Abstract
Background
Extremely preterm (EPT) birth is a major risk factor for neurodevelopmental impairments. The aim was to evaluate the predictive value of Prechtl General Movement Assessment (GMA), including the Motor Optimality Score—Revised (MOS-R), at 3 months corrected age (CA) for adverse neurodevelopmental outcome at the age of 12 years.
Methods
The GMA, including the MOS-R, was applied at 3 months CA and outcomes were assessed at 12 years by Touwen’s neurological examination, the Movement Assessment Battery for Children-2, and chart reviews.
Results
Fifty-three infants born EPT (33 boys, mean GA 25 weeks, mean body weight 805 ± 156 g) were included. Forty-two (79%) children participated in the follow-up (mean age 12.3 ± 0.4) and 62% of these had adverse outcomes. The MOS-R differed between groups (p = 0.007). The respective predictive values of GMA, aberrant FMs, and the MOS-R cut-off of 21 for adverse outcomes were positive predictive values (PPVs) of 1.00 and 0.77, negative predictive value of 0.47 and 0.63, sensitivity of 0.31 and 0.77, and specificity of 1.00 and 0.77.
Conclusions
Using the Prechtl GMA, including the MOS-R, at 3 months CA predicted an overall adverse neurodevelopment at 12 years, with a high PPV, specificity, and sensitivity in children born EPT.
Impact
-
The Prechtl GMA, including the MOS-R, can improve early identification of long-term adverse neurodevelopmental outcomes.
-
This is the first study to investigate the predictive value of the MOS-R for neurodevelopmental outcome at mid-school age in children born EPT.
-
Using the GMA, including the MOS-R, is suggested as one important part of the neurological assessment at 3 months CA in children born EPT.
-
Aberrant FMs in combination with a MOS of <21 is an indicator of an increased risk of future adverse neurodevelopment in children born EPT.
Similar content being viewed by others
Background
Extremely preterm (EPT) birth (<28 weeks of gestation) is a major risk factor for brain injuries and neurodevelopmental impairments; hence, early identification is crucial for initiating early targeted intervention.1,2 Sequalae of EPT birth that persist into later childhood include motor, sensory, cognitive, and behavioral impairments.3,4,5,6 The incidence of severe motor impairments, such as cerebral palsy (CP), are relatively low, at approximately 10%. However, the risk of neuropsychiatric disorders, such as autism spectrum disorders (ASDs) and attention deficit hyperactivity disorder (ADHD) are 40–50%.6,7 It is also well known that children born EPT often have other less severe motor difficulties than CP, such as minor neurological disorders (MND), including developmental coordination disorder (DCD), which often have an impact on children’s daily life.3,4 The Prechtl General Movement Assessment (GMA), including the Motor Optimality Score—Revised (MOS-R), is used to identify neurological impairments at an early stage and predict an infant’s later neurodevelopmental outcome.8,9,10,11,12 Between approximately 9 and 20 weeks of age, the GMA focuses on the presence or absence of fidgety movements (FMs) and in the literature it is described as a corner stone assessment for early identification of infants at risk of CP.11 FMs are continuous small movements of moderate speed in all directions. In addition to FMs, infants exhibit a repertoire of other spontaneous movement and behavioral components, which can be evaluated using the MOS-R.8,11,13 The MOS has shown to be associated with later gross and fine motor performance,14 the prognosis, including early type and severity of CP,11,15,16 the risk of MND,13,17 language development,9,18 and cognitive function at school age.19,20,21 Infants born EPT, or with an extremely low birth weight (ELBW) of <1000 g, have also demonstrated greater deviating movement and postural patterns and are more often likely to have aberrant FMs than children born full term.22,23 To our knowledge, only one study has examined the relationship between the GMA and the long-term outcomes in children born EPT and/or ELBW.5 Grunewaldt et al.5 showed that the children who displayed normal FMs but abnormal movement characters, compared to term-born controls, had an increased risk of later functional deficits and brain pathology at 10 years of age.5 However, to our knowledge no studies have presented predictive data of the Prechtl GMA, including the MOS-R, in children born EPT for long-term neurodevelopmental outcomes.
Information on long-term implications of using the GMA, including the MOS-R, in children born EPT, could assist parents with early support and health care personnel to plan for appropriate follow-up strategies and early interventions. The aim of this prospective cohort study was therefore to evaluate the predictive value of the Prechtl GMA, including the MOS-R, at 3 months corrected age for neurodevelopmental outcomes at the age of 12 in children born EPT. We have also compared a detailed assessment of the MOS-R in our cohort with healthy term-born controls. These results will be presented in due course.
