Abstract
Background
In neonatal intensive care units (NICUs), hygiene and disinfection measures are pivotal to protect neonates from nosocomial infections. This study aimed to evaluate the efficacy of the classical incubators disinfection procedure and to follow-up neonates housed in the incubators for the development of late-onset sepsis (LOS).
Methods
In a tertiary NICU, 20 incubators were monitored for bacterial contamination at three times: before disinfection, after disinfection, and 24 h after turning on and housing a new neonate. Clinical data of neonates housed in these incubators were retrieved from the medical records.
Results
All 20 incubators were contaminated at the 3 times of the study, mainly on mattresses and balances. Coagulase-negative Staphylococci, Enterococcus, and Bacillus-resisted disinfection while enterobacteria and Staphylococcus aureus were eradicated. After 24 h, the bacterial colonisation was similar to the one observed before disinfection. The bacteria isolated on incubators were also found on the caregivers’ hands. During the study, two preterm neonates developed a LOS involving a bacterial species that has been previously isolated in their incubator.
Conclusion
Pathogenic contaminants persist on incubators despite disinfection and represent a risk for subsequent infection in preterm neonates. Improvements are needed concerning both the disinfection process and incubator design.
Impact
-
Procedures of disinfection that are usually recommended in NICUs do not allow for totally eradicating bacteria from incubators.
-
Preterm neonates are housed in incubators colonised with potentially pathogenic bacteria.
-
The control of nosocomial infections in NICUs requires further researches concerning mechanisms of bacterial persistence and ways to fight against environmental colonisation.
Similar content being viewed by others
Introduction
In neonatal intensive care units (NICUs) late-onset sepsis (LOS) is a frequent issue associated with significant morbidity and mortality, especially in preterm very-low-birth-weight infants.1 These infections are frequently nosocomial and the most frequent pathogens involved are coagulase-negative Staphylococci (CoNS), Staphylococcus aureus, and enterobacteria.1
Previous studies have explored the possible sources and reservoirs of these pathogens within the NICU settings. On the one hand, caregivers could represent a possible source and/or vector of pathogens responsible for the contamination of neonates.2 On the other hand, several authors have identified the environment and inert surfaces of the NICU as the reservoir of some pathogens. For example, the clone S. capitis NRCS-A, an emerging CoNS responsible for LOS worldwide has been retrieved from several surfaces in the NICU, especially on the incubators.3,4,5 Other bacteria including S. aureus and enterobacteria involved in nosocomial outbreaks have also been isolated on NICU equipment.6,7 These findings point out a possible failure in the process of disinfection.3
In that respect, the aim of the present study was to evaluate the efficacy and weak points of the procedure of incubator disinfection usually recommended on the basis of environmental samples in a tertiary NICU. The secondary objectives of this study were: (i) to follow-up neonates housed in the incubators of the study, especially for the development of LOS, (ii) to describe the bacterial epidemiology of incubator’s contamination before and after disinfection, (iii) to investigate the hand skin colonisation among caregivers, and (iv) to determine other environmental sources of pathogens within the NICU.
Methods
Study setting and methods of sampling
The study was conducted in the Level III NICU of the Hôpital Femme-Mère-Enfant, Hospices Civils de Lyon, France during two consecutive weeks in June 2020. During this period, for each incubator that was disinfected five different sites were sampled (the rubber grommet, the left door handles, the temperature adjustment button, the mattress and the balance) using flocked swabs (ESwab, Copan®, United States) just before and just after the disinfection procedure. After 24 h during which the incubator was turned on and a neonate housed in (24H), the mattress and the rubber grommet were sampled again. In the same way, one heating table in the delivery room of this hospital was also sampled one time at five different sites (the mattress, the lateral door, the monitor, the timer button and the air-O2 blender) since it could represent a potential reservoir for the early colonisation of neonates. This heating table was chosen because it was the one used in the delivery room for neonates that require hospitalisation in the NICU. Of note, this table is disinfected between each patient using Surfanios Premium (ANIOS, Lezennes, France) impregnated wipes. Finally, the disinfection room where incubators are disinfected between two patients was sampled one time at two sites: the computer keyboard and the surface where pieces of incubators are placed during drying.
