Abstract
Background
Neonates admitted in the neonatal intensive care unit are vulnerable to acute kidney injury leading to worse outcomes. It is important to identify “at-risk” neonates for early preventive measures.
Methods
The study was a multicenter, national, prospective cohort study done in 11 centers in India. A multivariable logistic regression technique with step-wise backward elimination method was used, and a “Risk Prediction Scoring” was devised [the STARZ score].
Results
The neonates with admission in the NICU within <25.5 h of birth, requirement of positive pressure ventilation in the delivery room, <28 weeks gestational age, sepsis, significant cardiac disease, urine output <1.32 ml/kg/h or serum creatinine ≥0.98 mg/dl during the first 12 h post admission, use of nephrotoxic drugs, use of furosemide, or use of inotrope had a significantly higher risk of AKI at 7 days post admission in the multivariate logistic regression model. This scoring model had a sensitivity of 92.8%, specificity of 87.4% positive predictive value of 80.5%, negative predictive value of 95.6%, and accuracy of 89.4%.
Conclusions
The STARZ neonatal score serves to rapidly and quantitatively determine the risk of AKI in neonates admitted to the neonatal intensive care unit.
Impact
-
The STARZ neonatal score serves to rapidly and quantitatively determine the risk of AKI in neonates admitted to the neonatal intensive care unit.
-
These neonates with a higher risk stratification score need intense monitoring and daily kidney function assessment.
-
With this intensification of research in the field of AKI risk stratification prediction, there is hope that we will be able to decrease morbidity and mortality associated with AKI in this population.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is common in critically ill children and neonates, although the data on epidemiology of neonatal AKI is sparse.1,2,3,4 Neonates admitted in the neonatal intensive care unit (NICU) are more vulnerable to AKI than older children owing to the immature functioning kidneys.5 Consensus endorsement of Kidney Disease: Improving Global Outcomes (KDIGO) AKI definition has been instrumental in bringing uniformity in research and clinical trials in neonatal AKI.6,7,8
Multiple studies suggest that the incidence of neonatal AKI varies from 18 to 70%.9,10,11,12,13,14,15,16 AKI is associated with increased morbidity, longer length of stay in NICU, and mortality across all groups of neonates and is an independent predictor of survival.4,9,10,11,12,13,14,15,16 High-risk neonates who develop AKI have almost three times higher risk of dying than those who do not have AKI. The risk of death even in milder forms of AKI in neonates is similar to stage 3, highlighting the need to identify it early and take appropriate measures.4,9,10,11,12,13,14,15,16
Multiple risk factors affect the incidence and the overall outcomes in neonates with AKI. The incidence of AKI in critically ill neonates was meticulously studied in a multicenter AWAKEN (Assessment of the Worldwide AKI Epidemiology in Neonates) study and later the same group published risk factors specific to various subgroups.9,10,11,12 Prevalence of neonatal AKI varies among high-risk subgroups, like preterm neonates, very low birth weight, neonates with congenital diaphragmatic hernia on extra-corporeal membrane oxygenation, and those with perinatal asphyxia.13,14,15,16
Increasing incidence and marked vulnerability to AKI in neonates warrants extensive research on utility of new biomarker or risk stratification score for early detection/prediction of AKI.17 Various risk stratification score for mortality in critically ill children have been successfully implemented in critical care units across the globe. The two commonly used scoring system include Pediatric RISk of Mortality (PRISM) and Pediatric Index of Mortality (PIM 1, 2, 3).18,19,20,21 Although they are validated in various populations, the utility in assessing neonatal outcomes is poor. Illness severity and mortality risk scoring system studied in neonates include Neonatal Therapeutic Intervention Scoring System (NTISS),22 Score for Neonatal Acute Physiology (SNAP),23 the Transport Risk Index of Physiologic Stability (TRIPS),24 the Clinical Risk Index for Babies (CRIB),25 The simplified age–weight–sex (SAWS) score,26 and NMR-2000 score.27 None of the above-mentioned scores are practical enough to be used in resource-limited settings except for SAWS and NMR-2000.
