Abstract
Objective
To investigate the association between fluid and sodium status in the first 10 postnatal days and death/bronchopulmonary dysplasia (BPD) among infants born <29 weeks’ gestation.
Study design
Single center retrospective cohort study (2015–2018) of infants born 23–28 weeks’. Three exposure variables were evaluated over the first 10 postnatal days: cumulative fluid balance (CFB), median serum sodium concentration, and maximum percentage weight loss. Primary outcome was death and/or BPD. Multivariable logistic regression adjusting for patient covariates was used to assess the association between exposure variables and outcomes.
Results
Of 191 infants included, 98 (51%) had death/BPD. Only CFB differed significantly between BPD-free survivors and infants with death/BPD: 4.71 dL/kg (IQR 4.10–5.12) vs 5.11 dL/kg (IQR 4.47–6.07; p < 0.001). In adjusted analyses, we found an association between higher CFB and higher odds of death/BPD (AOR 1.56, 95% CI 1.11–2.25). This was mainly due to the association of CFB with BPD (AOR 1.60, 95% CI 1.12–2.35), rather than with death (AOR 1.08, 95% CI 0.54–2.30).
Conclusion
Among preterm infants, a higher CFB in the first 10 days after delivery is associated with higher odds of death/BPD.
Impact
-
Previous studies suggest that postnatal fluid status influences survival and respiratory function in neonates.
-
Fluid balance, serum sodium concentration, and daily weight changes are commonly used as fluid status indicators in neonates.
-
We found that higher cumulative fluid balance in the first 10 days of life was associated with higher odds of death/bronchopulmonary dysplasia in neonates born <29 weeks.
-
Monitoring of postnatal fluid balance may be an appropriate non-invasive strategy to favor survival without bronchopulmonary dysplasia.
-
We developed a cumulative fluid balance chart with corresponding thresholds on each day to help design future trials and guide clinicians in fluid management.
Similar content being viewed by others
Introduction
More than 50% of infants born less than 29 weeks gestational age (GA) will either die or develop bronchopulmonary dysplasia (BPD).1,2 Defined as the need for supplemental oxygen or respiratory support at 36 weeks corrected GA, BPD is a severe complication of prematurity independently associated with poor growth, neurodevelopmental impairment, and death.3,4,5 The etiology is multifactorial and abnormalities in postnatal fluid status may contribute to disease development.6 In the first week after delivery, an efflux of fluid from the intra- to the extracellular fluid (ECF) compartment occurs.6,7,8 This leads to the initiation of salt and water diuresis around 48–72 h after birth.7,8 However, in extremely preterm infants with immature kidneys, ECF contraction and weight loss are limited due to delayed diuresis.6,7,8,9,10,11,12,13 Fluid overload in the postnatal period may result in increased interstitial fluid in the lungs, impaired gas exchange, and higher needs of mechanical ventilation, which could inadvertently contribute to the development of BPD.6,12
Previous studies have evaluated water balance changes during the early postnatal period as a critical variable for respiratory function and survival among preterm infants.14,15,16,17,18,19,20,21,22,23,24,25,26,27 High cumulative fluid balance (CFB) and fluid intake during postnatal days 2–7 have been associated with BPD and mortality.14,15,16,17 Furthermore, there is evidence supporting associations of higher serum sodium21 and increased weight loss in the first postnatal week with lower risk of BPD.14,18,19 Because most analyses have been conducted nearly two decades ago,14,22,23,24,25,26,27 previous findings may not apply to current NICU settings and high-risk population for the outcome of death/BPD. Advances in perinatal care including the widespread use of surfactant, conservative management of patent ductus arteriosus, and introduction of high-performing incubators have changed postnatal fluid adaptation.3,28,29 Yet, no study has simultaneously assessed the relation between indicators of fluid status and death/BPD in a contemporary cohort, making it difficult to reach definite conclusions. We hypothesized that fluid retention during the early postnatal period, reflected by high CFB, low serum sodium concentration, and reduced weight loss, would be associated with higher odds of death/BPD. The objective of our study was to evaluate the association between these three clinical indicators of fluid status (fluid balance, serum sodium concentration, and weight loss) in the first 10 days after birth and the outcome of death/BPD among preterm infants born <29 weeks GA.