Procedure of incubator disinfection
In the study NICU, two types of incubators are currently used: GiraffeTM (General Electrics Healthcare, Limonest, France) and SATIS+ (Médipréma, Tauxigny, France). In this setting, incubators are changed every 10 days or earlier if the neonate did not need it any more. Between two patients, the incubators are disinfected using a 20-min disinfectant immersion bath using a diluted solution of Surfanios Premium (containing N-(3-aminopropyl)-N-dodécylpropane-1,3-diamine 51 mg/g and didecyldimethylammonium chloride 25 mg/g; final dilution 0.25%) (ANIOS, Lezennes, France). The protocol of disinfection of incubators is usual, according to the manufacturer recommendations. It consists in (i) an immersion of the removable parts of the incubator and (ii) decontamination of the frame and of the fragile parts with diluted Surfanios Premium impregnated wipes, as described elsewhere.3 The different incubator parts are then rinsed with filtered water and finally dried before the reassembly. When a neonate is housed in the incubator, the incubator is daily cleaned by nurses based on careful wipe disinfection using Surfanios Premium for the exterior of the incubator while the interior of the incubator (in contact with the neonate) is cleaned using sterile water.
Hand skin sampling among caregivers
During the study period, the hands of the caregivers involved in the process of disinfection were swabbed using sterile wet compresses twice each day, first after hand washing but before disinfection of the first incubator of the day, and second just after disinfection of the last incubator of the day, beforehand washing. Of note, in this NICU the same caregivers are involved either in incubator disinfection or in cares provided to neonates.
Bacterial culture of samples and bacterial identification
Contamination of a surface was defined by the presence of at least one bacterial strain belonging to a species that can be involved in LOS in neonates. That is why bacterial analyses aimed to identify the main pathogenic agents including CoNS (including S. capitis NRCS-A), S. aureus, Gram-negative bacteria and other bacteria of clinical relevance. Briefly, after 24 h of enrichment in brain heart infusion (BHI-T, BioMérieux, Marcy l’Etoile, France) at 37 °C, two agar plates were inoculated for each sample: one MRSA Brillance 2 agar plate (Oxoid®, Versel, Germany) to isolate methicillin-resistant CoNS including S. capitis NRCS-A as previously described8 and one CPSE agar plate (Chrom ID® CPS® Elite, Biomérieux, Marcy l’Etoile, France) to isolate the other bacteria. Species identification was performed using matrix-assisted laser desorption-ionisation - time of flight mass spectrometry (MALDI-TOF MS) using the VITEK® MS system (BioMérieux, Marcy l’Etoile, France).
Collection of clinical data
Clinical data were retrospectively collected concerning the neonates housed in the incubators of the study after the disinfection process. The clinical data were retrieved using the software ICCA (IntelliSpace Critical Care and Anesthesia, Philips®, Suresne, France) which is used to prospectively record medical information for patients in the study NICU. The following items were collected: gestational age and weight at birth, sex and small for gestational age status (according to revised Fenton curves)9 and age at the housing in the incubator. For each neonate, the occurrence of a LOS was searched. LOS was defined as the combination of (i) clinical signs of infection, whether hemodynamic, respiratory, or intestinal, and/or biological disorders (raised C-reactive protein and/or procalcitonin) in a patient older than three days of life; and (ii) a positive bacterial culture of blood sample; and (iii) antimicrobial treatment for more than five days or until the patient died.10 Positive culture of urinary sample and/or tracheobronchial suction and/or cerebrospinal fluid and/or operative site swabbing were also considered as infections if they were associated with clinical signs of infection and antimicrobial treatment more than five days or shorter if the patient died. For infected patients, additional items were collected: mode of delivery, premature membrane rupture more than 24 h before birth, intrapartum antimicrobial drugs administered to the mother, antimicrobial drugs administered to the neonate before the onset of sepsis, the delay between housing in the incubator and the onset of sepsis, age at sepsis, clinical signs, antibiotics administered for the sepsis.
Ethics statement
This study was approved by an ethical committee for biomedical research (Comité de Protection des Personnes Est I) under the number ID RCB 2020-A00818-31. Oral consent was obtained from the caregivers for the analysis of their hand skin samples. Written information was delivered to the parents of the infants involved in this study.