Methods
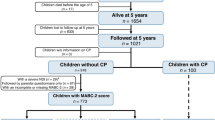
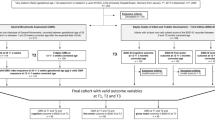
A total of 55 infants born EPT (<27 + 0 weeks) in 2004–2007 in Stockholm, Sweden were included. This cohort has been described in detail earlier by Skiöld et al.24 and the exclusion criteria included children with malformations, chromosome aberrations, malignant disorders, or congenital infections.24 Two recordings were of a poor quality and could not be scored for details, thus recordings of 53 infants (33 boys) born EPT at a gestational age of mean 25.0 ± 1.0 weeks were assessed, according to the Prechtl GMA,8 including the MOS-R.11 The recordings were made at a mean post-term age of 13.5 ± 1.9 weeks (range 11–21 weeks).
At 12 years of age, a follow-up assessment of neurodevelopmental outcome using the simplified version of the neurological examination described by Touwen,25 the Movement Assessment Battery for Children—2 (MABC-2),26 and a review of medical records were performed. The chart reviews based on the International Statistical Classification of Diseases and Related Health Problems—Tenth Revision (ICD-10) were specifically used to identify any potential diagnoses of CP, DCD, epilepsy, ASD, ADHD, blindness, and intellectual disability (ID).
Forty-two of the 53 (79%) of the children (25 boys) participated at follow-up at a mean age of 12.3 ± 0.4 years, as 7 children did not want to participate and 4 could not be reached. Thirty-two of the 42 (81%) children were assessed with MABC-2 and the simplified Touwen examination (children not tested: CP n = 5, blind n = 2, severe autism n = 1).
The clinical characteristics for the follow-up and drop-out groups were retrieved from the medical charts and no differences in the clinical variables or in total MOS (p = 0.49) were found between them (Table 1).
The Prechtl GMA, including the MOS-R
The videos were recorded on a single occasion in line with the GMA procedure.8 FMs and other observed movement and postural patterns in detail were assessed by the MOS-R.11 Assessment of the recordings were performed by the certified GMA expert and one of the inventors of the MOS-R (C.E.), who were blinded to the infants’ perinatal clinical histories and neurodevelopmental outcome.
The MOS-R is based on five subcategories; (i) temporal organization of FMs (maximum 12 points), (ii) observed movement patterns other than FMs (maximum 4 points), (iii) age-adequate movement repertoire (maximum 4 points), (iv) observed postural patterns (maximum 4 points), and (v) movement character (maximum 4 points).8 The assessment is summarized in a MOS with a maximum of 28 (best possible performance) and a minimum of 5 points. A MOS from 25 to 28 is considered optimal, from 20 to 24 as mildly reduced, from 9 to 19 as moderately, and from 5 to 8 as severely reduced.9,11,18 The MOS-R has proved to be valid and reliable when used in several different populations.9,11,13,14,27
Neurological examination according to Touwen and the MABC-2
The simplified Touwen examination is a neurological assessment that examines body posture, movements, tonus, reflexes, coordination, and cranial nerves in a structured way.25 The aim is to detect minor neurological impairments, such as simple and complex MND.25 The MABC-2 is a standardized instrument that evaluates a child’s motor function and has shown good measurement characteristics.26 It is divided into fine motor skills, ball skills, and balance. Raw points are converted and can be compared to standard scores in relation to the child’s age. A score below the 5th percentile indicates major motor difficulties.26 Both the simplified Touwen examination and the MABC-2 are part of the national follow-up program of high-risk infants in Stockholm, Sweden and are regularly used in clinics and research. The assessor had no information about the GMA and MOS-R results.
Statistics
Data were analyzed using SPSS Statistics, version 26.0 (IBM Corp, Armonk, NY). A significance level of 0.05 (two tailed) was used. The subjects were divided into two groups, based on the assessments at 12 years of age and the chart reviews (diagnoses according to ICD-10). These were normal outcome or adverse outcome, including CP, DCD (MABC-2 ≤5th percentile or a diagnose according to ICD-10), epilepsy, ASD, ADHD, blindness, ID, and complex MND.