AKI forms an important component of pediatric scores as it independently worsens patient outcomes.28 A dedicated AKI risk stratification score is the need of the hour. Renal Angina Index (RAI), a term borrowed from cardiac angina, is the only AKI-specific risk stratification score that has been extensively studied in multiple pediatric cohorts outperforming traditional markers of illness severity (PRISM and PIM), and KDIGO staging is used to classify AKI.29 RAI is an empiric clinical model of renal angina proposed to identify which critically ill patients would be at the greatest risk of AKI using patient demographic factors and early signs of injury, where the presence of a high score at admission may delineate patients at higher risk for subsequent severe AKI.30 With all the validation coming from pediatric (non-neonatal population) population, RAI still is not a recognized risk stratification score in neonates.
It is highly important to identify “at-risk” neonates and do periodic assessments in them to identify AKI early and start early preventive and therapeutic measures. A recent adaptation of the Nephrotoxic Injury Negated by Just-in-time Action (NINJA) program was applied in neonates in United States and it was found that it was effective in reducing the rates of AKI in neonates in the NICU.31 Incorporation of these systems into the electronic medical record (EMR) may be beneficial for all the practising neonatologists.
It is important to understand that the main basis of treatment of AKI in neonates is prevention, vigilant monitoring, and early detection.4,32 In the current study, we have attempted to generate a novel AKI-specific risk stratification score [The STARZ (Sethi, Tibrewal, Agarwal, Raina and Wazir) neonatal score] to fill this void in the neonatal AKI management, especially in resource-constraint settings.
Materials and methods
Study design
The study was a multicenter, national, prospective cohort study done in 11 centers all across the country (India). All the neonates who fulfilled the inclusion and exclusion criteria at each of the participating centers with level 2–4 NICUs from August 2018 to August 2019 were enrolled. An informed consent was obtained from the parents for participation in the study, and the study was approved by the Institutional Review Board of all the participating centers.
Inclusion criteria
All neonates (≤28 days) admitted to NICU who were cannulated and received intravenous (IV) fluids for at least 48 h for hydration and/or nutrition were included in the study.
Exclusion criteria
The following neonates were excluded
-
Death within 48 h of admission;
-
Neonates receiving routine care in the nursery;
-
Neonates who received intravenous hydration for <48 h or only for administration of medications;
-
Presence of any lethal chromosomal anomaly (including trisomy 13, trisomy 18, and anencephaly);
-
Neonates requiring congenital heart surgery within the first 7 days of life.
Data collection
Demographic details, including date of birth, age, male or female, and date of NICU admission, were recorded. Maternal particulars about age, parity, health conditions, peripartum infections, or complications were also noted. Maternal risk factors were defined as per standard American College of Obstetricians and Gynecologists guidelines. Neonatal characteristics, including mode and site of delivery (inborn or outborn), gestational age, birth weight, length and head circumference, data on resuscitation, temperature at admission, and cause of admission, were recorded. We defined AKI as an increase in serum creatinine of ≥0.3 mg/dl (≥26.5 μmol/l) or ≥50% from the previous lowest value, or a urinary output of <1 ml/kg/h on postnatal days 2–7, according to the KDIGO Workgroup AKI definition modified for neonates, as used in previous neonatal studies.9 Serum creatinine was recorded daily using enzymatic method till the resolution of AKI episode. We defined significant cardiac disease as hemodynamically significant patent ductus arteriosus [PDA], persistent pulmonary hypertension of the newborn [PPHN], cardiogenic shock, and other congenital cardiac disease. Weight, blood pressure, heart rate, fluid intake and output, and basic laboratory parameters (hemoglobin (Hb), blood urea nitrogen, electrolytes, and albumin) were also recorded. Information on use of nephrotoxic medications (colistin or vancomycin or amphotericin B), respiratory support, blood/urine, and cerebrospinal fluid cultures were noted. In-depth information was obtained about neonates with specific renal diagnoses (congenital anomaly, prior AKI episodes, and need for renal replacement therapy).
Data entry points
Data on the aforementioned variables were collected daily during the first week of hospitalization and thereafter first value was recorded for subsequent weeks (excepting serum creatinine) until any endpoint was reached.
Endpoints
Data collection was continued until any one of following endpoints was achieved—discharge to home, transfer to a facility not in liaison with the national collaboration, transfer out of the NICU, death, or 120 days of age.
Statistical analysis
The data were entered in an online collaborative database and exported to Microsoft excel, and Statistical software (SPSS version 20) was used for the statistical analyses. All the variables were tested for normality using Kolmogorov–Smirnov test. Categorical variables are summarized as frequencies and percentages, while continuous variables as medians and interquartile range (IQR; 25th to 75th percentiles), for the sake of uniformity.
