Abstract
Background
The objective of this study was to determine sex-specific differences in inflammatory cytokine responses to red blood cell (RBC) transfusion in preterm infants in the neonatal period and their relationship to later neurocognitive status.
Methods
Infants with a birth weight <1000 g and gestational age 22–29 weeks were enrolled in the Transfusion of Prematures (TOP) trial. The total number of transfusions was used as a marker of transfusion status. Nineteen cytokines and biomarkers were analyzed from 71 infants longitudinally during the neonatal period. Twenty-six infants completed the Bayley Scales of Infant & Toddler Development, 3rd Edition (Bayley-III) at 12 months’ corrected age.
Results
Nine cytokine levels were significantly elevated in proportion to the number of transfusions received. Of those, one cytokine showed a sex-specific finding (p = 0.004): monocyte chemoattractant protein-1, MCP-1, rose substantially in females (8.9% change per additional transfusion), but not in males (−0.8% change). Higher concentrations of MCP-1 exclusively were associated with worse Bayley-III scores: decreased cognitive raw scores (p = 0.0005) and motor scaled scores (p < 0.0001).
Conclusions
This study provides evidence of a sex-specific difference in the inflammatory response to RBC transfusions during neonatal life, with MCP-1 levels rising only in females and inversely correlating with neurocognitive status at 12 months old.
Impact
-
It is important to understand the risk factors for abnormal neurodevelopment in preterm infants, including anemia and RBC transfusion, in order to improve outcomes and provide potential targets for therapy.
-
Our study investigates and provides the first evidence of sex-specific differences in inflammatory cytokine responses to RBC transfusions in preterm infants in the neonatal period, and their relationship to later cognitive outcomes.
-
This study critically suggests that different transfusion thresholds may have a sex-specific effect on neurodevelopment: females have worse cognitive outcomes with increased number of transfusions, while males have worse outcomes with lower number of transfusions.
Similar content being viewed by others
Introduction
Preterm infants are at risk for several long-term morbidities including deficits in the motor, sensory, cognitive, and behavioral domains,1,2,3,4,5,6,7 suggesting that brain development is an area of vital importance in evaluating the medical management of premature infants. One possible etiology of altered brain development that can be managed in the neonatal intensive care unit (NICU) is the degree of neonatal anemia and, more specifically, its management: the red blood cell (RBC) transfusion. RBC transfusion is a common therapy for anemia in preterm infants in the first few weeks of life. Approximately 90% of infants with birth weights <1000 g born in the United States receive at least one RBC transfusion.8,9,10 But RBC transfusions in the preterm infant are not without risk, and the mechanisms by which RBC transfusions affect the developing preterm infant brain are not well understood.11 Therefore, a lack of understanding of the optimal RBC transfusion strategy is a critical barrier to improving these infants’ neurodevelopmental outcome.
Recently, the Neonatal Research Network Transfusion of Prematures (TOP) trial published the findings that the main outcome of death or neurodevelopmental impairment (NDI) [defined as Cognitive Bayley II <85, Cerebral Palsy, or Vision impairment with acuity <20/200, or bilateral hearing deficits requiring amplification/cochlear implant] was not different in extremely low-birth-weight (ELBW) infants randomized to either a low- or high-hemoglobin (Hb) threshold for RBC transfusions.12 Despite the overall negative finding on the primary outcome, it is important to highlight that the majority of the neurodevelopmental outcomes for premature birth are milder in severity than the level of NDI utilized in this trial. Deficits in executive function, visuomotor skills, intelligence quotients, language, and learning have been shown to occur in 50–70% of children born with ELBW. These children are also estimated to be four to six times more likely to exhibit attention-deficit symptoms than their term-born peers.13,14,15 Although clearly less severe than the NDI used as a primary outcome measure for the TOP trial, these less severe deficits can have lifelong, significant impacts on functioning, and should not be overlooked. Therefore, it is imperative to continue to investigate these neurodevelopmental outcomes in the context of prematurity and transfusion.
Perinatal inflammation due to infection is an established precursor to many later neurodevelopmental deficits in the preterm infant.16 Studies have consistently shown that perinatal infection and/or increased concentrations of inflammation-related proteins are associated with white matter damage in the brain.17,18,19 In preclinical models, increased pro-inflammatory cytokine concentrations lead to alterations in the blood–brain barrier, release of reactive oxygen species, and vascular dysfunction and may even sensitize the brain to secondary excitotoxic insults.20,21 In human neonates, pro-inflammatory cytokine production and endothelial activation after RBC transfusion, often referred to as transfusion-related immunomodulation, have been proposed to underlie several neonatal morbidities.22,23 It remains unclear if these transfusion-induced alterations in cytokine concentrations contribute to and exacerbate later adverse neurodevelopmental morbidities.