Differences in subcategories and items of the MOS-R (categorical data) between the groups were analyzed using Pearson chi-square test or Fisher’s exact test as appropriate. Student’s t test and Mann–Whitney U test were used, as appropriate, to identify differences in continuous data between the groups. A binary logistic regression model, with adverse outcome as dependent variable and total MOS as the independent variable, was fitted for the 42 children born EPT who were followed up at 12 years of age. The estimated probabilities were used to fit a receiver operating curve (ROC) and calculate sensitivities and specificities. An optimal cut-off probability was calculated by finding the largest Youden’s Index, namely, sensitivity plus specificity minus 1. The total MOS corresponded to the optimal cut-off probability and was chosen as the optimal total MOS cut-off for predicting adverse outcome. The ROC analysis indicated that this was 21. An odds ratio (OR) and 95% confidence interval (95% CI) was calculated to estimate the risk of having an adverse outcome at 12 years in the groups with a MOS of <21 points and ≥21 points. Calculation of sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were determined to evaluate the accuracy of the GMA (aberrant FMs), including the MOS-R (cut-off of 21 points), for prediction of adverse outcome at 12 years of age.
Results
Neurodevelopmental outcomes at 12 years in correlation to early motor performance
Twenty-six of the 42 children (62%) showed adverse neurodevelopmental outcomes at 12 years. There were no difference between the normal and adverse outcome groups with regard to gestational age or sex, but children with adverse outcomes had significantly lower body weights (p < 0.05; Table 2).
Of the 42 children, 11 (26%) had ASD, 6 (14%) ADHD, 15 (44%) DCD (8 children with CP, blindness, or severe autism excluded), 3 (7%) complex MND, 2 (5%) were blind, 2 (5%) had an ID, and 1 (2%) had epilepsy (Fig. 1). Nineteen of the 26 (73%) of the children had more than one single diagnosis. We found that 5 (12 %) children had developed CP: 1 had absent FMs (bilateral CP) and the other 4 had normal FMs (unilateral CP). All children with CP had a functional level of Gross Motor Function Classification System (GMFCS) I (mildest form). There were 16 (38%) children with normal neurodevelopment at 12 years.
The MOS-R
There was a significant difference in total MOS at 3 months corrected age between the normal and adverse outcome group at 12 years of age (p = 0.007), showing that children with an adverse outcome had a lower total MOS (median score of 17.5) than children with normal outcome (median score = 21.0). The total MOS was mildly reduced for one child with CP, moderately reduced for three, and severely reduced for one. There was no significant difference (p = 0.128) in total MOS scores between those with and without DCD.
All 16 children with normal outcome at 12 years had normal FMs at 3 months corrected age, which significantly differed from the adverse outcome group (p = 0.005) where 5 were scored as abnormal FMs and 3 as absent FMs. Children with adverse outcomes also showed a significantly reduced age-adequacy of movement repertoire compared to the other group (p = 0.05). Figure 1 shows how the MOS-R scores were associated with outcomes, and Table 3 presents details of the MOS-R subcategories in relation to normal and adverse outcomes.
Predictive value of the GMA, including the MOS-R, for outcomes at 12 years of age
The odds of having an adverse neurodevelopmental outcome was 5.6 (95% CI 1.4–21.7) times higher in the group with a total MOS <21 than the group with ≥21 points. Other predictive values are presented in Table 4.
Discussion
The aim of the present study was to evaluate the predictive value of Prechtl GMA, including the MOS-R, for neurodevelopmental outcomes at 12 years of age in children born EPT. In summary, our results show that abnormal or absent FMs at 3 months corrected age predicted an overall adverse neurodevelopment at 12 years of age with a high PPV and specificity; however, the sensitivity to detect adverse outcome per se is low. On the other hand, when assessing the MOS-R, i.e., FMs complemented with the repertoire of other spontaneous movement and behavioral components, the sensitivity was substantially increased.
Neurodevelopmental outcome at 12 years and early motor performance
It is well known that children born EPT have an increased risk of adverse neurodevelopmental outcomes. We also know that children with mild motor impairments often experience co-morbidities and delays in cognitive, communications skills, behavioral, and perceptual domains.6 Almost two-thirds (62%) of our cohort had some kind of neurological impairment at 12 years of age. To our knowledge, this was the first study to assess infants with such a low gestational age and birth weight, as our EPT group, and measure their outcomes at 12 years of age. Part of the cohort used in this study has been evaluated before by Skiöld et al.24 They investigated the GMA in relation to neurological outcome at the age of 30 months corrected age and magnetic resonance imaging (MRI) findings at term-equivalent age. In their study, GMA were evaluated according to another GMA approach described by Hadders-Algra,28 which makes comparison with our results difficult.