Results
Efficacy of incubator disinfection
During the 2-weeks study period, 20 incubators were disinfected and were thus included in the analysis. All 20 incubators were contaminated both before and immediately after disinfection (details presented for each incubator in Supplemental Fig. 1). CoNS were the most frequently found bacteria at each time of the study with 100%, 90%, and 95% of incubators that were contaminated with CoNS before, after disinfection and at 24H, respectively (Fig. 1). Among the CoNS, S. epidermidis was the most frequent species followed by S. capitis and S. haemolyticus. S. aureus and enterobacteria (in particular Enterobacter, Escherichia, and Klebsiella strains) were identified before disinfection but were eradicated from the sites of sampling after disinfection. However, S. aureus and enterobacteria were isolated again in, respectively, 15% and 30% of the 20 incubators at 24H. E. faecalis and Bacillus were also isolated at the 3 time-points of the study even if disinfection was associated with a decreased frequency of isolation.
Percentage of incubators that were colonised with each bacterial species or group among the 20 incubators of the study at the different times of the study: before disinfection (BD), after disinfection (AD), and after 24 h during which the incubator was turned on and a neonate housed in (24H). Details of the species identified on incubators are presented in Supplemental Table 1.
Concerning the different parts of the incubators, the mattress and the balance remained the most frequently contaminated sites after the disinfection procedure with, respectively, 100% and 90% of positive samples just after disinfection (Fig. 2a). For all sampled sites, CoNS were once again the most frequently isolated bacteria (Fig. 2b). In particular, S. capitis NRCS-A was the most frequent bacteria isolated on the mattress both before disinfection (24% of all isolates) and at 24H (23% of all isolates). Of note, S. aureus was isolated before disinfection on the grommet, the temperature adjustment button and the mattress but was absent elsewhere (Fig. 2b). At 24H, the number of contaminated sites increased in comparison with the results just after disinfection and the repartition of the bacterial species tended to the one observed before disinfection (Fig. 2).
Each site was sampled before disinfection (BD) and after disinfection (AD). The grommet and the mattress were also sampled after 24 h during which the incubator was turned on and a neonate housed in (24H). a Percentage of contaminated sites. The number of samples contaminated per sites for the 20 incubators is expressed in per cent. The percentage of total positive samples BD, AD, and 24H includes the 5 sites of the 20 incubators. b Percentage of bacteria isolated on the different incubator parts. For each site, the percentage of each bacterial group or species was calculated compared to the total number of isolates. Each species or group is represented by a specific colour. Details of the species identified on incubators are presented in Supplemental Table 1.
Other environmental reservoirs of bacteria
To detect other environmental putative reservoirs in the NICU, additional samples were performed. Inside the disinfection room, S. epidermidis was isolated on the computer keyboard and on the bench where pieces of incubators are placed during drying. Moreover, Bacillus cereus was isolated on the bench. In the delivery room, we identified S. hominis on the air-CO2 blender and Micrococcus luteus on the lateral door and on the timer button.
Hand colonisation among caregivers
During the study period, seven different caregivers were involved in the process of incubator disinfection and a total of 22 hand skin samples was obtained. The hand swabbing from caregivers showed a predominance of CoNS, Bacillus, Enterococcus faecalis, enterobacteria, and S. aureus before disinfection of the first incubator of the day (Fig. 3). At the end of the day, the diversity and repartition of bacteria remained similar, except for an increase in the frequency of isolation of S. aureus and the absence of enterobacteria. Even if CoNS were frequently isolated, S. capitis was isolated only on two caregivers’ hand skin samples at the end of the day.
The hands of the caregivers were sampled before disinfection of the first incubator of the day and after disinfection of the last incubator of the day. The percentage of each bacterial group or species was calculated compared to the total number of isolates. Each species or group is represented by a specific colour. Details of the species identified on the hand samples of caregivers are presented in Supplemental Table 1.
LOS occurrence during the study period
The 20 neonates housed in the incubators of the study were preterm (median gestational age 28 weeks, extremes 24–34 weeks) with a median birth weight of 1020 g (extremes 530–2075 g). Nine (45%) were small for gestational age. They were housed in one of the study incubators at a median of 13 days of life (extremes 0–43 days). Among these 20 patients, three extreme preterm patients developed a LOS during their housing in the incubator. The characteristics of these three neonates are presented in Table 1. Interestingly, for two of these three infected patients, the bacterial species involved in the sepsis has been also previously isolated in the housing incubator. The three neonates recovered from the infection.