One vital aspect in the study of neurodevelopment of the preterm infant is sex-specific differences in outcomes. Our group recently reported on sex-specific effects of prematurity on infant brain morphology. We found that white matter was more vulnerable in females, while gray matter was more vulnerable in males.24 We have also reported on long-term outcomes of differential Transfusion of Prematures (TOP) infants, with females showing worse outcomes if transfused to maintain higher Hb levels, and cerebral white matter being the most affected region.25 It is unknown what role inflammatory responses play in sex-specific outcomes of prematurity. Two potential factors could be that white matter in females is more vulnerable to inflammation, or that inflammatory processes are more robust in females.
Although several studies have shown increased serum cytokine concentrations in preterm infants after RBC transfusion,26,27,28 no study has evaluated whether these changes occur in a sex-specific fashion. The purpose of this study was to first compare inflammatory cytokine responses to RBC transfusion in preterm male and female neonates, and secondly, examine the relationship between cytokine levels early in life and later cognitive status at 12 months corrected age.
Methods
Participants
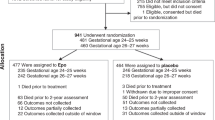
The current study was carried out at the NICU at the University of Iowa Stead Family Children’s Hospital. Infants were enrolled in the multicenter National Institute of Child Health and Human Development Neonatal Research Network Transfusion of Prematures, or TOP trial, a large two-armed randomized clinical trial comparing transfusion guidelines based on higher or lower Hb thresholds in ELBW infants.29 Infants fulfilled the following inclusion criteria: (a) birth weight <1000 g and gestational age (GA) ≥22 weeks but <29 completed weeks, and (b) <48 h of age. Exclusion criteria included (a) considered nonviable by attending neonatologist, (b) cyanotic congenital heart disease, (c) parental opposition to transfusion of blood, (d) parents with hemoglobinopathy or congenital anemia, (e) fetal transfusion, (f) twin-to-twin transfusion syndrome, (g) isoimmune hemolytic disease, (h) severe acute hemorrhage, acute shock, sepsis with coagulopathy, or need for perioperative transfusion, (i) prior blood transfusion on clinical grounds beyond the first 6 h of life. From the 99 eligible infants, those with NEC or sepsis were excluded (n = 25) as these inflammatory processes could potentially confound the results. Two infants received no RBC transfusions, and one infant had no cytokines drawn after transfusion (see Fig. 1 for flow diagram of participants).
Transfusion, blood banking, and sampling
A standard transfusion volume of 15 ml/kg was given according to the criteria determined by the TOP trial protocol.29 All RBCs transfused were tested and screened according to the policies of the hospital’s blood bank. Blood samples used in this study were collected for clinically indicated analyses only, by venous, arterial, or prewarmed heel capillary sampling, or indwelling umbilical catheters if present. Clinically indicated blood samples were obtained according to NICU policy at least weekly for the first month, and were not timed in any prescribed way relative to the blood transfusions. Therefore, the risks of phlebotomy were minimized by coordinating phlebotomy for research purposes with phlebotomy for clinical purposes. Clinical blood samples were stored at 4 °C and processed (plasma was separated) within 4 h of sample collection time. Plasma samples were subsequently stored at −80 °C until analysis. Secondary outcomes of the TOP trial, which included the number of transfusions and the number of donor exposures by RBC donors or other blood product, have been previously reported for infant participants in this study; there was no statistical difference in the number of donor exposures between infants in the high- and low-Hb threshold groups in the TOP trial.12
All of the parents in this study agreed to have their infants’ scavenged blood assessed for cytokines and biomarkers. Samples for Hb and cytokine levels were obtained longitudinally throughout the NICU stay. Adequate blood samples (all left over from clinical samples) were obtained for all infants. Hb obtained directly prior to each transfusion—termed the pre-transfusion Hb (ptHb)—was used as a marker of the degree of anemia prior to transfusion in each infant. The cumulative number of transfusions up to the point of cytokine measurement was used in statistical analyses.