The follow-up rate of the present study was 79%, with no clinical differences between the follow-up and drop-out groups. This indicated a low risk of selection bias. However, we did note that children with adverse outcomes had a significantly lower birth weight than those with normal outcomes. This was in line with previous findings which showed that children born small-for-gestational age had an increased risk of developmental deficits.29 The most common diagnoses found at 12 years of age in our study were DCD (36%), ASD (26%), and ADHD (14%) and several combined diagnoses were common (73%). No group comparisons could be performed due to the low number of participants in the other specific diagnose groups.
Children included in the present study were also part of a sub-cohort from the Swedish national population-based study EXPRESS.30 Hafstrom et al.31 reported that 10.5% of the EXPRESS population, which comprised 467 eligible children alive at 1 year of age, had a life-time diagnosis of CP and 76% were ambulatory.31 A similar prevalence of CP (12%) was found in our cohort.
Children born EPT with MND often show signs of DCD4 and this was the case for all three children with complex MND in our study. Previous studies on early motor performance and MND have shown that it is more common with a jerky and monotonous movement character in children who later develop MND. Interestingly, in our cohort, all children were scored as having an abnormal movement character, either jerky and/or monotonous, regardless of whether they had MND or DCD. We also found that 4/15 children with DCD also had aberrant FMs: 2 absent and 2 abnormal. Yuge et al.32 studied 41 children with DCD at the age of 5 years, including 11 born preterm, and reported that 1 in 3 children had absent FMs at 3–5 months of age. The present study found no significant difference (p = 0.128) in total MOS-R scores between those with or without DCD. To our knowledge, no other studies on children born EPT with DCD have been published in relation to the Prechtl GMA and there is a need for more studies in this field.
Almost half of the 15 children later diagnosed with ASD and/or ADHD had aberrant FMs: 2 absent and 5 abnormal. Up to now, there have only been a few studies that have evaluated early motor behavior and its association with ASD.33 One pilot study by Phagava et al.34 evaluated 20 children with ASD during FM age and found similar numbers to ours regarding abnormal FMs. They showed that 21% had absent FMs and 29% had abnormal FMs, compared to the 3 and 27%, respectively, reported in our study. Abnormal GMs appear to be common in infants who are later diagnosed with ASD or related neurodevelopmental disorders.33 However, since early abnormalities, as in abnormal GMs, occur in infants with other impairments such as in CP or MND, e.g., this is not a specific sign for ASD. A review by Einspieler et al.33 suggested that more studies using GMA including the detailed assessment of MOS-R in children with ASD are needed in the future.33 More specific results and discussions about the relationship between ASD and/or ADHD and early infant motor performance will be presented in due course.
The presence of abnormal, exaggerated FMs in blind infants has been described by Prechtl et al.35 The 14 totally blind term and preterm infants in their cohort demonstrated an exaggerated type of FMs and a clear delay in head control around 2 months’ post-term. Later, postural control was characterized by a prolonged period of ataxic features. Those findings were reflected by the present study, as the 2 blind children had abnormal FMs, abnormal movement and postural patterns, and movement character as well as a reduced repertoire at 3 months of corrected age.
It is well known that children born EPT often have cognitive impairments.6 In our cohort, only 2 children had a clinical diagnose of ID at 12 years. One of them had normal FMs and the other one had abnormal FMs. They both displayed abnormal movement and postural patterns and movement character, as well as a reduced movement repertoire at 3 months corrected age. However, these 2 children had a number of co-morbidities, such as ASD, ADHD, blindness and epilepsy, which made it impossible to conclude whether their altered motor performance was a specific sign of ID.
All of the 16 (38%) children in the present study who had normal neurodevelopmental outcomes at 12 years had normal FMs at 3 months of corrected age. This was significantly different from the group with adverse outcome, as five had abnormal FMs and three had absent FMs. However, it is important to highlight the altered quality of movements and postures in infants born EPT who had developed normally at 12 years of age. All of them had normal FMs, but none displayed an optimal smooth and fluent movement character.
Approximately two-thirds of the normally development group also had abnormal postural patterns and nearly half of them showed a reduced age-adequacy of the movement repertoire for corrected age. Abnormal movement character has previously been described in children born preterm with normal development. Bruggink et al.17 have showed that nearly half of their cohort of infants without CP or complex MND, who showed an abnormal movement character at 2–5 months of age, were later classified as normal. Groen et al.36 reported that 11/15 (73%) children with a normal neurodevelopmental outcome had a jerky movement pattern when they were 2–4 months old.36
However, all the children in our cohort displayed abnormal movement character, which suggests that character alone does not seem to be a good indicator of further development in EPT infants.
