Abstract
Apart from its known actions as a pulmonary vasodilator, nitric oxide (NO) is a key signal mediator in the neonatal brain. Despite the extensive use of NO for pulmonary artery hypertension (PAH), its actions in the setting of brain hypoxia and ischemia, which co-exists with PAH in 20–30% of affected infants, are not well established. This review focuses on the mechanisms of actions of NO covering the basic, translational, and clinical evidence of its neuroprotective and neurotoxic properties. In this first part, we present the physiology of transport and delivery of NO to the brain and the regulation of cerebrovascular and systemic circulation by NO, as well the role of NO in the development of the immature brain.
Impact
-
NO can be transferred from the site of production to the site of action rapidly and affects the central nervous system.
-
Inhaled NO (iNO), a commonly used medication, can have significant effects on the neonatal brain.
-
NO regulates the cerebrovascular and systemic circulation and plays a role in the development of the immature brain.
-
This review describes the properties of NO under physiologic conditions and under stress.
-
The impact of this review is that it describes the effects of NO, especially regarding the vulnerable neonatal brain, and helps understand the conditions that could contribute to neurotoxicity or neuroprotection.
Similar content being viewed by others
Introduction
Nitric oxide (NO) is produced endogenously by a variety of cells following the reaction of the amino acid l-arginine with molecular oxygen with production of l-citrulline by NO synthases (NOS). There are three types of NOS that produce endogenous NO in the brain: neuronal NOS (nNOS, or NOS1), inducible NOS (iNOS or NOS2), and endothelial NOS (eNOS or NOS3). The enzyme eNOS is a membrane-bound isoform found in endothelial cells, and nNOS is a cytosolic isoform first described in neuronal tissues. All of these isoforms are activated by intracellular calcium-dependent binding of calmodulin (CaM), whereas iNOS is inducible in a variety of cells including endothelial and innate immune cells.1
One of the key intracellular effects of NO involves the production of cyclic GMP (cGMP), which is responsible for its biological actions. Because of its vasodilatory properties, inhaled NO (iNO) has been used for the treatment of pulmonary artery hypertension,2,3,4,5,6 an intervention that has decreased the need for extracorporeal membrane oxygenation.7,8 Indeed, iNO improves cardiovascular function in sepsis,9,10 and after ischemia,11 facilitates blood flow to ischemic tissues,12 improves renal function,13 increases the oxygen affinity of sickle cell erythrocytes,14 improves mesenteric flow,15 decreases trypsinogen activation peptides in pancreatitis,16 and has a significant impact in hemopoietic tissues, including platelets, neutrophils, and platelet–leukocyte interactions.17,18,19 In the brain, NO plays an important role as a biochemical neurotransmitter. On the other hand, NO is also associated with direct neurotoxic effects, which are attributed in part to energy failure, lipid peroxidation, increased production of peroxynitrite, and protein nitrosylation.20,21,22 The underlying mechanisms of NO transport and the effects on brain function are the subject of this review.
Mechanisms of regulation and transport of nitric oxide
In order to prove an effect of iNO arising from the primary organ of action—the lung, to a distal organ—the brain, it is important to determine the mechanisms of biological interactions and transport of NO from one site to the other. The extracellular fate of iNO is hindered by its short half-life, which is estimated to be <5 s, thereby necessitating a transport mechanism to influence more distal sites. NO transport involves the formation of complexes with proteins into nitrosothiols (usually termed as R-SNO). The formation of R-SNO occurs via mechanisms that involve the reaction of NO with iron that is bound to proteins. The most abundant of these proteins is hemoglobin (Hgb). Hgb contains both iron and a thiol group and is therefore ideal for transport and regulation of NO.23,24 S-nitrosothiol-modified Hgb (SNO-Hgb) or NO-related metabolites account for the distant transfer of NO and explain its extrapulmonary effects. NO blood concentrations and its transport are regulated via complex mechanisms, which involve key allosteric interactions with Hgb and oxygen (O2). Heme concentrations in blood are ~2 mM, while those of NO are <100 nM.23,25 NO production rates cannot explain the NO concentrations found in plasma where there are always unoccupied heme sites, even in the arterial circulation. As a result, Hgb cannot be viewed just as a reservoir for NO.23,25,26 The physiologic transport of NO via Hgb was first described by Stamler and co-workers23 in 1996 who identified that NO binds at a highly conserved cysteine residue (β93 position) to form SNO-Hgb.27 Gow and Stamler27 and Stamler and co-workers28 suggested that the actions of Hgb in terms of NO regulation vary depending on context with delivery, storage, or consumption of NO.