Patients and methods
Study population and eligibility criteria
This retrospective cohort study included a convenience sample of infants born between 230/7 and 286/7 weeks GA admitted to the Montreal Children’s Hospital tertiary-level NICU from June 1, 2015 to December 31, 2018. Infants who died within 24 h of admission, with major congenital anomaly and/or admitted to the NICU > 1 day after birth were excluded. This study was approved by the Research Ethics Board of the McGill University Health Center.
Data collection
Data on infant characteristics and outcomes were obtained from the local Canadian Neonatal Network (CNN) database where patient information is entered electronically by trained abstractors into a data-entry program with built-in error checking, high reliability, and internal consistency.30 The following characteristics were extracted: use of antenatal steroids (partial or complete), multiple births, mode of delivery, outborn status, GA at birth, birth weight, small for GA status (SGA; defined as <10th percentile for GA and sex),31 infant sex, 5 min Apgar <7, and use of surfactant. Additional information on daily fluid intake, fluid output, serum sodium concentration, and weight over the first 10 days of life was collected from individual patient chart review. After initial complete data collection, all data on fluid status (40 data points per patient) from a random sample of 10% of infants were re-abstracted and showed high internal consistency (error rate of 0.23%).
Standard of care and exposure variables definitions
During the study period, standard of care involved initial total fluid intake of 80–100 mL/kg/day and increased by 20 mL/kg/day daily based on clinical status. There was no added sodium in the parenteral nutrition used during the first 24 h. Sodium intake typically started at 0.5–1.0 mmol/kg/day on day 2 or 3, and was subsequently adjusted up to 3 mmol/kg/day by clinicians based on daily fluid status. Infants were placed in a servo-controlled incubator with a relative humidity of 70–80% during the first 7 days and 50% afterwards. Infants were weighed on the same scale every evening, and fluid balance was calculated at least once a day by the bedside nurse. Serum electrolyte levels were measured every 12 to 48 h and additionally as clinically indicated. In our center, hydrocortisone for BPD prevention or nonsteroidal anti-inflammatory for intraventricular hemorrhage prophylaxis are not standard of care. Patent ductus arteriosus was managed conservatively (no drug therapy) in the first 10 postnatal days. Hence, no infant was exposed to nonsteroidal anti-inflammatory drugs during this period.
The three main exposures evaluated for each patient during the first 10 postnatal days were: (a) fluid balance, (b) serum sodium concentration, and (c) weight loss. Values for each exposure were summarized for each postnatal day (days 1 through 10) and overall over the 10 days. Whereas most previous studies evaluated indicators in the first 2–7 days,15,16,17,18,19,20,21,22,23,24 we prolonged our observation period to 10 days in order to detect potential differences in postnatal fluid adaptation. For each exposure, day 1 corresponded to the day of birth, and a day was defined as the period from midnight to 23:59. Daily fluid balance was calculated as the difference between total fluid intake (dL/day) and total fluid output (dL/day) divided by birth weight (kg). Birth weight was used for all daily fluid balance calculations for consistency, and deciliters were used instead of milliliters to facilitate interpretation of calculated odds ratios. Days when data on fluid intake or output were not available were not included in the calculation. The total fluid intake was calculated as the sum of blood products, intravenous fluids, fluids boluses (dextrose, saline, or other), and enteral nutrition administered to the infants. We did not include medications and flushes given with intravenous medications, which typically consist of 0.3–0.5 mL of normal saline. Total fluid output was the sum of daily urine volumes. We did not record blood volume drawn for blood tests and our transfusion practices were based on thresholds recommended by the Canadian Pediatric Society.32 Finally, the daily CFB for days 1 through 10 was calculated. To limit variability between patients who had several serum sodium values per day, we collected both the daily highest serum sodium concentration (maxNa) and lowest serum sodium concentration (minNa) for each day. If a patient had only a single serum sodium concentration recorded for a given day, that value was used as both the maxNa and minNa. Median Na was defined as the median of all serum sodium values (maxNa and minNa) in the first 10 postnatal days. Daily weight change was calculated as a percentage relative to birth weight (difference between daily weight and birth weight, divided by birth weight), where negative values represent weight loss and positive values represent weight gain. The maximum percentage weight loss (%wtloss) was the absolute value of the lowest daily percentage weight change in the first 10 days.