Three Meso Scale Diagnostic, LLC© Multiplex Kits were used for all cytokine measurements: V-PLEX Plus Pro-inflammatory Panel 1 Human Kit/Base Catalog#K15049G, V-PLEX Plus Vascular Injury Panel 2 Human Kit/Base Catalog#K15198G, and U-PLEX Biomarker Group 1 (hu) Assays/Base Catalog#K15067L. These kits made it possible to measure multiple cytokines using very small plasma samples (~25 μL). The following 19 cytokines and endothelial markers were analyzed in the infant samples: interleukin-1 beta (IL-1β), interleukin-2 (IL-2), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), interleukin-12 (IL-12), interleukin-13 (IL-13), interleukin-17 (IL-17), interleukin-18 (IL-18), interferon gamma (IFN-γ)-induced protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), IFN-γ, tumor necrosis factor-alpha (TNF-α), tumor necrosis factor-beta (TNF-β), soluble intercellular adhesion molecule 1 (sICAM-1), soluble vascular cell adhesion molecule 1 (sVCAM-1), vascular endothelial growth factor (VEGF), and thrombopoietin (TPO).
Statistical analysis
The first analysis evaluated the percentage change in cytokines directly after transfusion. The second analysis evaluated whether sex differentially predicted changes in cytokines. All cytokine measures showed skewed distributions and were log-transformed for analysis. A single cytokine determination was the unit of analysis. We employed repeated-measures regression analysis with the cumulative number of RBC transfusions as the primary independent variable, with adjustment for sex, birth weight, GA at birth, day of age, and ptHb within 24 h preceding the most recent transfusion before the cytokine determination. The model allowed for interactions of sex with the independent variable (cumulative transfusions) and with the latter three covariates. We used a spatial power covariance structure to account for within-subject clustering, i.e., correlation between two data points decreasing geometrically with the number of days’ separation. To reduce the influence of extreme cytokine values, we applied an iterative outlier-deletion algorithm, equivalent to robust regression with M-estimation using the Talworth weighting function. To compensate for multiple comparisons, we applied the Holm step-down procedure to calculate adjusted p values and limit the familywise Type I error rate to 5% (probability of at least one false inference among the cytokines being evaluated).30 We performed the adjustment separately for each covariate and interaction effect. We also calculated the false discovery rate (probability of at least one null effect occurring among those declared statistically significant).31
The Bayley Scales of Infant & Toddler Development, 3rd Edition (Bayley-III) were used to evaluate the relationship between cytokines early in life and later cognitive status. Infants in the current study returned for a follow-up visit at ~12 months’ corrected age for Bayley-III assessments of cognitive, motor, and language function. Serum cytokine concentrations during NICU stay were directly compared with Bayley-III scores at 12 months. Linear regression models were used to evaluate the association of mean log levels of the three cytokines which showed a sex-specific finding, in addition to the one additional cytokine that showed a trend in males, with Bayley-III scores at 12 months old. In order to reduce the influence of outliers in this small sample, as well as account for wide leverage points in the data, robust linear regression models with MM-estimations were used in these analyses. All analyses controlled for gestational age, birth weight, number of transfusions, and sex.
Results
Table 1 shows the demographics of the 71 infant participants. There were no significant differences between males and females in gestational age, birth weight, number of transfusions, or chronological or corrected age at follow-up visit. For the 19 cytokine analyses, the number of samples per infant ranged between 5.2 and 11.0 (median 10.5), for a total sample number ranging between 301 and 775 per cytokine (median 746).
Table 2 shows the percent change in serum cytokine concentrations per additional transfusion, for all infants. Eight cytokines had significantly changed serum concentrations associated with increasing number of transfusions: IL-8, TNF-β, IL-6, IP-10, IL-18, TPO, MCP-1, and IFN-γ, in addition to the endothelial marker sICAM-1. All of these cytokines increased after transfusion, with IL-6 by >10% per transfusion in both sexes. No cytokine decreased after transfusion.
Three cytokines showed a significant sex-specific finding: MCP-1, IP-10, and IL-6 (see Table 3). All three cytokines showed a significantly elevated response after transfusion in females only, but not males. Only one cytokine (MCP-1) survived Holm adjustment for multiple comparisons, with females having higher levels of MCP-1 (8.9% change per additional transfusion) as compared to males (−0.8% change). Figure 2 illustrates these regression models of MCP-1 by cumulative transfusions, as separated by sex. Of all nine cytokines, only one cytokine (TNF-β) showed a higher response in males as compared to females after transfusion, with males having a 10% increase in levels after transfusion (p < 0.0001) and females having a 5.8% increase after transfusion (p = 0.006). However, the sex interaction did not reach significance in this sample.