The Motor Optimality Score—Revised
When we compared the children with normal and adverse neurodevelopment at 12 years of age, we found significant differences in the total MOS-R scores for FMs and age-adequacy of movement repertoire at 3 months of corrected age. The children with adverse outcomes were more likely to have a reduced MOS-R score, together with aberrant FMs and reduced age-adequacy of the movement repertoire. However, there were no differences between the two groups with regard to observed movement and postural patterns or movement character.
According to earlier studies, a MOS of 25–28 is considered to be optimal and scores ≤24 are indicating a reduced performance.8 Despite the significant difference in total MOS between our groups, the median MOS for the whole group was 20, which means that the majority of the children did not have an optimal score regardless of outcome. A significantly reduced total MOS in infants born EPT in comparison to term-born healthy controls have been reported before.22,37
A low total MOS has also been associated with worse cognitive outcomes in very low birth weight (<1500 g) infants at 10 years of age17 and with adverse neurological outcome in preterm infants at 7–11 years of age. However, this has not been reported for children born EPT.13,17 The optimal cut-off value of a MOS <21 points for predicting adverse outcome was assessed by using a binary logistic regression model fitted for the study. The estimated probabilities were then used to fit a ROC curve. A larger cohort is needed in the future to validate this model.
Predictive value of the GMA, including the MOS-R, for outcomes at 12 years of age
Many of the earlier studies that dealt with the predictive validity of GMs for different aspects of neurodevelopmental outcome focused on less preterm groups, only FMs, and/or outcome assessments at earlier ages.13,17,19,21,32,38,39,40,41,42,43,44 To our knowledge, our study was the first to investigate the predictive value of the GMA, including the MOS-R, for neurodevelopmental outcome at mid-school age in children born EPT. Grunewalt et al.5 studied 31 children born preterm and ELBW with a mean gestational age of 26.1 weeks and a mean birth weight of 773 g. They used early motor performance, assessed with GMA including the parts of the MOS that focuses on FMs and movement character, to find associations with functional outcome measures and MRI results at 10 years. They found that children born with an ELBW without CP, and with normal FMs but an abnormal movement character, showed more functional impairments and brain pathology at 10 years of age, compared to those with smooth and fluent movement character.
Bruggink et al.19 also suggested that an early abnormal movement character was an early predictor for cognition at 7–11 years of age in children born very preterm.19 Other studies have also shown that children born preterm with normal FMs were unlikely to develop CP. However, if they also displayed abnormal movement character there was an increased risk of other neurodevelopmental impairments, such as decreased cognition and language difficulties9,18 and developing MND later in childhood.17 Since all children in our cohort showed abnormal movement characters, this does not seem to be a specific sign in children born EPT.
Our results showed a 100% specificity and PPV for predicting adverse outcome using GMA at the age of 12 when we focused on the presence of aberrant FMs. However, the sensitivity and NPV were lower, at 31 and 47%, respectively, which meant that we missed 69% of the children with an adverse outcome at 12 years when we only looked for aberrant FMs. On the other hand, when included the MOS-R, i.e., FMs complemented with the other spontaneous co-occurring movements, the sensitivity increased substantially, from 31 to 77%.
This highlights the importance of combining assessment methods and are in line with the published guidelines for the early detection of CP.45 The guidelines recommend that high-risk infants are assessed according to the Prechtl GMA and to the Hammersmith Infant Neurological Examination at 3 months corrected age, in combination with term-age brain imaging.
One striking finding of our study, and a possible explanation for the lower sensitivity of aberrant FMs, is that four out of five infants with CP were not identified by just the absence of normal FMs. However, they did show a reduced total MOS of 17–21. The inclusion of the MOS-R could increase the number of infants who face a high risk of CP, but are missed, especially children born EPT with mild CP. It was not possible to specifically analyze the prediction values for the 5 children with CP, as it was only a small number, but we can speculate that the absence of normal FMs in GMs were less sensitive in predicting milder forms of CP. This was the case in all the children in our study, since they all had a functional level of GMFCS I.
This confirms the findings of Einspieler et al.11 and Stoen et al.41 Einspieler et al.11 evaluated early specific markers for ambulation, gross motor function, topography, and the type of CP in a worldwide cohort of 468 infants. They found that 95% of children with CP did not display FMs, but 100% of them had a non-optimal total MOS. They also found a strong correlation between GMFCS level and MOS, where a MOS >14 was most likely to be associated with GMFCS levels I and II.11 A study by Stoen et al.41 of 405 Norwegian high-risk infants, including 46% who were born EPT, showed that the presence of normal FMs was a strong marker for a non-CP outcome at approximately 2 years of age but did not exclude milder CP types.41 They confirmed that 10.4% of their cohort had CP but speculated that a longer follow-up period might have resulted in more cases of mild CP, which could have resulted in poorer performance of GMs.41 In our cohort, 3 of the 5 children with CP were diagnosed before 3 years of age.