Outcomes
The primary outcome was a composite of death and/or BPD. Death was defined as mortality prior to discharge from the NICU, and BPD was defined as the need for supplemental oxygen or respiratory support at 36 weeks postmenstrual age or at time of discharge.33 A composite outcome was selected because both are competing events in extremely preterm infants: BPD is diagnosed at 36 weeks postmenstrual age and death typically occurs prior to that age.34 Evaluating BPD alone could induce a selection bias, since it is likely that critically ill infants who die prematurely would have otherwise developed BPD.34 We examined the individual components of the primary composite outcome as secondary outcomes.
Statistical analyses
Categorical variables are presented as n (%) and continuous variables as median and interquartile range (IQR). The Pearson correlation coefficient (r) was used to assess independence between pairwise combinations of the three main exposure variables (CFB on day 10, median Na, and maximum %wtloss). Unadjusted comparisons between BPD-free survivors and infants with death/BPD were made with the Pearson chi-squared test for categorical variables and the Wilcoxon rank-sum test for continuous variables. For daily exposure variables (CFB, maxNa, minNa, and percentage weight change), unadjusted comparisons between groups were made using the Wilcoxon rank-sum test and adjusted associations using logistic regression (adjusted for antenatal steroid use, mode of delivery, GA, SGA status, sex, 5-min Apgar <7, and use of surfactant).
Logistic regression analyses were used to explore the association between each exposure (used as a continuous variable) and each outcome. Crude odds ratios (OR), adjusted odds ratios (AOR), and corresponding 95% confidence intervals (CI) were calculated. The ORs were adjusted for patient covariates, including antenatal steroid use, mode of delivery, GA, SGA status, sex, 5-min Apgar <7, use of surfactant, as well as the three main exposure variables. Collinearity between independent variables was assessed with values of the variance inflation factor >5.35 Model performance was evaluated using the area under the receiver operating characteristic curve (AUC), and goodness-of-fit was assessed using the Hosmer–Lemeshow test.36 We did not impute missing data since the number of missing data points for each day was low (2.3% of daily fluid balances, 1.7% of weights, and all patients had at least one sodium value during the first 10 days) (Supplementary Table 1).37
To better understand the contribution of each component of fluid balance (intake and output), we conducted additional analyses evaluating the association of median daily fluid intake and daily fluid output per kilogram of birth weight with death/BPD. Additionally, since primary analyses showed that daily CFB was associated with death/BPD, we proposed different classification thresholds of daily CFB (identified using Youden’s index and the 25th, 50th, and 75th percentile of values) and reported the corresponding sensitivities and specificities for association with death/BPD.38 Statistical analyses were conducted using R version 3.6.0. (R Foundation for Statistical Computing, Vienna, Austria). A two-sided p value <0.05 was considered statistically significant.
Results
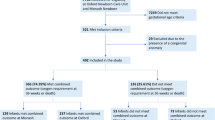
There were 195 infants born 23–28 weeks GA and admitted to the NICU during the study period. Four infants were excluded: two died within 24 h of admission and two had a major congenital anomaly (Supplementary Fig. 1). The final study sample included 191 infants, of whom 98 (51%) died and/or developed BPD; 26 (14%) infants died, 75 (39%) developed BPD, and three died after BPD diagnosis. Among infants that died, 16 died in the first 10 days (Supplementary Table 2). Of them, nine died of respiratory failure, four of brain injury, and three of infection. The characteristics of infants with death/BPD and BPD-free survivors are presented in Table 1. Compared to BPD-free survivors, infants with death/BPD were born at a lower GA and birth weight, and were more frequently delivered by cesarean and exposed to surfactant.
The pattern of each daily exposure variable for these infants over the first 10 days is presented in Fig. 1. Daily CFB gradually increased from day 2 to 10 in both groups. However, BPD-free survivors had a significantly lower daily CFB compared to infants with death/BPD between postnatal days 2 and 10. In both groups, maxNa and minNa increased from days 1 to 3 after delivery, reaching a peak at day 3 followed by a gradual decrease until day 10. There were no differences in daily maxNa and minNa between BPD-free survivors and infants with death/BPD (except minNa on day 2). Infants experienced increasing weight loss from days 2 to 4, then progressively gained weight from days 4 to 10. BPD-free survivors had a greater percentage weight loss than infants with death/BPD on days 6 and 7. There was a weak correlation between each pairwise combination of the three main exposure variables (all |r| <0.24, Supplementary Fig. 2).
a Cumulative fluid balance. b Maximum daily serum sodium concentration. c Minimum daily serum sodium concentration. d Weight change percentage. Data presented as median and IQR. Unadjusted p values calculated using Wilcoxon rank-sum test and adjusted p value calculated with multivariable logistic regression adjusted for steroid use, mode of delivery, gestational age, Apgar at 5 min <7, use of surfactant, sex, and small for gestational age status.