Of the three cytokines that showed a sex-specific increase after transfusion in females only, MCP-1 showed strongly significant negative relationships with multiple Bayley-III measures (see Table 4). Higher concentrations of MCP-1 were associated with decreased cognitive raw scores (B = −15.24, SE 3.45, p = 0.0005), decreased language scaled scores (B = −13.00, SE 4.43, p = 0.011), and decreased motor scaled scores (B = −31.69, SE = 3.35, p < 0.0001). Figure 3a shows the negative association of MCP-1 with Bayley-III cognitive scores. There were no significant associations of IP-10 and IL-6 with Bayley-III scores at 12 months. TNF-β, which was the only cytokine that was higher in males than females after transfusion, showed a strong positive association with multiple Bayley-III measures, including cognitive raw scores (B = 11.24, SE = 5.25, p = 0.049) and receptive communication scores (B = 6.22, SE = 2.14, p = 0.011). That is, the higher the TNF-β concentration, the higher the Bayley-III cognitive and receptive communication scores in these infants. Figure 3b shows the positive association of TNF-β levels with Bayley-III cognitive scores.
a One cytokine, MCP-1, showed a strong negative correlation with Bayley-III: the higher the MCP-1 concentrations, the lower the Bayley-III cognitive score (beta coefficient = −15.24, standard error = 3.45, p = 0.0005). b The single cytokine elevated in males only, TNF-β, was associated with higher Bayley-III cognitive scores (beta coefficient = 11.24, standard error = 5.25, p = 0.049). Sex: blue diamond, males; red diamond, females.
Discussion
The current study demonstrates that several pro-inflammatory cytokines are elevated in the plasma in response to RBC transfusions in preterm infants randomized at birth to a higher or lower Hb threshold for RBC transfusion, confirming the results of several previous studies.26,27,28 This study provides the first evidence of a sex-specific difference in the neonatal response to RBC transfusion with a rise in MCP-1, IL-6, and IP-10, all of which were limited to females. Further, MCP-1 was uniquely inversely associated with Bayley-III cognitive, language, and motor scores at 12 months in these preterm infants.
Pro-inflammatory cytokine production and endothelial activation after RBC transfusion, often referred to as transfusion-related immunomodulation, has been proposed to underlie several neonatal morbidities.22,23 Chemokines are small cytokines that act as chemoattractants for leukocyte migration. Recent studies indicate that chemokines and their receptors are also produced by brain cells, and are involved in various neurological disorders,32 supporting the notion that perinatal inflammation is a potential precursor for later neurodevelopmental abnormalities. Elevated MCP-1 levels have been shown to be associated with brain injury, in premature infants33 and after traumatic brain injuries.34,35 This study provides evidence that one chemokine, MCP-1, may well be involved in the pathophysiology that leads to the poor developmental outcomes for females that receive high-Hb threshold RBC transfusions, as previously reported in a long-term outcome study.25 Previous studies have also demonstrated other cytokines of interest in brain injury, including findings of higher concentrations of IL-1β, IL-6, TNF-α, and IL-8 in neonates with encephalopathy associated with later abnormal neurodevelopmental outcomes,36 as well as a meta-analysis demonstrating that IL-6 genetic polymorphisms are associated with the development of cerebral palsy.37 Maternal elevations of IL-6 are also associated with larger amygdala volumes in neonates, and subsequently, lower impulse control at 24 months of age.38 Finally, increased IP-10 and IL-6 amniotic fluid concentrations have been associated with chronic and acute chorioamnionitis, respectively, a known risk factor for the development of cystic periventricular leukomalacia and cerebral palsy.39,40 However, in the current study, there were no significant associations of IL-6 and IP-10 with Bayley-III cognitive scores. Further investigations are needed to elucidate the role of these cytokines in brain development of female preterm infants.