Strengths and limitations
Our study had a number of strengths. We focused on a unique group of children born EPT, with a lower gestational age than previously described. There was a high follow-up rate and we measured long-term outcome at a higher age than most studies. In addition, the assessor was blinded to the patients’ clinical histories. An even higher follow-up rate would have strengthened our study.
One limitation was that only 5 children had been diagnosed CP by 12 years of age, which made it impossible to analyze the data for this sub-cohort. Another possibility would have been to analyze the cohort divided into major (CP, blindness, deafness) vs. minor disabilities (ASD, ADHD, DCD, and ID). However, all children with CP in our cohort had the mildest form of CP (GMFCS1) and the cohort would still be too small for a sub-cohort analysis. Larger cohorts are therefore needed to study prediction data in more specific subgroups of children born EPT.
Another possible limitation was that the analyses were based on single GM recording, which meant we could not study the GM trajectory. Kwong et al.23 showed a trend of less aberrant FMs with increasing age. It would have been interesting to evaluate that for this cohort and this should be considered for future studies.
In future studies, neonatal morbidities, such as, e.g., bronchopulmonary dysplasia, severe retinopathy of prematurity and brain injury, cognitive outcomes, and neuropsychiatric disabilities, and their relation to early infant motor development also need to be studied further in this specific population.
Clinical implications
The GMA, including the MOS-R, is a cost-effective and non-intrusive tool, which can be partly performed in home settings, for example, by using a smart-phone app.46 Asking parents to record their children just before a regular 3 months follow-up in the clinic would greatly enrich their clinical assessment. Carrying out a detailed assessment of the MOS-R is more time-consuming than scoring the presence of FMs and requires an assessor more experienced. However, it can make the assessment more precise in this specific group of children born EPT. In combination with other assessment such as brain imaging and neurological assessments, the MOS-R can improve how to identify infants with early abnormal motor performances and increased risk of long-term adverse neurodevelopmental outcomes.
This could also allow for entry to early intervention programs and studies during the period of greatest neuroplasticity and may also help limit unnecessary referrals.
Conclusion
This study evaluated the early motor performance in children born EPT at 3 months corrected age and the association with long-term neurological outcomes at 12 years of age. Almost two-thirds of the children born EPT demonstrated adverse neurodevelopmental outcomes at 12 years. Our findings show that aberrant FMs predicted overall adverse neurodevelopment at 12 years of age in children born EPT with a high PPV and specificity. However, by including the more detailed assessment, the MOS-R, the sensitivity was further enhanced. These findings need to be confirmed in larger cohorts of children born EPT.
Disclosure
The manuscript has not been submitted or published elsewhere, except as an abstract.
References
Morgan, C. et al. Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Dev. Med. Child Neurol. 58, 900–909 (2016).
Spittle, A. J., Orton, J., Anderson, P., Boyd, R. & Doyle, L. Early developmental intervention programs post hospital discharge to prevent motor and cognitive impairments in preterm infants. Cochrane Database Syst. Rev. 1, 1–56 (2015).
Bolk, J., Farooqi, A., Hafström, M., Aden, U. & Serenius, F. Developmental coordination disorder and its association with developmental comorbidities at 6.5 years in apparently healthy children born extremely preterm. JAMA Pediatr. 172, 765–774 (2018).
Broström, L., Vollmer, B., Bolk, J., Eklöf, E. & Ådén, U. Minor neurological dysfunction and associations with motor function, general cognitive abilities, and behaviour in children born extremely preterm. Dev. Med. Child Neurol. 60, 826–832 (2018).
Grunewaldt, K. H. et al. Follow-up at age 10 years in ELBW children - functional outcome, brain morphology and results from motor assessments in infancy. Early Hum. Dev. 90, 571–578 (2014).
Serenius, F. et al. Neurodevelopmental outcomes among extremely preterm infants 6.5 years after active perinatal care in Sweden. JAMA Pediatr. 170, 954–963 (2016).
Padilla, N., Alexandrou, G., Blennow, M., Lagercrantz, H. & Ådén, U. Brain growth gains and losses in extremely preterm infants at term. Cereb. Cortex 25, 1897–1905 (2015).
Einspieler, C., Prechtl, H. F. R., Bos, A. F. & Ferrarri, F. C. G. Prechtl’s Method on the Qualitative Assessment of General Movements in Preterm, Term and Young Infants (MacKeith Press; Cambridge University Press, 2004).