A higher CFB at day 10 was associated with higher adjusted odds of death/BPD and of BPD alone (Table 2). In the adjusted analyses, a 1 dL/kg increase in CFB at day 10 was associated with a 56% increase in the odds of death/BPD, and a 60% increase in the odds of BPD. There was no association with mortality (AOR 1.08, 95% CI 0.54–2.30). Median Na and maximum %wtloss were not associated with the adjusted odds of death/BPD, mortality, or BPD (Table 2). Additional adjusted analyses did not show significant associations between time to regain birth weight and death/BPD (Supplementary Table 3).
In the analysis assessing fluid intake and output separately, there were no differences in daily fluid intake between BPD-free survivors and infants with death/BPD (Fig. 2). Daily fluid output in the first three postnatal days was significantly higher among BPD-free survivors compared to infants with death/BPD. Sensitivity and specificity for the risk of death/BPD using different daily CFB thresholds were calculated (Supplementary Table 4), and used to create a CFB chart (Supplementary Fig. 3). A CFB in the 25th percentile on day 5 (below 1.01 dL/kg = 101 mL/kg) had high sensitivity (86%) but low specificity (35%) to predict death/BPD.
a Total fluid intake. b Total fluid output. Data presented as median and IQR. Unadjusted p values calculated using Wilcoxon rank-sum test and adjusted p value calculated with multivariable logistic regression adjusted for steroid use, mode of delivery, gestational age, Apgar at 5 min <7, use of surfactant, sex, and small for gestational age status.
Discussion
In this contemporary cohort of extremely preterm infants, a lower CFB over the first 10 days after birth was associated with lower odds of death/BPD. The association between CFB and death/BPD was mainly attributable to the association with BPD. Our results suggest that among the three clinical measures of fluid status evaluated, CFB may be the clinical indicator that is most associated with death/BPD among infants born <29 weeks GA.
Our findings are consistent with previous studies that have investigated the association of fluid status with death/BPD, although only a few directly assessed fluid balance. Oh et al.14 studied 1382 infants born <1000 g (ELBW) and found that higher fluid intake in the first 10 days of life was associated with higher odds of death/BPD. Another study including 226 ELBW infants demonstrated an association between high fluid intake during the first week of life and BPD severity.15 However, none of these studies evaluated fluid balance as an exposure. Extremely preterm infants have immature kidneys, and may be unable to accommodate high fluid intake in the postnatal period leading to fluid accumulation over time.6 A recent investigation of 219 ELBW infants demonstrated an association between higher CFB in the first 3 days with mortality and prolonged mechanical ventilation in the first 7 postnatal days.17 Notably, our results indicate that infants with death/BPD had a lower fluid output than BPD-free survivors, but fluid intake was not different between the groups leading to an increased CFB. The association between CFB and death/BPD appeared to be mainly driven by the association with BPD, rather than with death. These findings support previous work suggesting that reduced renal capacity during early postnatal period may contribute to low pulmonary function.6,20 Hence, high fluid prescriptions, without considerations of fluid output, in the first days after delivery may result in fluid overload and ECF retention. This can contribute to increased interstitial fluid within the lungs, pulmonary overflow, reduced lung compliance, and increased need for oxygen and ventilator support.6,14,15,16,17 Consequently, aiming for a CFB of 1.01 dL/kg by day 5 may be an appropriate non-invasive strategy to favor BPD-free survival among extremely preterm infants. However, fluid intake must allow for appropriate caloric intake, and careful monitoring is needed to avoid risks of dehydration.