Univariate analysis (using chi-square or Fisher exact test for categorical variables and Wilcoxon’s rank-sum test for continuous variables) was first carried out to assess the unadjusted relationship between the variables and AKI incidence at 7 days post admission in NICU. There were missing data for almost all of the continuous variables, and instead of imputing the missing data, we have excluded those patients or variables due to relatively larger sample size. So, prior to conducting the multivariate regression analysis, following steps were carried out sequentially: (a) the variables with missing data in >50% of the patients were ignored; (b) patients with any of the missing variable out of the significant variables in the univariate analysis were excluded; (c) significant continuous variables were converted into categorical variables based on the cut-off threshold value (sensitivity and specificity is observed to be highest) identified by receiver operating characteristic (ROC) analysis.
A multivariable logistic regression technique with step-wise backward elimination method was used to determine the relationship between the independent variables and AKI incidence. The step-wise method resulted in several models, each derived from a set of independent variables. Of these models, the best fit model was selected based on (a) Hosmer–Lemeshow goodness-of-fit statistic (which statistically compares the predicted probability with actual probability within population subgroups; the larger the p value, the better the fit) and (b) R-square (R2) (a statistical measure of how close the data are to the fitted regression line; the higher the R2, the better the model fits the data). The predictive validity of the best fit model was computed based on the standard statistical measures (sensitivity, specificity, positive predictive value, negative predictive value, accuracy) and area under the ROC curve.
For the construction of a scoring system to predict the probability of AKI incidence, a score was assigned to each of the significant variable in the best fit model. This score was given the acronym STARZ score (Sethi, Tibrewal, Agarwal, Raina and Wazir score), using the initials of the authors driving this study. For this, the logistic regression coefficient (β) estimate of each variable was multiplied by ten, and the resultant value was summed up for all the variables.33 To obtain the score of each variable out of 100, the formula [(resultant value of each variable × 100)/summed up value for all the variables] was used, and the obtained value was rounded to the nearest integer. A score of zero was assigned to the reference group of each of the variable. The total score for this scoring system ranged from 0 to 100, where higher score indicates greater probability of AKI incidence. The cut-off score for this scoring system (score at which the sum of sensitivity and specificity is highest) for categorizing the patients into high and low probability of AKI incidence was determined based on the ROC statistics. The predictive validity of the scoring system was also computed based on the standard statistical measures and area under the ROC curve.
Results
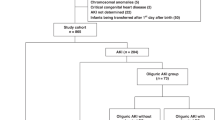
The study flow chart is shown in Fig. 1. We included 763 neonates for analysis, of which 24.5% had AKI (n = 187) at 7 days post admission in the NICU. For the included neonates (n = 763), the median (IQR) values for different variables were age at entry: 19 (6–76) h; gestational age at birth: 35 (32–37) weeks, birth weight: 2150 (1520–2794) g, Apgar score at 5 min: 8 (7–9), admission weight: 2125 (1520–2700) g, and duration in NICU: 10 (6–20) days. The proportions of neonates for different variables were outborn delivery: 52.4% (400/763), vaginal delivery: 43.1% (329/763), males: 68.8% (525/763), required respiratory support in NICU: 67.5% (515/763), significant cardiac disease: 43.3% (330/763), and use of inotrope(s): 2.4% (18/763).
Univariate analysis
The neonates who had AKI were observed to have significantly lower age at NICU admission [median (IQR): 13 (5–33) vs. 23 (6–100) h; p = 0.003], lower temperature [36.5 (36.4–36.5) vs. 36.5 (36.5–36.6) °C; p < 0.001], higher IV fluid intake [60 (50–80) vs. 60 (45–70) ml/kg/day; p = 0.003], lower enteral feed intake [0 (0–0) vs. 0 (0–24) ml/kg/day; p < 0.001], lower urine output [1.4 (1.01–2.0) vs. 1.7 (1.2–2.3) ml/kg/h; p < 0.001], higher serum urea [45.45 (30.0–86.75) vs. 24.5 (18.11–31.88) mg/dl; p < 0.001], higher serum creatinine [1.2 (1.0–1.8) vs. 0.6 (0.5–0.8) mg/dl; p < 0.001], higher serum sodium [139.0 (134.8–147.0) vs. 136.0 (132.0–141.0) meq/l; p < 0.001], greater serum potassium [5.2 (4.4–5.8) vs. 4.5 (4.2–5) meq/l; p < 0.001], and lesser lowest Hb [16.3 (13.45–18.0) vs. 17.5 (15.2–18.7) g/dl; p = 0.001] during the first 12 h post admission in a univariate analysis [Supplementary Table 1; for continuous variables].
