of difference in fABAS means, it is highly unlikely that data lost from visits missed due to COVID-19 would have changed the results. Because the aims of this trial were based on maternal–infant DHA status and neurodevelopmental outcomes, the eligibility requirements were limited to English-speaking, healthy women carrying singleton pregnancies. Further, the diversity of women enrolling in PANDA did not reflect the racial and ethnic population of the greater metropolitan area, making the results of this trial less generalizable to the overall obstetric public.
Conclusions
We were able to increase the incidence of maternal–infant DHA equilibrium using 800 mg DHA compared to 200 mg, but equilibrium status had no effect on fetal neurodevelopment. Maternal–infant DHA equilibrium is not a reliable biomarker of insufficient status when maternal DHA is >6%. However, the failure to achieve equilibrium when maternal DHA is below 6% in this study is consistent with previous population studies and is a likely indicator of low maternal DHA status. It is possible that group differences may emerge later in life, as infants will continue to accumulate DHA from their postnatal diet. We will gain additional knowledge through planned secondary data analyses that will explore the effect of DHA and other dietary nutrients on fetal neurodevelopment and ongoing measures of brain physiology, cognitive development, and behavior during the first year of life.
Data availability
We are willing to share deidentified data from the study with a signed data access agreement that includes the study principal investigators, contingent on approval of the planned use of the data. As the data are entered into an electronic system, a specific request to K.M.G. (kgustafson@kumc.edu) or D.N.C (dchristifano@kumc.edu) would be needed to generate a data output for other investigators. We plan to publish secondary results and longitudinal outcomes of the trial and cannot share some data until the study is final.
References
Gil-Sanchez, A. et al. Mechanisms involved in the selective transfer of long chain polyunsaturated fatty acids to the fetus. Front. Genet. 2, 57 (2011).
Gil-Sanchez, A., Koletzko, B. & Larque, E. Current understanding of placental fatty acid transport. Curr. Opin. Clin. Nutr. Metab. Care 15, 265–272 (2012).
Markhus, M. W. et al. Docosahexaenoic acid status in pregnancy determines the maternal docosahexaenoic acid status 3-, 6- and 12 months postpartum. Results from a longitudinal observational study. PLoS ONE 10, e0136409 (2015).
Innis, S. M. Plasma and red blood cell fatty acid values as indexes of essential fatty acids in the developing organs of infants fed with milk or formulas. J. Pediatr. 120, S78–S86 (1992).
Crawford, M. A., Hassam, A. G. & Williams, G. Essential fatty acids and fetal brain growth. Lancet 1, 452–453 (1976).
Luxwolda, M. F. et al. A maternal erythrocyte dha content of approximately 6 g% is the DHA status at which intrauterine DHA biomagnifications turns into bioattenuation and postnatal infant DHA equilibrium is reached. Eur. J. Nutr. 51, 665–675 (2012).
Kuipers, R. S. et al. Maternal DHA equilibrium during pregnancy and lactation is reached at an erythrocyte DHA content of 8 g/100 g fatty acids. J. Nutr. 141, 418–427 (2011).
Gustafson, K. M. et al. Effects of docosahexaenoic acid supplementation during pregnancy on fetal heart rate and variability: a randomized clinical trial. Prostaglandins Leukot. Essent. Fatty Acids 88, 331–338 (2013).
Hoyer, D. et al. Fetal functional brain age assessed from universal developmental indices obtained from neuro-vegetative activity patterns. PLoS ONE 8, e74431 (2013).
Schmidt, A. et al. Universal characteristics of evolution and development are inherent in fetal autonomic brain maturation. Auton. Neurosci. 212, 32–41 (2018).
Hoyer, D., Schmidt, A., Schneider, U. & Gustafson, K. Fetal developmental deviations reflected in a functional autonomic brain age score. In Computing in Cardiology 1–4 (IEEE, Maastricht, Netherlands, 2018).
Hoyer, D. et al. Heart rate variability categories of fluctuation amplitude and complexity: diagnostic markers of fetal development and its disturbances. Physiol. Meas. 40, 064002 (2019).
Diet History Questionnaire. National Institutes of Health, Epidemiology and Genomics Research Program, National Cancer Institute (2010).
Diet*calc analysis program. National Cancer Institute, Epidemiology and Genomics Research Program (2012).
Tarvainen, M. P. et al. Kubios HRV–heart rate variability analysis software. Comput. Methods Prog. Biomed. 113, 210–220 (2014).
Schneider, U. et al. Developmental milestones of the autonomic nervous system revealed via longitudinal monitoring of fetal heart rate variability. PLoS ONE 13, e0200799 (2018).
Nijhuis, J. G., Prechtl, H. F., Martin, C. B. Jr & Bots, R. S. Are there behavioural states in the human fetus? Early Hum. Dev. 6, 177–195 (1982).
Pillai, M. & James, D. The development of fetal heart rate patterns during normal pregnancy. Obstet. Gynecol. 76, 812–816 (1990).
Schneider, U. et al. Human fetal heart rate variability-characteristics of autonomic regulation in the third trimester of gestation. J. Perinat. Med. 36, 433–441 (2008).
Schneider, U. et al. Fetal heart rate variability reveals differential dynamics in the intrauterine development of the sympathetic and parasympathetic branches of the autonomic nervous system. Physiol. Meas. 30, 215–226 (2009).