Einspieler, C. et al. GM Zika Working Group. Association of infants exposed to prenatal Zika virus infection with their clinical, neurologic, and developmental status evaluated via the general movement assessment tool. JAMA Netw. Open 2, e187235 (2019).
Einspieler, C. & Prechtl, H. F. R. Prechtl’s assessment of general movements: a diagnostic tool for the functional assessment of the young nervous system. Ment. Retard. Dev. Disabil. Res. Rev. 11, 61–67 (2005).
Einspieler, C. et al. Cerebral palsy: early markers of clinical phenotype and functional outcome. J. Clin. Med. 8, 1616 (2019).
Prechtl, H. F. R. et al. An early marker for neurological deficits after perinatal brain lesions. Lancet 349, 1361–1363 (1997).
Bruggink, J. L. M. et al. Quantitative aspects of the early motor repertoire in preterm infants: do they predict minor neurological dysfunction at school age? Early Hum. Dev. 85, 25–36 (2009).
Zang, F. F. et al. Very low birth weight infants in China: the predictive value of the motor repertoire at 3 to 5 months for the motor performance at 12 months. Early Hum. Dev. 100, 27–32 (2016).
Bruggink, J. L. M. et al. Early motor repertoire is related to level of self-mobility in children with cerebral palsy at school age. Dev. Med. Child Neurol. 51, 878–885 (2009).
Yang, H. et al. Cerebral palsy in children: movements and postures during early infancy, dependent on preterm vs. full term birth. Early Hum. Dev. 88, 837–843 (2012).
Bruggink, J. L. M. et al. The quality of the early motor repertoire in preterm infants predicts minor neurologic dysfunction at school age. J. Pediatr. 153, 32–39 (2008).
Salavati, S. et al. The association between the early motor repertoire and language development in term children born after normal pregnancy. Early Hum. Dev. 111, 30–35 (2017).
Bruggink, J. L. M., Van Braeckel, K. N. & Bos, A. F. The early motor repertoire of children born preterm is associated with intelligence at school age. Pediatrics 125, e1356–e1363 (2010).
Butcher, P. R. et al. The quality of preterm infants’ spontaneous movements: an early indicator of intelligence and behaviour at school age. J. Child Psychol. Psychiatry 50, 920–930 (2009).
Fjørtoft, T. et al. Assessment of motor behaviour in high-risk-infants at 3 months predicts motor and cognitive outcomes in 10 years old children. Early Hum. Dev. 89, 787–793 (2013).
Fjørtoft, T. et al. High prevalence of abnormal motor repertoire at 3 months corrected age in extremely preterm infants. Eur. J. Paediatr. Neurol. 20, 236–242 (2016).
Kwong, A. K. L. et al. Occurrence of and temporal trends in fidgety general movements in infants born extremely preterm/extremely low birthweight and term-born controls. Early Hum. Dev. 135, 11–15 (2019).
Skiöld, B., Eriksson, C., Eliasson, A. C., Ådén, U. & Vollmer, B. General movements and magnetic resonance imaging in the prediction of neuromotor outcome in children born extremely preterm. Early Hum. Dev. 89, 467–472 (2013).
Fily, A. et al. Neurological assessment at five years of age in infants born preterm. Acta Paediatr. 92, 1433–1437 (2003).
Hendersen, S. E. Movement Assessment Battery for Children - 2 2nd edn (Pearson, 2007).
Fjørtoft, T., Einspieler, C., Adde, L. & Strand, L. I. Inter-observer reliability of the “Assessment of Motor Repertoire - 3 to 5 Months” based on video recordings of infants. Early Hum. Dev. 85, 297–302 (2009).
Hadders-Algra, M. General movements: a window for early identification of children at high risk for developmental disorders. J. Pediatr. 145, 12–18 (2004).
Løhaugen, G. C. C. et al. Small for gestational age and intrauterine growth restriction decreases cognitive function in young adults. J. Pediatr. 163, 447–454 (2013).
The Express Group. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 301, 2225–2233 (2009).
Hafström, M. et al. Cerebral palsy in extremely preterm infants. Pediatrics 141, e20171433 (2018).
Yuge, M. et al. Movements and postures of infants aged 3 to 5 months: to what extent is their optimality related to perinatal events and to the neurological outcome? Early Hum. Dev. 87, 231–237 (2011).
Einspieler, C. et al. Highlighting the first 5 months of life: general movements in infants later diagnosed with autism spectrum disorder or Rett Syndrome. Res. Autism Spectr. Disord. 8, 286–291 (2014).