Likewise, the neonates who received positive pressure ventilation (PPV) in the delivery room [unadjusted relative risk (RR) (95% confidence interval (CI)): 1.78 (1.32–2.40); p < 0.001]; with <28 completed weeks of gestational age at birth [1.81 (1.21–2.72); p = 0.01]; who were males [1.61 (1.18–2.22); p = 0.002]; who required respiratory support in NICU [2.62 (1.82–3.78); p < 0.001]; who had sepsis during the NICU stay [1.35 (1.03–1.76); p = 0.008]; who had significant cardiac disease [2.19 (1.69–2.84); p < 0.001], such as hemodynamically significant PDA [1.46 (1.07–1.98); p = 0.02], PPHN [2.29 (1.72–3.04); p < 0.001], cardiogenic shock [1.67 (1.31–2.14); p < 0.001] and other congenital cardiac disease [1.65 (1.06–2.57); p = 0.04]; who had multiple seizures during the first 12 h post admission (defined as >1 seizure episode in the first 12 h) [1.90 (1.43–2.53); p < 0.001]; who had an evidence of fluid overload (>10%) during the first 12 h post admission [3.09 (2.26–4.24); p < 0.001]; and who had received nephrotoxic drugs, such as vancomycin or colistin or amphotericin B [2.20 (1.71–2.82); p < 0.001], ibuprofen [2.48 (1.63–3.77); p = 0.001], furosemide [2.41 (1.82–3.20); p < 0.001], and use of inotrope [2.45 (1.92–3.13); p < 0.001] had a significantly higher risks of AKI at 7 days post admission in a univariate analysis, while the neonates whose mother had a history of maternal diabetes [0.46 (0.22–0.99); p = 0.02] or maternal hypertension [0.57 (0.36–0.91; p = 0.01] during antenatal period, who was born in an institutional setting [0.66 (0.51–0.86); p = 0.001], and who required supplemental O2 in the delivery room [0.72 (0.51–1.00); p = 0.04)] had a significantly lower risk of AKI at 7 days post admission in a univariate analysis. [Supplementary Table 2; for categorical variables; and Supplementary Fig. 1].
Multivariate analysis
Prior to the multivariate analysis, the following steps were carried out: (a) ignoring two variables (enteral fluid intake, and serum urea) for further analysis due to missing data in >50% of patient, (b) removing patients with any of the missing variable out of the remaining 33 significant variables in the univariate analysis, and (c) converting remaining 8 significant continuous variables into categorical variables based on the cut-off threshold value (sensitivity and specificity is observed to be the highest) identified by ROC analysis. The neonates with admission in the NICU within <25.5 h of birth [adjusted RR (95% CI): 2.06 (1.05–2.97); p = 0.048); requirement of PPV in the delivery room [1.91 (1.05–2.32); p = 0.045]; <28 weeks of gestational age [2.01 (1.02–2.74); p = 0.029]; sepsis during the NICU stay [1.98 (1.04–2.54); p = 0.04]; significant cardiac disease [2.81 (1.06–3.56); p = 0.04]; urine output during the first 12 h post admission <1.32 ml/kg/h [2.29 (1.21–3.01); p = 0.017]; serum creatinine during the first 12 h post admission ≥0.98 mg/dl [13.44 (8.44–15); p < 0.001]; use of nephrotoxic drugs [2.76 (1.6–3.19); p = 0.004]; use of furosemide [2.39 (1.06–2.98); p = 0.045]; or use of inotropes [2.79 (1.01–2.91); p = 0.049] had a significantly higher risk of AKI at 7 days post admission in the multivariate logistic regression model. This model was observed to be the best fit model for the AKI prediction based on Hosmer–Lemeshow goodness-of-fit test [chi-square = 1.835; df = 8; p = 0.986] and Nagelkerke R2 = 0.855 [Table 1]. This best fit model demonstrated high calibration between predicted and observed probability [Fig. 2].
This best fit model was observed to have a sensitivity of 91.9% [102/111], specificity of 96.0% [191/199], positive predictive value of 92.7% [102/110], negative predictive value of 95.5% [191/200], and accuracy of 94.5% [293/310] [Table 2]. This model had an area under the ROC curve of 0.974 (95% CI: 0.958–0.990) [p < 0.001], indicating high discriminative power [Fig. 3].