Discussion
The present work highlights the partial ineffectiveness of the disinfection process of incubators that are currently performed in most NICUs as well as the potential consequences and risks for hospitalised neonates.
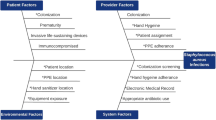
Several hypotheses could explain this partial failure in incubators’ disinfection. First, incubators are composed of many delicate parts and recesses that cannot be immersed in the disinfectant bath and that have to be manually disinfected with impregnated wipes. Two major examples are the mattress and the balance that were the most contaminated parts of the incubators after disinfection in our study and previous ones.3 Due to its tissue composition and the presence of seams, the mattress is probably a primary reservoir for bacteria. In that regard, a recent study conducted in a NICU setting showed that among 42 incubator mattresses tested, all were contaminated both on the cover and in the foam.6 Second, bacteria could resist the disinfection and persist on incubators because of their capacity to produce biofilm. Previous studies have demonstrated that bacteria embedded in biofilm matrix are less sensitive to disinfectant than planktonic ones11,12 and are able to persist in NICU environment.2,13 Third, the failure of disinfection could be related to a relative bacterial tolerance to disinfectants compounds. In particular resistance mechanisms against quaternary ammoniums have already been reported in NICU isolates.14 Finally, in light of our findings showing a wide bacterial diversity on incubators after disinfection, we have to consider a possible early recolonisation from the environment and/or caregivers. This hypothesis is reinforced by the isolation of several pathogenic species both in staff hands and incubators samples, especially CoNS that are part of the normal skin commensal microbiota. The transient carrying of CoNS pathogenic strains in caregivers has already been highlighted.3 Hira et al.2 demonstrated both a higher prevalence of multidrug-resistant CoNS on the hands of caregivers in comparison of the general population, and a 90% genetic correlation between CoNS strains isolated from caregivers and those responsible for LOS in a NICU. Thereby, we hypothesised that in our study, CoNS isolated on incubators (in particular methicillin-resistant strains, growing on MRSA Brillance 2 agar plates) may come from caregivers. This reminds the importance of the strict respect of standard hygiene measures in order to avoid cross-transmission not only during patient cares but also when touching medical devices. One limitation of our study is the absence of data concerning the hand skin colonisation of parents, who can also be involved in the steps of contamination of incubators and/or neonates. The improvement of hand hygiene of all people touching newborns and/or environmental NICU surfaces (caregivers, parents and visitors) has already been reported as one of the most important strategies to avoid cross-transmission and to decrease infection risks for patients in NICUs.15,16
In our study along with other ones, reporting such defective disinfection is of major concerns since it reflects a supplementary LOS risk factor for preterm neonates who are particularly vulnerable to infections due to their immature immune system and skin barrier.1 In our study, 2 of the 20 patients developed sepsis involving a bacterial species previously isolated in the incubator. Because of the absence of strain molecular analysis and the lack of data concerning the previous microbiota composition of these infected patients, we can neither attest nor exclude the causality between incubator contamination and patient subsequent infection. Nonetheless, the major part of bacteria isolated at the different times of our study are known as potential pathogens responsible for neonatal sepsis and have already been isolated from NICU incubators in previous studies.3,6,17 In particular, even if CoNS are part of the normal environmental colonisers, they have to be considered since they are frequently involved in LOS. The link between environmental colonisation (especially incubators) and outbreak in NICUs has previously been demonstrated involving sometimes virulent and multidrug-resistant strains.6,17,18,19 Interestingly here we highlight that while the disinfection process was effective for common pathogens (S. aureus, enterobacteria) immediately after disinfection, those pathogens quickly recolonised. This suggests that in addition to an improved process of incubator disinfection, it is necessary to perform a more frequent/in-depth cleaning of incubators during housing infants.