Recently, the Neonatal Research Network TOP trial published the findings that the main outcome of death or NDI [defined as Cognitive Bayley II < 85, cerebral palsy, or vision impairment with acuity <20/200, or bilateral hearing deficits requiring amplification/cochlear implant] was not significantly different in the randomized trial in which very low-birth-weight infants were assigned to either a low- or high-Hb threshold for RBC transfusions.12 These TOP trial primary outcome results have been reproduced by another similar clinical trial, the Effects of Transfusion Thresholds on Neurocognitive Outcomes of Extremely Low-Birth-Weight Infants (ETTNO) trial, which utilized similar primary outcome criteria as the TOP trial.41 Despite the overall negative findings on the primary neurodevelopmental outcome measures in these trials, it is important to emphasize that both the ETTNO and TOP clinical trials were designed to determine optimal clinical guidelines and the safe utilization of RBC transfusions in the neonatal period; that is, does a low-Hb or higher Hb threshold strategy in ELBW infants improve survival or gross neurodevelopmental outcome at 24 months of corrected age? However, the majority of the neurodevelopmental adverse outcomes for premature birth are “high prevalence, low severity” cognitive dysfunctions, such as learning disabilities, low average to borderline intelligence quotients, academic underachievement, attention-deficit hyperactivity disorder, and neuropsychological deficits and behavioral concerns.13 While these deficits are milder in severity, they are estimated to occur in up to 50–70% of children born with ELBW and many persist into adolescence and adulthood. Although clearly less severe than the NDI used as a primary outcome measure for the TOP trial, the impact that these less severe deficits can have lifelong should not be overlooked or underestimated. The current study was uniquely designed to highlight these subtle, more specific brain developmental outcomes these infants remain at risk for, by using continuous longitudinal markers of inflammatory markers very early in life during a critical time point in development and their relationship to later cognition, as opposed to discrete two-group comparisons in brain function between two different transfusion strategies. There are implications for these possible mechanisms of early injury to ongoing, long-term brain development and later function in these infants. As a direct correlate to potential RBC transfusion effects on the brain, it has been shown that early iron deficiency anemia (i.e., late prenatal and neonatal) leads to various long-term neurobehavioral abnormalities.42,43 For example, children with iron deficiency or iron deficiency anemia in infancy perform worse on specific cognitive functions such as spatial memory, selective attention, and executive function tasks, specifically ones requiring inhibition and planning. They show slower processing speeds, behavioral deficits, and disrupted sleep–wake rhythms, often in the context of normal IQs. Moreover, many of these deficits persist into adulthood.44 Therefore, it is imperative to continue to investigate these subtler neurodevelopmental outcomes in the context of prematurity and RBC transfusion.
In the current study, although most cytokines rose after transfusion for both males and females, there was no significant elevation in cytokines based on cumulative transfusions observed for only male preterm infants as compared to females. However, differential transfusion may still affect outcomes in males. It is important to note that TNF-β was the single cytokine that was descriptively higher in males than females after transfusion. This cytokine is a member of the TNF superfamily (TNFSF), which plays a pivotal role in adaptive and innate immunity. Pro-apoptotic TNFSF members are essential for cytotoxic effector cell function in apoptosis, immune surveillance and tumor regression, and protection from viral and bacterial infections through activation of signaling pathways including TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2).45,46,47 Further, almost all the ligands for TNFSF are produced by hematopoietic cells, and are essential in interactions regulating their proliferation. More specifically, TNF-β, also known as lymphotoxin alpha, is involved in organogenesis of secondary lymphoid tissues.48 Finally, TNFR2 expression and activation in astrocytes has been shown to be important for the proliferation of oligodendrocyte progenitor cells after demyelination.46,49 Therefore, increased levels of this signaling molecule in preterm infants after transfusion may potentially be a critical protective mechanism to maturational insults to the developing neonatal brain. The findings here support this by showing greater levels of TNF-β associated with increased cognitive scores at 12 months’ corrected age.
The current findings and previous research suggest that neurodevelopmental outcomes associated with different transfusion thresholds may have a sex-specific effect, where females have worse outcomes with high-Hb thresholds for RBC transfusion, while males have worse outcomes with low-Hb thresholds, as previously documented by our group in multiple long-term brain structure and function outcome studies in children who previously had higher or lower Hb thresholds for RBC transfusion.25,50,51 In the context of the current analysis, we hypothesize that for females, a higher Hb concentration threshold for RBC transfusion is associated with more transfusions and higher MCP-1 levels, a possible inflammatory response that is detrimental to brain development; for males, lower Hb thresholds for transfusion lead to less transfusions and a relative reduction of TNF-β levels, and presumably, more tissue hypoxia.52,53 Given that TNF-β is beneficial to brain development, a relative reduction in TNF-β levels would manifest as poorer developmental outcomes. Although other factors are likely involved, the sex-specific roles of cytokines and their effect on later cognitive status as seen in the current study are directly in line with the previous sex-specific brain outcomes of higher or lower thresholds for RBC transfusion. Therefore, while this study may be considered associative and hypothesis-generating, it provides the first direct evidence that brain status may be due to a differential mounted inflammatory response between sexes. However, it remains to be determined whether this significant factor affecting early brain development in these infants is the primary pathogenesis to injury, or whether it acts in concert with the potential additional differences between sexes in inherent vulnerability of the brain itself during this critical time of development.