Scoring model
In order to construct a scoring system, each of the significant variables based on the best fit model was assigned a score as per the method described in the “Statistical analysis” section, and a score of zero was assigned to the reference group. The total score for this model ranged from 0 to 100, where higher score indicates greater risk of AKI incidence at 7 days [Table 3]. The patients having a cut-off score ≥31.5 in this model were at higher probability of AKI [AUC: 0.959 (95% CI: 0.939–0.979); p < 0.001; Fig. 4]. The mean, median, and range of the score among all the patients were 31, 28 and 0–78, respectively. The probability of AKI was <20% up to score 37, 20–<40% for score 38–42, 40–<60% for score 43–48, 60–<80% for score 49–53, and ≥80% for score ≥54. This scoring model was observed to have a sensitivity of 92.8% [103/111], specificity of 87.4% [174/199], positive predictive value of 80.5% [103/128], negative predictive value of 95.6% [174/182], and accuracy of 89.4% [277/310] [Table 4]. The snapshot of user friendly dashboard for the scoring system is depicted in Fig. 5.
Mortality and length of stay
The risk of mortality was observed to be significantly higher among those with AKI as compared to those without AKI [19.8 vs. 2.4%; RR (95% CI): 3.44 (2.76–3.95)]. However, the median (IQR) length of stay in the NICU was not significantly different between those with vs. without AKI [10 (5–19) vs. 11 (6–20) days; p = 0.10].
Discussion
Almost 2·5 million neonatal deaths occur each year all across the world, among which 78% of the neonatal deaths are in sub-Saharan Africa and southern Asia. Out of the neonatal deaths in sub-Saharan Africa and southern Asia, 80% of these deaths occur in babies with a low birth weight. Given that 73% of neonatal deaths occur within the first 7 days of life, an early recognition of a severe illness and rapid initiation of evidence-based interventions is crucial to improve survival in these babies.27 AKI is now known to be an independent marker of survival in neonatal population.4,9,10,11,12,13,14,15,16 Thus, there is a need to develop a neonatal AKI risk stratification score to identify these babies early and to initiate preventive and therapeutic measures. This study is the first ever initiative carried out in a prospective, multicentric manner with 11 centers all across the country, recruiting newborn babies with a standard criteria.
Multiple neonatal disease severity scorings have been used in clinical practice to assess neonatal mortality.27,34 Therapy-based scores include NTISS, which categorizes the neonatal illness based on the quantity and type of treatment administered.22 SNAP and the TRIPS use objective measurable parameters, which may deteriorate with the illness severity, e.g., blood pressure.23,24,35 However, CRIB combines the physiological parameters with clinical parameters, e.g., birth weight, and then defines the mortality rate.25,36 CRIB-II, SNAP-II, and SNAPPE-II (Score for neonatal acute physiology – Perinatal extension- II) are most commonly used in clinical practice. None of the above-mentioned scores are practical or were developed exclusively for routine use in low- and middle-income countries. Only two scores—SAWS score and NMR-2000—are validated neonatal mortality scores designed exclusively for low-resource settings.26,27 SAWS derivation cohort included neonates from Bangladesh and NMR-2000 score from Gambia.26,27 AKI being an independent predictor of short- and long-term outcomes, there was a need to have a neonatal acute kidney risk stratification score designed for the low-resource settings.
The variables in our neonatal AKI risk prediction score (STARZ Score) are simple to extract and use. The variables include clinical parameters (age at entry in NICU in hours, need for PPV in the delivery room, gestational age, sepsis, significant cardiac disease, urine output in the first 12 h post admission <1.32 ml/kg/h, use of nephrotoxic drugs, use of furosemide or inotropes, and one laboratory parameter (serum creatinine in the first 12 h post admission ≥ 0.98 mg/dl).