Few studies have assessed the association of serum sodium concentration with risk of BPD among preterm infants. In ELBW infants, lower serum sodium concentrations were observed in the first seven postnatal days among BPD-free infants, compared to infants with BPD.15 Conversely, another study reported a non-significant increase in serum sodium concentrations during the first four postnatal days among BPD-free infants, compared to infants with BPD.21 Low median serum sodium concentrations may reflect increased fluid retention in early postnatal days, while higher values may indicate effective kidney function with optimal sodium reabsorptive capacity.6,21 As urine is hypo-osmotic during postnatal adaptation, appropriate diuresis in the first days of life may lead to increased serum sodium concentrations and lower risks of fluid overflow within the lungs.6,7,8,9,10 However, our results did not show any association between median Na in the first 10 days of life with death/BPD. The lack of difference in serum sodium concentrations between infants who died and/or developed BPD and BPD-free survivors in our cohort may be attributed to the fact that we did not collect daily sodium intake. In clinical practice sodium intake is adjusted daily, and therefore may influence serum sodium values. The low variability in the daily serum sodium concentration measurements in our study population (median for first 10 days: 137 mmol/L [IQR 134–139]) might be related to daily adjustments made by the clinicians. A recent study suggested that sodium intake, rather than fluid intake, was the main determinant of serum sodium concentration in extremely preterm infants.39 Our data suggest that the median Na correlated poorly with CFB and maximum %wtloss, thus sodium intake and concentration might not be a strong indicator of postnatal fluid overload in preterm infants.
The lack of association between maximum %wtloss and death/BPD contrasts with the Oh et al.14 study, which found an inverse association between these variables. Their observed effect may in part be due to a greater percentage weight lost by neonates in their cohort, where the mean weight loss on day 5 was 8.5% compared to 4.2% in our sub-group of infants born <1000 g (results not shown). Differences in weight loss among infants admitted in 1999–2001 compared to a 2015–2018 cohort are likely due to the advent of widespread high-performing incubator use, which considerably reduces insensible water loss by maintaining higher humidity.29 Indeed, more recent investigations evaluating the association of maximum %wtloss with BPD have reported mixed results, which may also be explained by the use of different weight measurement methods.18,19,20 Our results show differences in patterns of weight change from days 6 and 7 between BPD-free survivors and infants with death/BPD. This is consistent with previous literature that has reported increased weight gain between days 6 and 10 of life among infants with death/BPD.14 However, adjusted analyses did not show an association between time to regain birth weight and death/BPD.
Limitations and strengths
This study has some limitations. We could not account for errors related to urine volume measurements and weighing of unstable babies, which can be sometimes imprecise. Our site carefully documents fluid balance on a daily basis whereas other sites may prioritize different clinical indicators of fluid status, thus affecting generalizability. We did not measure sodium intake which can affect serum sodium concentration.39 Fluid flushes used during the administration of antibiotics, caffeine, and other medications were not calculated because of inconsistent reporting in the medical charts. This may significantly increase the overall fluid and sodium intake, especially in smaller and sicker infants receiving multiple medications. We also lacked data on the blood volume drawn for testing during the first 10 postnatal days, which can represent a significant volume in smaller babies. Due to the observational nature of the study, we cannot infer causality and ascertain that aiming for a lower CFB would decrease death/BPD. However, there are several strengths that should be highlighted. The study included a large number of infants using validated data in a contemporary setting. Our results were consistent across multiple analyses to address biases including adjusted models for confounders, analysis of exposures for each individual day, and separate evaluations of fluid intake and output. Moreover, our study simultaneously assessed CFB, serum sodium concentration, and weight loss. All these variables are potential risk factors for BPD, and our analysis was able to determine that CFB best correlated with death/BPD in extremely preterm infants.
Conclusion
Higher CFB over the first 10 days of life was associated with death/BPD among extremely preterm infants. Our results suggest that careful monitoring of fluid balance over the first 10 days with a particular focus on the first 5 days may increase BPD-free survival. Results of this study can help planning randomized trials comparing different fluid management strategies to determine optimal targets and assess outcomes in this high-risk population.
References
Shah, P. S. et al. Outcomes of preterm infants <29 weeks gestation over 10-year period in Canada: a cause for concern? J. Perinatol. 32, 132–138 (2012).
Lee, S. K. et al. Outcomes and care practices for preterm infants born at less than 33 weeks’ gestation: a quality-improvement study. CMAJ 192, E81–E91 (2020).
Stoll, B. J. et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA 314, 1039–1051 (2015).
Isayama, T. et al. Revisiting the definition of bronchopulmonary dysplasia: effect of changing panoply of respiratory support for preterm neonates. JAMA Pediatr. 171, 271–279 (2017).