It is important to recognize the limitations of the current study. A significant limitation of the neurocognitive outcome investigations is that the association between Bayley-III measures and cytokine levels could not be determined in the entire sample, as there were only 26 infants with 12-month follow-up Bayley-III assessment and cytokine levels. Therefore, this association is based on a 37% follow-up rate at 12 months. As such, there is the potential for selection bias introduced by this low follow-up rate. It may also be argued that the neurocognitive deficits would be generalizable to all infants with elevated MCP-1 levels, and not simply the female infants. A statistical model that included an interaction term of cytokine by sex would add weight to the hypothesis that the association between Bayley score and cytokine (MCP-1) is sex-specific to females. Conversely, it should be noted that there were no significant differences between participants and nonparticipants in all demographic characteristics, including gestational age, birth weight, number of transfusions received, and ptHb. Of all 19 cytokines, participants showed lower levels of TNF-β and higher levels of IL-17. However, even given the lower levels of TNF-β in participants, increases in this cytokine were demonstrated in association with cumulative transfusions in this study (and, conversely, no associations were shown with IL-17). We are also unable to answer the question of whether or not anemia itself causes inflammation, as most extremely low-birth-weight infants will receive at least one RBC transfusion during their neonatal intensive care hospital stay. However, recent studies using mouse models of phlebotomy-induced anemia suggest that increasing severity of anemia in the preterm infant may correlate with levels of IFN-γ, a key pro-inflammatory cytokine that may predispose an infant’s to necrotizing enterocolitis, another comorbid condition in preterm infants.53 Additionally, the samples used for cytokine measurements used were obtained from scavenged blood from clinically drawn samples. Therefore, time after transfusion varied based on clinical practices for each of these blood draws. However, the purpose of this study was to determine the mounting inflammatory response in preterm infants by using increased number of transfusions as an independent predictor. While the elevations in cytokine levels may be multifactorial, using the number of transfusions and averaged cytokine levels throughout NICU stay may provide better evidence that the increased cytokine levels observed are a direct and sustained result of the transfusions, and not a transient response to another biologic process in the infant. In much the same way, a strength of this study is that outcomes were determined using statistical models that utilized an independent predictor accounting for a continuous measure of ptHb, rather than simple discrete group differences (i.e., high-Hb or low-Hb thresholds).
Finally, this study investigated the inflammatory response (as measured by cytokine plasma level) to differential transfusion by sex in preterm infants in the neonatal period, supporting the potential that these immunomodulatory effects may be involved in the pathogenesis of morbidities seen in this population. This study was not designed to determine the incidence of these morbidities in association with serum cytokine concentrations. Therefore, future investigations in larger cohort of infants will be necessary to truly elucidate the direct associations of inflammatory biomarkers with adverse outcomes, both in the neonatal period and later in development.
References
Saigal, S. & Doyle, L. W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371, 261–269 (2008).
Orchinik, L. J. et al. Cognitive outcomes for extremely preterm/extremely low birth weight children in kindergarten. J. Int. Neuropsychol. Soc. 17, 1067–1079 (2011).
Arpino, C. et al. Preterm birth and neurodevelopmental outcome: a review. Childs Nerv. Syst. 26, 1139–1149 (2010).
Aarnoudse-Moens, C. S., Weisglas-Kuperus, N., van Goudoever, J. B. & Oosterlaan, J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 124, 717–728 (2009).
Allen, M. C. Neurodevelopmental outcomes of preterm infants. Curr. Opin. Neurol. 21, 123–128 (2008).
Anderson, P. & Doyle, L. W. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA 289, 3264–3272 (2003).
Bhutta, A. T. et al. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA 288, 728–737 (2002).
Maier, R. F. et al. Changing practices of red blood cell transfusions in infants with birth weights less than 1000 g. J. Pediatr. 136, 220–224 (2000).
Strauss, R. G. Transfusion therapy in neonates. Am. J. Dis. Child. 145, 904–911 (1991).
Keir, A. K. et al. Temporal changes in blood product usage in preterm neonates born at less than 30 weeks’ gestation in Canada. Transfusion 55, 1340–1346 (2015).