An improvement in incubator disinfection in NICUs worldwide is urgently required given that some authors previously reported its benefits in controlling outbreaks and in preventing nosocomial infections in NICU settings.6,19,20,21 An interesting method of disinfection could be the steam technology which consists in spraying steam at high temperature (130–150 °C) and high pressure (4–6 bars) to reach the recesses that are difficult to access with the classical chemical method.21 It has been shown to significantly reduce the bacterial load on incubators in a previous study21 and to be effective in the fight against NICU outbreaks involving either S. capitis NRCS-A or vancomycin-resistant Enterococci.19,20 Moreover it is more cost-efficient and less water consuming.21,22 Finally, it avoids exposure of neonates and staff to toxic and potentially allergenic chemicals able to adsorb on incubators surfaces.6,20,21
However, steam could not be recommended for mattress disinfection according to Cadot et al.6 who suggested that formation of moistures on the inner layers was related to the use of this method. Another approach to improve the efficacy of incubator disinfection could be an in-depth consideration about incubators’ design with fewer recesses and maybe single-use mattresses or mattress protective covers. To this end, further studies should be conducted to help in the choice of the materials constituting the different incubators’ parts to select the ones preventing microbial adherence. The combination with immobilised non-toxic antimicrobial molecules might also contribute to inhibit microbial presence in these surfaces as suggested in recent studies.23,24,25
Our work presents several limits. First of all, only five sites were sampled for each incubator which is less than other studies26 and our method of analysis only provides qualitative data. However, these sites were chosen because they are either the most frequently touched or because they have previously been identified as bacterial reservoirs within the incubators.3,6,26 Second, the discrepancy that may appear when comparing the bacteria isolated on the same incubator at the three times of the study suggests a limit of sensitivity in our protocol of sampling and bacterial identification. Third, since CoNS are part of the normal skin and environmental colonisers, our study does not allow the identification of potentially pathogenic strains among all CoNS. However, considering all CoNS isolated here is the most rigorous method to avoid underestimation of risks for neonates. In a future study, a better characterisation of the CoNS strains coming from incubators, caregivers, parents and the general population would be useful to better understand the origin and ways of neonatal contamination with CoNS in NICUs. Finally, the absence of molecular analysis of the strains prevents us to attest the link between the strains involved in infections and those identified from incubators.
Conclusions
The process of decontamination as it is currently recommended is not sufficient to eradicate bacterial colonisation from incubators and in particular pathogenic strains. Incubators could thereby represent an important reservoir of the pathogen. Incubator disinfection is a milestone in the fight against nosocomial contamination and infection. Future research should focus on re-evaluating the strategy of incubators disinfection as well as incubators materials choice and design.
References
Shah, J., Jefferies, A. L., Yoon, E. W., Lee, S. K. & Shah, P. S. Risk factors and outcomes of late-onset bacterial sepsis in preterm neonates born at < 32 weeks’ gestation. Am. J. Perinatol. 32, 675–682 (2015).
Hira, V. et al. Coagulase-negative staphylococcal skin carriage among neonatal intensive care unit personnel: from population to infection. J. Clin. Microbiol. 48, 3876–3881 (2010).
Butin, M. et al. Sources and reservoirs of Staphylococcus capitis NRCS-A inside a NICU. Antimicrob. Resist. Infect. Control 8, 157 (2019).
Carter, G. P. et al. Genomic analysis of multiresistant Staphylococcus capitis associated with neonatal sepsis. Antimicrob. Agents Chemother. 62, e00898–e00918 (2018).
Wirth, T. et al. Niche specialization and spread of Staphylococcus capitis involved in neonatal sepsis. Nat. Microbiol. 5, 735–745 (2020).
Cadot, L. et al. Extended spectrum beta-lactamase-producing Klebsiella pneumoniae outbreak reveals incubators as pathogen reservoir in neonatal care center. Eur. J. Pediatr. 178, 505–513 (2019).
Lin, D., Ou, Q., Lin, J., Peng, Y. & Yao, Z. A meta-analysis of the rates of Staphylococcus aureus and methicillin-resistant S aureus contamination on the surfaces of environmental objects that health care workers frequently touch. Am. J. Infect. Control 45, 421–429 (2017).
Butin, M. et al. Chromogenic detection procedure for the multidrug-resistant, neonatal sepsis-associated clone Staphylococcus capitis NRCS-A. Diagn. Micr. Infect. Dis. 90, 81–82 (2018).
Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13, 59 (2013).