Schmidt, B. et al. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA 289, 1124–1129 (2003).
Lorenz, J. M. Fluid and electrolyte therapy in the very low-birthweight neonate. NeoReviews 9, e102–e108 (2008).
Chawla, D., Agarwal, R., Deorari, A. K. & Paul, V. K. Fluid and electrolyte management in term and preterm neonates. Indian J. Pediatr. 75, 255 (2008).
Fusch, C. & Jochum, F. Water, sodium, potassium and chloride. World Rev. Nutr. Diet. 110, 99–120 (2014).
Chow, J. & Douglas, D. Fluid and electrolyte management in the premature infant. Neonatal Netw. 27, 379–386 (2008).
Hartnoll, G. The physiology of fluid management in preterm infants. Curr. Paediatr. 13, 179–183 (2003).
Ross, B., Cowett, R. M. & Oh, W. Renal functions of low birth weight infants during the first two months of life. Pediatr. Res. 11, 1162–1164 (1977).
Gien, J. & Kinsella, J. P. Pathogenesis and treatment of bronchopulmonary dysplasia. Curr. Opin. Pediatr. 23, 305 (2011).
Oh, W. Fluid and electrolyte management of very low birth weight infants. Pediatr. Neonatol. 53, 329–333 (2012).
Oh, W. et al. Association between fluid intake and weight loss during the first ten days of life and risk of bronchopulmonary dysplasia in extremely low birth weight infants. J. Pediatr. 147, 786–790 (2005).
Al-Jebawi, Y., Agarwal, N., Groh Wargo, S., Shekhawat, P. & Mhanna, M. Low caloric intake and high fluid intake during the first week of life are associated with the severity of bronchopulmonary dysplasia in extremely low birth weight infants. J. Perinat. Med. 13, 207–214 (2020).
Guo, M. M.-H. et al. Severe bronchopulmonary dysplasia is associated with higher fluid intake in very low-birth-weight infants: a retrospective study. Am. J. Perinatol. 32, 155–162 (2015).
Matsushita, F. Y., Krebs, V. L. J., Ferraro, A. A. & de Carvalho, W. B. Early fluid overload is associated with mortality and prolonged mechanical ventilation in extremely low birth weight infants. Eur. J. Pediatr. 179, 1665–1671 (2020).
Aksoy, H. T. et al. The association of early postnatal weight loss with outcome in extremely low birth weight infants. Pediatr. Neonatol. 60, 192–196 (2019).
Selewski, D. T. et al. The impact of fluid balance on outcomes in premature neonates: a report from the AWAKEN study group. Pediatr. Res. 87, 550–557 (2020).
Askenazi, D. et al. Acute kidney injury is associated with bronchopulmonary dysplasia/mortality in premature infants. Pediatr. Nephrol. 30, 1511–1518 (2015).
Rocha, G., Ribeiro, O. & Guimarães, H. Fluid and electrolyte balance during the first week of life and risk of bronchopulmonary dysplasia in the preterm neonate. Clinics (Sao Paulo). 65, 663–674 (2010).
Lorenz, J. M., Kleinman, L. I., Kotagal, U. R. & Reller, M. D. Water balance in very low-birth-weight infants: relationship to water and sodium intake and effect on outcome. J. Pediatr. 101, 423–432 (1982).
Van Marter, L. J., Leviton, A., Allred, E. N., Pagano, M. & Kuban, K. C. Hydration during the first days of life and the risk of bronchopulmonary dysplasia in low birth weight infants. J. Pediatr. 116, 942–949 (1990).
Spitzer, A. R., Fox, W. W. & Delivoria-Papadopoulos, M. Maximum diuresis—a factor in predicting recovery from respiratory distress syndrome and the development of bronchopulmonary dysplasia. J. Pediatr. 98, 476–479 (1981).
Tammela, O. K. & Koivisto, M. E. Fluid restriction for preventing bronchopulmonary dysplasia? Reduced fluid intake during the first weeks of life improves the outcome of low‐birth‐weight infants. Acta Paediatr. 81, 207–212 (1992).
Bell, E. F. & Acarregui, M. J. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2014, CD000503 https://doi.org/10.1002/14651858.CD000503.pub3 (2014).
Barrington, K. J., Fortin‐Pellerin, E. & Pennaforte, T. Fluid restriction for treatment of preterm infants with chronic lung disease. Cochrane Database Syst. Rev. 2, (2017).