Howarth, C., Banerjee, J. & Aladangady, N. Red blood cell transfusion in preterm infants: current evidence and controversies. Neonatology 114, 7–16 (2018).
Kirpalani, H. et al. Higher or lower hemoglobin transfusion thresholds for preterm infants. N. Engl. J. Med. 383, 2639–2651 (2020).
Aylward, G. P. Update on neurodevelopmental outcomes of infants born prematurely. J. Dev. Behav. Pediatr. 35, 392–393 (2014).
Hutchinson, E. A. et al. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics 131, e1053–e1061 (2013).
Aylward, G. P. Neurodevelopmental outcomes of infants born prematurely. J. Dev. Behav. Pediatr. 26, 427–440 (2005).
Carlo, W. A. et al. Cytokines and neurodevelopmental outcomes in extremely low birth weight infants. J. Pediatr. 159, 919–25 e3 (2011).
Leviton, A. et al. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J. Pediatr. 158, 897–903.e1 (2011).
Basu, S. et al. Elevated plasma and cerebrospinal fluid interleukin-1 beta and tumor necrosis factor-alpha concentration and combined outcome of death or abnormal neuroimaging in preterm neonates with early-onset clinical sepsis. J. Perinatol. 35, 855–861 (2015).
Hagberg, H. et al. The role of inflammation in perinatal brain injury. Nat. Rev. Neurol. 11, 192–208 (2015).
Aden, U. et al. Systemic inflammation sensitizes the neonatal brain to excitotoxicity through a pro-/anti-inflammatory imbalance: key role of TNFalpha pathway and protection by etanercept. Brain Behav. Immun. 24, 747–758 (2010).
Van Steenwinckel, J. et al. Brain damage of the preterm infant: new insights into the role of inflammation. Biochem. Soc. Trans. 42, 557–563 (2014).
Kinjo, T. et al. Serum chemokine levels and developmental outcome in preterm infants. Early Hum. Dev. 87, 439–443 (2011).
Dammann, O. & Leviton, A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr. Res. 75, 376–380 (2014).
Benavides, A. et al. Sex-specific alterations in preterm brain. Pediatr. Res. https://doi.org/10.1038/s41390-018-0187-5 (2018).
Nopoulos, P. C. et al. Long-term outcome of brain structure in premature infants: effects of liberal vs restricted red blood cell transfusions. Arch. Pediatr. Adolesc. Med. 165, 443–450 (2011).
Keir, A. K., McPhee, A. J., Andersen, C. C. & Stark, M. J. Plasma cytokines and markers of endothelial activation increase after packed red blood cell transfusion in the preterm infant. Pediatr. Res. 73, 75–79 (2013).
Dani, C. et al. Red blood cell transfusions can induce proinflammatory cytokines in preterm infants. Transfusion 57, 1304–1310 (2017).
Yu, H. et al. Serum concentrations of cytokines in infants with retinopathy of prematurity. APMIS 122, 818–823 (2014).
Transfusion of Prematures Trial (TOP). National Library of Medicine (US). https://clinicaltrials.gov/ct2/show/NCT01702805 (2012).
Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 (1979).
Benjamini, Y. & Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 29, 1165–1188 (2001).
Cerri, C., Caleo, M. & Bozzi, Y. Chemokines as new inflammatory players in the pathogenesis of epilepsy. Epilepsy Res. 136, 77–83 (2017).
Lu, H. et al. Relationship between premature brain injury and multiple biomarkers in cord blood and amniotic fluid. J. Matern. Fetal Neonatal Med. https://doi.org/10.1080/14767058.2017.1359532, 1–7 (2017).
Semple, B. D. et al. Role of CCL2 (MCP-1) in traumatic brain injury (TBI): evidence from severe TBI patients and CCL2-/- mice. J. Cereb. Blood Flow. Metab. 30, 769–782 (2010).
Ho, L. et al. Elevated plasma MCP-1 concentration following traumatic brain injury as a potential “predisposition” factor associated with an increased risk for subsequent development of Alzheimer’s disease. J. Alzheimer’s Dis. 31, 301–313 (2012).
Bartha, A. I. et al. Neonatal encephalopathy: association of cytokines with MR spectroscopy and outcome. Pediatr. Res. 56, 960–966 (2004).
Wu, D. et al. The association of genetic polymorphisms with cerebral palsy: a meta-analysis. Dev. Med. Child Neurol. 53, 217–225 (2011).
Graham, A. M. et al. Maternal systemic interleukin-6 during pregnancy is associated with newborn amygdala phenotypes and subsequent behavior at 2 years of age. Biol. Psychiatry 83, 109–119 (2018).