Hoyer, D. et al. Fetal development of complex autonomic control evaluated from multiscale heart rate patterns. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R383–R392 (2013).
Schmidt, A. et al. Developing fetal motor-cardiovascular coordination analyzed from multi-channel magnetocardiography. Physiol. Meas. 35, 1943–1959 (2014).
Christifano, D. N. et al. Higher maternal weight is related to poorer fetal autonomic function. J. Dev. Orig. Health Dis. 12, 1–3 (2020).
Berry, S. M. & Berry, D. A. Accounting for multiplicities in assessing drug safety: a three-level hierarchical mixture model. Biometrics 60, 418–426 (2004).
Tomedi, L. E. et al. Pre-pregnancy obesity and maternal nutritional biomarker status during pregnancy: a factor analysis. Public Health Nutr. 16, 1414–1418 (2013).
Mackay, V. A. et al. Preeclampsia is associated with compromised maternal synthesis of long-chain polyunsaturated fatty acids, leading to offspring deficiency. Hypertension 60, 1078–1085 (2012).
Min, Y. et al. Efficacy of docosahexaenoic acid-enriched formula to enhance maternal and fetal blood docosahexaenoic acid levels: randomized double-blinded placebo-controlled trial of pregnant women with gestational diabetes mellitus. Clin. Nutr. 35, 608–614 (2016).
Min, Y. et al. Unfavorable effect of type 1 and type 2 diabetes on maternal and fetal essential fatty acid status: a potential marker of fetal insulin resistance. Am. J. Clin. Nutr. 82, 1162–1168 (2005).
Leveille, P., Rouxel, C. & Plourde, M. Diabetic pregnancy, maternal and fetal docosahexaenoic acid: a review of existing evidence. J. Matern. Fetal Neonatal Med. 31, 1358–1363 (2018).
Zhang, Z., Fulgoni, V. L., Kris-Etherton, P. M. & Mitmesser, S. H. Dietary intakes of EPA and DHA omega-3 fatty acids among us childbearing-age and pregnan't women: an analysis of NHANES 2001-2014. Nutrients 10, 416 (2018).
Carlson, S. E. et al. DHA supplementation and pregnancy outcomes. Am. J. Clin. Nutr. 97, 808–815 (2013).
Carlson, S. E. et al. Higher dose docosahexaenoic acid supplementation during pregnancy and early preterm birth: a randomised, double-blind, adaptive-design superiority trial. Eclinicalmedicine 36, 100905 (2021).
Simmonds, L. A. et al. Omega-3 fatty acid supplementation in pregnancy-baseline omega-3 status and early preterm birth: exploratory analysis of a randomised controlled trial. BJOG 127, 975–981 (2020).
Hoge, A. et al. Imbalance between Omega-6 and Omega-3 Polyunsaturated Fatty Acids in Early Pregnancy Is Predictive of Postpartum Depression in a Belgian Cohort. Nutrients 11, 876 (2019).
Otto, S. J., de Groot, R. H. & Hornstra, G. Increased risk of postpartum depressive symptoms is associated with slower normalization after pregnancy of the functional docosahexaenoic acid status. Prostaglandins Leukot. Essent. Fatty Acids 69, 237–243 (2003).
Acknowledgements
We are grateful for the support of study personnel who were responsible for initiation of the study, recruiting participants, communicating with them monthly, and collecting the data critical for the study, especially Sheri Copeland, Sara Macone, Jocelyn Thodosoff, Beth Kerling, Sarah Crawford, and Sarah Touchette. The success of this trial is due to their diligence and care. We recognize the nurses who cared for the women in the trial and collected the blood samples. We are grateful to DSM Nutritional Products for providing the study capsules. We appreciate the time and effort of our DSMB; Michael Georgieff, MD of the University of Minnesota Masonic Children’s Hospital, Minneapolis, MN; Vishal Pandey, MD, Courtney Marsh, MD, and Jianghua He, PhD, of the University of Kansas Medical Center, Kansas City, KS. Finally, we are most grateful to the 300 women who enrolled in the study.
Funding information
This study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Author information
Authors and Affiliations
Contributions
K.M.G. was the principal investigator and designed the study with input from S.E.C., J.C., D.H., and B.J.G.; D.N.C. and N.B.M. maintained regulatory documents, oversaw conduct of study operation, and verified the data entered from medical records; K.M.G., D.N.C., and N.B.M. obtained all maternal–fetal measures at the study visits and processed the MCG data; D.H. and A.S. performed the fABAS analysis; S.A.S. performed the RBC fatty acid analysis; A.R.B. and D.P.M. were responsible for verifying the data within Velos; D.P.M. developed the electronic records for data entry in conjunction with A.R.B.; L.C.-H. and B.J.G. were responsible for the statistical analysis; K.M.G., D.N.C., S.E.C., L.C.-H., J.C., and B.J.G. wrote the manuscript, but all authors contributed their insights to the final version.
Corresponding author
Ethics declarations
Competing interests
S.E.C. and J.C. have received honorariums for presentations about DHA in infancy and pregnancy. The remaining authors declare no competing interests.
Consent statement
All participants signed written informed consent prior to enrollment.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gustafson, K.M., Christifano, D.N., Hoyer, D. et al. Prenatal docosahexaenoic acid effect on maternal-infant DHA-equilibrium and fetal neurodevelopment: a randomized clinical trial. Pediatr Res 92, 255–264 (2022). https://doi.org/10.1038/s41390-021-01742-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01742-w