Sung, S. I., Lee, M. H., Ahn, S. Y., Chang, Y. S. & Park, W. S. Effect of nonintervention vs oral ibuprofen in patent ductus arteriosus in preterm infants: a randomized clinical trial. JAMA Pediatr. 174, 755–763 (2020).
Antonucci, R., Porcella, A. & Fanos, V. The infant incubator in the neonatal intensive care unit: unresolved issues and future developments. J. Perinat. Med. 37, 587–598 (2009).
Shah, P. S. et al. Internal audit of the Canadian Neonatal Network data collection system. Am. J. Perinatol. 34, 1241–1249 (2017).
Kramer, M. S. et al. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 108, e35–e35 (2001).
Whyte, R. K., Jefferies, A. L., Canadian Paediatric Society & Fetus and Newborn Committee. Red blood cell transfusion in newborn infants. Paediatr. Child Health 19, 213–217 (2014).
Shennan, A. T., Dunn, M. S., Ohlsson, A., Lennox, K. & Hoskins, E. M. Abnormal pulmonary outcomes in premature infants: prediction from oxygen requirement in the neonatal period. Pediatrics 82, 527–532 (1988).
Das, A. et al. Methodological issues in the design and analyses of neonatal research studies: experience of the NICHD Neonatal Research Network. Semin. Perinatol. 40, 374–384 (2016).
Institute for Digital Research and Education. Regression with SPSS. http://stats.idre.ucla.edu/spss/webbooks/reg/chapter2/spss-webbooksregressionwith-spsschapter-2-regression-diagnostics/. Accessed May 12, 2020.
Lemeshow, S. & Hosmer, D. W. Jr A review of goodness of fit statistics for use in the development of logistic regression models. Am. J. Epidemiol. 115, 92–106 (1982).
Dong, Y. & Peng, C.-Y. J. Principled missing data methods for researchers. Springerplus 2, 222 (2013).
Fluss, R., Faraggi, D. & Reiser, B. Estimation of the Youden Index and its associated cutoff point. Biom. J. 47, 458–472 (2005).
Späth, C., Sjöström, E. S., Ahlsson, F., Ågren, J. & Domellöf, M. Sodium supply influences plasma sodium concentration and the risks of hyper- and hyponatremia in extremely preterm infants. Pediatr. Res. 81, 455–460 (2017).
Acknowledgements
The authors gratefully acknowledge the Canadian Neonatal Network (CNN) for data support. M.B. holds an Early Career Investigator Grant from the CIHR Institute of Human Development, Child and Youth Health (IHDCYH), a research grant funding from the FRSQ Clinical Research Scholar Career Award Junior 1, and an Early Career Investigator Grant from the Montreal Children’s Hospital Foundation. S.S. received the Class of Medicine 1996 Research Bursary and Nicholas McCutcheon Summer Research Bursary from McGill University for this project.
Author information
Authors and Affiliations
Contributions
S.S. acquired the data with the supervision of M.B., S.S, M.B., and S.P. designed the study. S.S. and S.P. realized the data analysis with input from M.B. M.C., L.W., and G.S.A. helped in the interpretation of the results. S.S. and S.P. wrote the preliminary versions of the manuscript, and M.B., M.C., L.W., and G.S.A. critically appraised the manuscript, adding significant intellectual contributions to the content. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Patient consent was not required.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Soullane, S., Patel, S., Claveau, M. et al. Fluid status in the first 10 days of life and death/bronchopulmonary dysplasia among preterm infants. Pediatr Res 90, 353–358 (2021). https://doi.org/10.1038/s41390-021-01485-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01485-8
This article is cited by
-
The association between BMI trajectories and bronchopulmonary dysplasia among very preterm infants
Pediatric Research (2023)
-
Positive fluid balance and diuretic therapy are associated with mechanical ventilation and mortality in preterm neonates in the first fourteen postnatal days
Pediatric Nephrology (2023)
-
Neonatal fluid overload—ignorance is no longer bliss
Pediatric Nephrology (2023)
-
Postnatal maximal weight loss, fluid administration, and outcomes in extremely preterm newborns
Journal of Perinatology (2022)
-
Fluid balance in early postnatal life: Should we keep the babies dry to prevent bronchopulmonary dysplasia?
Pediatric Research (2021)