Need for PPV and inotropes are important variables in the current score, given a score 7 and 17, respectively. It is well known that neonates who are sick and asphyxiated are at risk of AKI.37,38,39
Gestational age <28 weeks is one of the significant variable in our neonatal risk score with an assigned score of 7. It is well known that prematurity and low birth weight are risk factors for AKI in neonates. Both prematurity and low birth weight increase due to impaired nephrogenesis and reduced nephron endowment leading to reduced glomerular filtration and tubular function.40,41,42 In the largest dataset to predict mortality in neonates (NMR-2000) also, birth weight was found to be one of the three variable in the score predicting mortality. Birth weight is important with reference to low-income countries, since there is often late presentation for antenatal care, poor recall of the maternal last menstrual period, and unavailability of ultrasonography.27
It is well known that nephrotoxin exposure is the highest in neonates, and in fact the youngest babies have the highest exposure.9 There is a need for EMR-based initiatives, e.g., Baby NINJA, which can alert a clinician using a system-based alert to reduce the nephrotoxin exposure in “at-risk” babies.31 These “at-risk” babies should be screened with serial serum creatinine evaluations, so that episodes of AKI are not missed. We looked at multiple nephrotoxins received during the NICU stay and found that use of colistin, vancomycin, and amphotericin B were most significant variables impacting the occurrence of AKI later in the stay. Gram-negative organisms are the most common organisms causing neonatal sepsis in resource-limited settings, which have high degree of antimicrobial resistance (nearly 50–80% are multidrug resistant, even to reserve drugs, such as carbapenems).43 In view of such high degree of antimicrobial resistance, drugs such as colistin are being used in these settings.
It is interesting to note that we found that a threshold of urine output <1.32 ml/kg/h was a significant variable in the neonatal AKI risk prediction score. Another study done from a single-center NICU also proposed that the urine output threshold should be higher in newborns (<1.5 ml/kg/h) as opposed to older children.44 There is a need to have more research in this field to validate the ideal urine output threshold in babies. Moreover, it is important to realize that a fluid balance state should be kept in these babies keeping in mind the lower urinary concentration ability and looking at “the net cumulative fluid overload”, which has been reported to have poor outcomes in sick babies.45 We did look at the cumulative fluid overload in the first 12 h in these babies, but the variables, although significant on univariate analysis, lost their effect on the multivariate analysis.
Babies born to maternal diabetes and chronic hypertension had less AKI. This finding is important to note and also has been noted previously in multiple studies, including the AWAKEN cohort.9,45,46 There are speculations that such protective effect could have arisen from prenatal ischemic pre-conditioning of the kidney or medications and/or other interventions received for the management of maternal pre-eclampsia, diabetes, or chronic hypertension.45,46
The strengths of this study are the size of the cohort and it being a prospective, multicenter cohort with a uniform inclusion criterion. Robust number of values of serum creatinine using the enzymatic method were available for analysis allowing us to characterize the AKI rates in all the centers. The major strength of the study has been to be able to get the urine output variable in the neonates and also strengthening is the fact that even urine output <1.32 ml/kg/h in first 12 h may be an ominous sign and should be looked for in “at-risk” neonates. We were also able to identify a threshold value of serum creatinine (sensitivity and specificity is observed to be the highest) by ROC analysis and used the value of 0.98 mg/dl for categorization and use in risk prediction score. These parameters should be kept in consideration in future studies on neonatal AKI definitions.
Despite these strengths, we acknowledge the following limitations. We did not have access to cystatin-C, despite the awareness that use of biomarkers may enhance the sensitivity to diagnose AKI in neonates. Lastly, we do acknowledge that, although the definition we used is currently the most accepted neonatal AKI definition, this definition may need to be refined over time, looking at maybe a change in urine output criterion, rather than keeping an arbitrary cut-off for oliguria and a serum creatinine threshold.
The STARZ neonatal score serves to rapidly and quantitatively determine the risk of AKI in neonates admitted to the NICU. There is a need to apply the score in developed countries also to validate its applicability. These neonates with a higher-risk stratification score need intense monitoring and daily kidney function assessment. With this intensification of research in the field of AKI prediction, there is hope that we will be able to decrease morbidity and mortality associated with AKI in this population.
To conclude, we feel that the neonatal AKI risk prediction score shall be useful for all populations, especially the low-resource populations to identify neonates at risk of AKI and mortality early, and help initiate preventive and therapeutic measures. Validation of this scoring system is required in future studies of at-risk neonatal populations.
Change history
14 September 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41390-021-01674-5
References
Schneider, J., Khemani, R., Grushkin, C. & Bart, R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit. Care Med. 38, 933–939 (2010).
Andreoli, S. P. Acute renal failure in the newborn. Semin. Perinatol. 28, 112–123 (2004).
Akcan-Arikan, A. et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 71, 1028–1035 (2007).
Charlton, J. R. et al. Incidence and risk factors of early onset neonatal AKI. Clin. J. Am. Soc. Nephrol. 14, 184–195 (2019).