Stoll, B. J. et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 110, 285–291 (2002).
Wong, H. S., Townsend, K. M., Fenwick, S. G., Trengove, R. D. & O’Handley, R. M. Comparative susceptibility of planktonic and 3-day-old Salmonella Typhimurium biofilms to disinfectants. J. Appl. Microbiol. 108, 2222–2228 (2010).
Costa, D. M. et al. Biofilm contamination of high-touched surfaces in intensive care units: epidemiology and potential impacts. Lett. Appl. Microbiol. 68, 269–276 (2019).
Seng, R. et al. Biofilm formation of methicillin-resistant coagulase negative staphylococci (MR-CoNS) isolated from community and hospital environments. PLoS ONE 12, e0184172 (2017).
Lepainteur, M. et al. Prevalence of resistance to antiseptics and mupirocin among invasive coagulase-negative staphylococci from very preterm neonates in NICU: the creeping threat? J. Hosp. Infect. 83, 333–336 (2013).
Polin, R. A., Denson, S. & Brady, M. T. Committee on fetus and newborn; committee on infectious diseases, epidemiology and diagnosis of health care–associated infections in the NICU. Pediatrics 129, e1104–e1109 (2012).
Lam, B. C. C., Lee, J. & Lau, Y. L. Hand hygiene practices in a neonatal intensive care unit: a multimodal intervention and impact on nosocomial infection. Pediatrics 114, e565–e571 (2004).
Golan, Y., Doron, S., Sullivan, B. & Snydman, D. R. Transmission of vancomycin-resistant Enterococcus in a neonatal intensive care unit. Pediatr. Infect. Dis. J. 24, 566–567 (2005).
Svenningsen, N. W., Békássy, A. N., Christensen, P. & Kamme, C. Nosocomial Klebsiella pneumoniae infection: clinical and hygienic measures in a neonatal intensive care unit. Scand. J. Infect. Dis. 16, 29–35 (1984).
Ory, J. et al. Successful implementation of infection control measure in a neonatal intensive care unit to combat the spread of pathogenic multidrug resistant Staphylococcus capitis. Antimicrob. Resist. Infect. Control 8, 1–6 (2019).
Gillespie, E. et al. Microfiber and steam for a neonatal service: an improved and safe cleaning methodology. Am. J. Infect. Control 45, 98–100 (2017).
Braux, C., Lagier, A., Andrini, P., Debillon, T. & Croizé, J. Entretien des incubateurs de néonatalogie à l’aide d’un générateur de vapeur. Hygienes XVI, 241–247 (2008).
Gillespie, E., Brown, R., Treagus, D., James, A. & Jackson, C. Improving operating room cleaning results with microfiber and steam technology. Am. J. Infect. Control 44, 120–122 (2016).
Salwiczek, M. et al. Emerging rules for effective antimicrobial coatings. Trends Biotechnol. 32, 82–90 (2014).
Swartjes, J. et al. Current developments in antimicrobial surface coatings for biomedical applications. Curr. Med. Chem. 22, 2116–2129 (2015).
Qu, Y. et al. Hyperosmotic infusion and oxidized surfaces are essential for biofilm formation of Staphylococcus capitis from the neonatal intensive care unit. Front. Microbiol. 11, 920 (2020).
Fattorini, M. et al. Public health since the beginning: neonatal incubators safety in a clinical setting. J. Infect. Public Health 11, 788–792 (2018).
Acknowledgements
This work was supported by ANR (Agence Nationale de la Recherche) project NeoSCap [grant number ANR 19-CE17-0004-01].
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data: M.C., M.R., J.T., and M.B. Drafting the article or revising it critically for important intellectual content: M.C., M.B., A.T., and O.C. Final approval of the version to be published: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent statement
Written information was delivered to the parents of the infants involved in this study. Oral consent was obtained from the caregivers for the analysis of their hand skin samples.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chavignon, M., Reboux, M., Tasse, J. et al. Persistent microbial contamination of incubators despite disinfection. Pediatr Res 90, 1215–1220 (2021). https://doi.org/10.1038/s41390-021-01407-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01407-8
This article is cited by
-
Disinfection of incubators in neonatal intensive care units: impact of steam pulverization on bacterial colonization
Antimicrobial Resistance & Infection Control (2023)