Romero, R. et al. CXCL10 and IL-6: markers of two different forms of intra-amniotic inflammation in preterm labor. Am. J. Reprod. Immunol. 78, https://doi.org/10.1111/aji.12685 (2017).
McAdams, R. M. & Juul, S. E. The role of cytokines and inflammatory cells in perinatal brain injury. Neurol. Res. Int. 2012, 561494 (2012).
Franz, A. R. et al. Effects of liberal vs restrictive transfusion thresholds on survival and neurocognitive outcomes in extremely low-birth-weight infants: the ETTNO Randomized Clinical Trial. JAMA 324, 560–570 (2020).
Georgieff, M. K. Long-term brain and behavioral consequences of early iron deficiency. Nutr. Rev. 69, S43–S48 (2011).
Lozoff, B. & Georgieff, M. K. Iron deficiency and brain development. Semin. Pediatr. Neurol. 13, 158–165 (2006).
Lozoff, B. et al. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 64, S34–S43 (2006).
Aggarwal, B. B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3, 745–756 (2003).
Ward-Kavanagh, L. K., Lin, W. W., Sedy, J. R. & Ware, C. F. The TNF receptor superfamily in co-stimulating and co-inhibitory responses. Immunity 44, 1005–1019 (2016).
Etemadi, N. et al. Lymphotoxin alpha induces apoptosis, necroptosis and inflammatory signals with the same potency as tumour necrosis factor. FEBS J. 280, 5283–5297 (2013).
Tumanov, A. V. et al. Dissecting the role of lymphotoxin in lymphoid organs by conditional targeting. Immunol. Rev. 195, 106–116 (2003).
Patel, J. R. et al. Astrocyte TNFR2 is required for CXCL12-mediated regulation of oligodendrocyte progenitor proliferation and differentiation within the adult CNS. Acta Neuropathol. 124, 847–860 (2012).
McCoy, T. E. et al. The relationship between brain structure and cognition in transfused preterm children at school age. Dev. Neuropsychol. 39, 226–232 (2014).
McCoy, T. E. et al. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychol. 17, 347–367 (2011).
Dani, C. et al. Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion 50, 1220–1226 (2010).
Arthur, C. M. et al. Anemia induces gut inflammation and injury in an animal model of preterm infants. Transfusion 59, 1233–1245 (2019).
Acknowledgements
The design and conduct of this research study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript were made possible by National Institute for Heart, Lung, and Blood (NHLBI) Parent Project Grant #5P01HL046925-22, Sub-Project ID 5779, “Immunologic and Neurodevelopmental Consequences of Neonatal Anemia and Thrombocytopenia and Their Treatments—Project 4,” and National Institute for Mental Health (NIMH) Program Grant #5T32MH019113-26 “Iowa Neuroscience Specialty Program in Research Education (INSPIRE) Program Grant.” We confirm that this manuscript has not been previously published and has not been submitted simultaneously for publication elsewhere. I, A.B., had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Contributions
A.B. was involved in conceptualization/design, methodology, data curation and formal analysis, and writing initial draft of this manuscript, and confirms that all authors participated in the conceptualization/design, review, and editing of this manuscript. Additionally, D.N. provided significant expertise in the methodology of transfusion, blood banking, and cytokine sampling used in this study. H.A.F. contributed significantly to the supervision/oversight, statistical methodology, and formal analysis of all data presented in this manuscript. As part of multi-institutional program project grant, H.A.F., M.K.G., C.D.J., P.N., S.R.S., and M.S.-V. provided acquisition of funding for this manuscript, as well as critical supervision/oversight and feedback for revision throughout the manuscript writing and submission process. All authors have reviewed and approved the final version of the manuscript as submitted and agree with its submission to Pediatric Research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Statement of consent
Parents of all subjects in this study provided informed consent for participation, in full accordance with institutional review board requirements for human subject research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Benavides, A., Bell, E.F., Georgieff, M.K. et al. Sex-specific cytokine responses and neurocognitive outcome after blood transfusions in preterm infants. Pediatr Res 91, 947–954 (2022). https://doi.org/10.1038/s41390-021-01536-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01536-0
This article is cited by
-
Identification and prediction model of placenta-brain axis genes associated with neurodevelopmental delay in moderate and late preterm children
BMC Medicine (2023)
-
New frontiers in neonatal red blood cell transfusion research
Journal of Perinatology (2023)