Kaur, S. et al. Evaluation of glomerular and tubular renal function in neonates with birth asphyxia. Ann. Trop. Pediatr. 31, 129–134 (2011).
Walker, M., Clark, R. & Spitzer, A. Elevation in plasma creatinine and renal failure in premature neonates without major anomalies: terminology, occurrence and factors associated with increased risk. J. Perinatol. 31, 199–205 (2011).
Gawadia, J., Mishra, K., Kumar, M. & Saikia, D. Prediction of severe acute kidney injury using renal angina index in a pediatric intensive care unit. Indian Pediatr. 56, 647–652 (2019).
KDIGO AKI Work Group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2, 1–138 (2012).
Jetton, J. G. et al. Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicenter, multinational, observational cohort study. Lancet Child Adolesc. Health 1, 184–194 (2017).
Kirkley, M. J. et al. Acute kidney injury in neonatal encephalopathy: an evaluation of the AWAKEN database. Pediatr. Nephrol. 34, 169–176 (2019).
Stoops, C. et al. The association of intraventricular hemorrhage and acute kidney injury in premature infants from the Assessment of the Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study. Neonatology 116, 321–330 (2019).
Starr, M. C. et al. Acute kidney injury is associated with poor lung outcomes in infants born ≥32 weeks of gestational age. Am. J. Perinatol. 37, 231–240 (2020).
Bruel, A. et al. Critical serum creatinine values in very preterm newborns. PLoS ONE 8, e84892 (2013).
Carmody, J. B., Swanson, J. R., Rhone, E. T. & Charlton, J. R. Recognition and reporting of AKI in very low birth weight infants. Clin. J. Am. Soc. Nephrol. 9, 2036–2043 (2014).
Gadepalli, S. K., Selewski, D. T., Drongowski, R. A. & Mychaliska, G. B. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J. Pediatr. Surg. 46, 630–635 (2011).
Selewski, D. T., Jordan, B. K., Askenazi, D. J., Dechert, R. E. & Sarkar, S. Acute kidney injury in asphyxiated newborns treated with therapeutic hypothermia. J. Pediatr. 162, 725.e1–729.e1 (2013).
Parikh, C. R. & Han, G. Variation in performance of kidney injury biomarkers due to cause of acute kidney injury. Am. J. Kidney Dis. 62, 1023–1026 (2013).
Pollack, M. M., Ruttimann, U. E. & Getson, P. R. Pediatric risk of mortality (PRISM) score. Crit. Care Med. 16, 1110–1116 (1988).
Shann, F., Pearson, G., Slater, A. & Wilkinson, K. Pediatric index of mortality (PIM): a mortality prediction model for children in intensive care. Intensive Care Med. 23, 201–207 (1997).
Slater, A. A revised version of the Pediatric Index of Mortality. Intensive Care Med. 29, 278–285 (2003).
Straney, L. et al. Pediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care. Pediatr. Crit. Care Med. 14, 673–681 (2013).
Gray, J. E., Richardson, D. K., McCormick, M. C., Workman-Daniels, K. & Goldmann, D. A. Neonatal therapeutic intervention scoring system: a therapy-based severity-of-illness index. Pediatrics 90, 561–567 (1992).
Richardson, D. K., Corcoran, J. D., Escobar, G. J. & Lee, S. K. SNAP-II and SNAPPE-II: simplified newborn illness severity and mortality risk scores. J. Pediatr. 138, 92–100 (2001).
Lee, S. K. et al. Transport Risk Index of Physiologic Stability, version II (TRIPS-II): a simple and practical neonatal illness severity score. Am. J. Perinatol. 30, 395–400 (2013).
Parry, G., Tucker, J. & Tarnow-Mordi, W. CRIB II: an update of the clinical risk index for babies score. Lancet 361, 1789–1791 (2003).
Rosenberg, R. E. et al. Simplified age-weight mortality risk classification for very low birth weight infants in low-resource settings. J. Pediatr. 153, 519–524 (2008).
Medvedev, M. M. et al. Development and validation of a simplified score to predict neonatal mortality risk among neonates weighing 2000 g or less (NMR-2000): an analysis using data from the UK and The Gambia. Lancet Child Adolesc. Health 4, 299–311 (2020).
Alkandari, O. et al. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit. Care 15, R146 (2011).
Basu, R. K. et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 85, 659–667 (2014).