Phagava, H. et al. General movements in infants with autism spectrum disorder. Georgian Med. News 3, 100–105 (2008).
Prechtl, H. F. R., Cioni, G., Einspieler, C., Bos, A. F. & Ferrari, F. Role of vision on early motor development: lessons from the blind. Dev. Med. Child Neurol. 43, 198–201 (2001).
Groen, S. E., de Blécourt, A. C. E., Postema, K. & Hadders-Algra, M. General movements in early infancy predict neuromotor development at 9 to 12 years of age. Dev. Med. Child Neurol. 47, 731–738 (2005).
Sharp, M., Coenen, A. & Amery, N. General movement assessment and motor optimality score in extremely preterm infants. Early Hum. Dev. 124, 38–41 (2018).
Crowle, C., Galea, C., Walker, K., Novak, I. & Badawi, N. Prediction of neurodevelopment at one year of age using the general movements assessment in the neonatal surgical population. Early Hum. Dev. 118, 42–47 (2018).
Soleimani, F., Badv, R. S., Momayezi, A., Biglarian, A. & Marzban, A. General movements as a predictive tool of the neurological outcome in term born infants with hypoxic ischemic encephalopathy. Early Hum. Dev. 91, 479–482 (2015).
Spittle, A. J. et al. General movements in very preterm children and neurodevelopment at 2 and 4 years. Pediatrics 132, e452–e458 (2013).
Støen, R. et al. The Predictive accuracy of the general movement assessment for cerebral palsy: a prospective, observational study of high-risk infants in a clinical follow-up setting. J. Clin. Med. 8, 1790 (2019).
Morgan, C. et al. Sensitivity and specificity of general movements assessment for diagnostic accuracy of detecting cerebral palsy early in an Australian context. J. Paediatr. Child Health 52, 54–59 (2016).
Kwong, A. K. L., Fitzgerald, T. L., Doyle, L. W., Cheong, J. L. Y. & Spittle, A. J. Predictive validity of spontaneous early infant movement for later cerebral palsy: a systematic review. Dev. Med. Child Neurol. 60, 480–489 (2018).
van Dyk, J. et al. Prediction of long-term neurodevelopmental outcome in preterm infants using trajectories of general movement assessments. J. Perinatol. 38, 1398–1406 (2018).
Novak, I. et al. Early, accurate diagnosis and early intervention in cerebral palsy: advances in diagnosis and treatment. JAMA Pediatr. 171, 897–907 (2017).
Kwong, A. K. L. et al. The Baby Moves smartphone app for general movements assessment: engagement amongst extremely preterm and term-born infants in a state-wide geographical study. J. Paediatr. Child Health 55, 548–554 (2019).
Acknowledgements
We are grateful to all children and parents who participated in the study. We would also like to thank Christina Eriksson and Ann-Christine Eliasson as well as our research nurse Lena Swartling Schlinzig for their valuable contribution. M.Ö. was supported by Region Stockholm (clinical research appointment), Sällskapet Barnavård and the Promobilia Foundation Sweden. U.Å. was supported by grants from the Swedish Medical Research Council (grant number 2017-03043), the Stockholm County Council and the Karolinska Institutet, the Swedish Order of Freemasons in Stockholm, the Swedish Brain Foundation (grant number, FO2019-0045), and The Philipsson Foundation. The funders played no role in any aspect of the study or paper.
Author information
Authors and Affiliations
Contributions
M.Ö. and U.Å. conceptualized the study. All authors made substantial contributions of analysis and interpretation of data. M.Ö. drafted the initial manuscript, which was then critically reviewed and revised by all authors. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
C.E. is a co-inventor of the MOS-R and member and certified tutor of the General Movements Trust. The other authors have no conflicts to declare.
Consent statement
The study was approved by The Regional Ethics Committee in Lund and Stockholm (2015/488, 2018/2547). All parents or guardians gave their written consent of participation.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Örtqvist, M., Einspieler, C. & Ådén, U. Early prediction of neurodevelopmental outcomes at 12 years in children born extremely preterm. Pediatr Res 91, 1522–1529 (2022). https://doi.org/10.1038/s41390-021-01564-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01564-w
This article is cited by
-
Using the center of pressure movement analysis in evaluating spontaneous movements in infants: a comparative study with general movements assessment
Italian Journal of Pediatrics (2023)
-
General Movement Assessment in Prediction of Neurodevelopmental Disability and Cerebral Palsy
Indian Pediatrics (2022)