Sethi, S. K. et al. Fluid overload and renal angina index at admission are associated with worse outcomes in critically ill children. Front. Pediatr. 6, 118 (2018).
Stoops, C. et al. Baby NINJA (nephrotoxic injury negated by just-in-time action): reduction of nephrotoxic medication-associated acute kidney injury in the neonatal intensive care unit. J. Pediatr. 215, e226 (2019).
Raina, R. et al. Treatment of AKI in developing and developed countries: an international survey of pediatric dialysis modalities. PLoS ONE 12, e0178233 (2017).
Mehta, H. B., Mehta, V., Girman, C. J., Adhikari, D. & Johnson, M. L. Regression coefficient-based scoring system should be used to assign weights to the risk index. J. Clin. Epidemiol. 79, 22–28 (2016).
Dorling, J. S., Field, D. J. & Manktelow, B. Neonatal disease severity scoring systems. Arch. Dis. Child. Fetal Neonatal Ed. 90, F11–F16 (2005).
Richardson, D. K., Gray, J. E., McCormick, M. C., Workman, K. & Goldmann, D. A. Score for neonatal acute physiology: a physiologic severity index for neonatal intensive care. Pediatrics 91, 617–623 (1993).
The International Neonatal Network. The CRIB (clinical risk index for babies) score: a tool for assessing initial neonatal risk and comparing performance of neonatal intensive care units. Lancet 342, 193–198 (1993).
Karlowicz, M. G. & Adelman, R. D. Nonoliguric and oliguric acute renal failure in asphyxiated term neonates. Pediatr. Nephrol. 9, 718–722 (1995).
Gupta, B. D., Sharma, P., Bagla, J., Parakh, M. & Soni, J. P. Renal failure in asphyxiated neonates. Indian Pediatr. 42, 928–934 (2005).
Gluckman, P. D. et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicenter randomised trial. Lancet 365, 663–670 (2005).
Perico, N., Askenazi, D., Cortinovis, M. & Remuzzi, G. Maternal and environmental risk factors for neonatal AKI and its long-term consequences. Nat. Rev. Nephrol. 14, 688–703 (2018).
Harer, M. W. et al. Improving the quality of neonatal acute kidney injury care: neonatal-specific response to the 22nd Acute Disease Quality Initiative (ADQI) conference. J. Perinatol. https://doi.org/10.1038/s41372-020-00810-z (2020).
Marlow, N. et al. Perinatal outcomes for extremely preterm babies in relation to place of birth in England: the EPICure 2 study. Arch. Dis. Child. Fetal Neonatal Ed. 99, F181–F188 (2014).
Investigators of the Delhi Neonatal Infection Study (DeNIS) Collaboration. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: a cohort study. Lancet Glob. Health 4, e752–e760 (2016).
Bezerra, C. T., Vaz Cunha, L. C. & Liborio, A. B. Defining reduced urine output in neonatal ICU: importance for mortality and acute kidney injury classification. Nephrol. Dial. Transpl. 28, 901–909 (2013).
Askenazi, D. J. et al. Fluid overload and mortality are associated with acute kidney injury in sick near-term/term neonate. Pediatr. Nephrol. 28, 661–666 (2013).
Lee, C. C. et al. Incidence and outcomes of acute kidney injury in extremely-low-birth-weight infants. PLoS ONE 12, e0187764 (2017).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data and drafting the article or revising it critically for important intellectual content. All authors gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Consent was taken from the parents for inclusion in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Tables 1 and 3 have been revised. In the Discussion section, the information on urine output was changed and Figure 5 was corrected.
Supplementary information
Rights and permissions
About this article
Cite this article
Wazir, S., Sethi, S.K., Agarwal, G. et al. Neonatal acute kidney injury risk stratification score: STARZ study. Pediatr Res 91, 1141–1148 (2022). https://doi.org/10.1038/s41390-021-01573-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01573-9
This article is cited by
-
Use of furosemide in preterm neonates with acute kidney injury is associated with increased mortality: results from the TINKER registry
Pediatric Nephrology (2024)
-
Acute kidney injury during the first week of life: time for an update?
Pediatric Nephrology (2024)
-
Management of Acute Kidney Injury in Critically Ill Children
Indian Journal of Pediatrics (2023)
-
Artificial intelligence in early detection and prediction of pediatric/neonatal acute kidney injury: current status and future directions
Pediatric Nephrology (2023)
-
Validation of the STARZ neonatal acute kidney injury risk stratification score
Pediatric Nephrology (2022)