NO transport and availability depend on changes of Hgb between “relaxed” and “tense” states and the presence of a “thermodynamic” linkage.29 SNO-Hgb is the predominant form in oxygenated arterial blood, while iron-nitrosyl-Hgb is higher in venous blood. In Fig. 1, we present the complex interactions of NO, including (a) the reaction with the iron of the alpha subunits of deoxy-Hgb in red blood cells (RBCs) to form iron-nitrosyl-Hgb, (b) the reaction with cysteine resulting in the formation of SNO-Hgb, (c) its oxidation to nitrate (NO3−) contributing to the formation of the ferric form of Hgb (Met Hgb, Hgb[Fe(III)]); this reaction is not significant under physiologic conditions since Met Hgb can be recycled back to deoxy-Hgb by intra-erythrocyte Hgb reductase with simultaneous production of NO and consumption of nitrite,27 and (d) the reaction of NO with superoxide (O2.−) to form peroxynitrite (OONO−). Under physiologic conditions, the formation of peroxynitrite is limited because the superoxide dismutase (SOD)/catalase system is usually able to limit the concentration of the superoxide. Any increase in the production of superoxide, as happens in metabolic stress, or if SOD is decreased or deactivated, can result in the production of peroxynitrite. The interactions described in (a) and (b) represent the predominant forms that exist and contribute to transport and biological actions of NO, while (c) and (d) are related to NO toxicity. A key intermediate contributing to the protective role of NO in the brain is the formation of S-nitroso-glutathione (GSNO) (Fig. 1, [4]). GSNO is 100 times more potent free radical scavenger than glutathione itself,30 operates as an endogenous NO reservoir, and protects against oxidative stress.31,32 Using these mechanisms, NO protects the brain from lipid peroxidation by scavenging lipid peroxyl radicals. GSNO can also neutralize peroxynitrite, which explains the weak oxidative effects of peroxynitrite in the brain in vivo and in vitro.33,34
The presence of molecular oxygen and other factors such as CO2 contribute to the allosteric conversion of Hgb from a tense deoxygenated state to the relaxed oxygenated form. Oxygen also serves as electron donor to convert the iron-nitrosyl hemoglobin to S-nitroso-hemoglobin, SNO-Hgb (product [b], Reaction [1]). In lungs exposed to high oxygen tension Hgb is S-nitrosylated with a simultaneous decline of iron-nitrosyl hemoglobin (product [a]). This can occur by intramolecular transport of NO to cysteine (Cysβ93). NO is released by the cysteine residue when red blood cell is under conditions of deoxygenation (Reactions [2] and [3]). SNO-Hgb is also in balance with other S-nitroso-thiols such as glutathione (GSH). High concentrations of glutathione shift the reaction towards the deoxy (Tense) form (Reactions [4] and [5]) providing an endogenous protective mechanism for NO. Reaction [c] shows alternative ways of production or consumption of NO in different redox states of the cell, either with production of Met-Hg and nitrate or by consumption of nitrite and Met-Hg. Reaction [d] shows the production of peroxynitrite upon reaction with superoxide. Glutathione as well as the product of Reaction [4], S-nitroso-glutathione (GSNO), exacerbates the vasodilatory effect of SNO-Hgb. In SNO-Hgb, NO may exert its actions via nitrosonium ion (NO+) rather than the free radical NO. Nitrosonium is created by the reaction of NO with molecules that carry a metal center such as hemoglobin or ceruloplasmin, typically with the formation of intermediate thiols, which can create SNO if there is an electron acceptor such as oxygen, adequate flavin adenine dinucleotide (FAD), or nicotinamide adenine dinucleotide (NAD). NO is unlikely to be able to escape the RBCs as a free radical and is likely released as nitrosonium via the formation of iron-nitrosyl Hg as intermediate or as SNO-Hgb (Reaction [2]).
Both iron-nitrosyl-Hgb and SNO-Hgb concentrations have been measured in the circulation.23,24,35 In umbilical cord blood samples that were obtained from newborns delivered between 37 and 42 weeks of gestation, SNO-Hgb was almost twice as high in the umbilical vein than in the artery.36 NO in the blood activates soluble guanylyl cyclase in vascular smooth muscle to maintain normal vascular tone and oxygen delivery, whereas excess NO is sequestered by Hgb and could contribute to more effective distal delivery of O2 and NO.37
The most likely source of NO that is physiologically transferred via the RBCs is eNOS, although inhaled NO is another possibility (Fig. 1, [3]). As RBCs pass through lung capillaries in close proximity to alveoli, both NO and O2 are taken up. Because of the high tension of O2, the allosteric conformation of Hgb changes and NO is taken up to form SNO-Hgb. In peripheral tissues, RBC oxygen falls as O2 is delivered, causing conformational change to iron-nitrosyl Hgb and NO is released, resulting in regional vasodilatation so as to match its function with the metabolic demand of the tissue.
NO exists in different redox forms that are derived from the oxidation states of nitrogen and mimic those of oxygen: free radical NO·, nitrosonium NO+, and nitroxyl anion NO−. Of these, nitrosonium ion contributes to the formation of SNOs by being a potent nitrosating agent. Nitrosonium is created by the reaction of NO with molecules that carry a metal center such as Hgb or ceruloplasmin,27,38 with the formation of intermediate thiols that can create SNO if there is an electron acceptor such as oxygen with adequate flavin adenine dinucleotide and nicotinamide adenine dinucleotide.39 NO is unlikely to be able to escape the RBCs as a free radical and is speculated to be released as nitrosonium via the formation of iron-nitrosyl Hgb as an intermediate or as SNO-Hgb (Fig. 1, [2]).40,41
The transport of NO as SNO-Hgb from the RBC to the site of action occurs using two mechanisms (a) as thiol carriers (glutathione disulfide-stimulated ATPases), which are exported by the RBCs,42 or (b) by direct association of Hgb with the red cell membrane, which will be able to donate the NO group from Cysβ93.43
The local production of NO in the brain has multiple possible sources. The obvious site of production, the endothelium, appears to contribute only partially as shown in studies of arteries with denuded endothelium44 or destruction of the endothelium with different methods such as light-dye techniques.45 NO most likely also arises from sites where NOS has been identified, such as neurons, astrocytes, and perivascular nerves.46 As noted earlier, another stimulus for intravascular NO production is the shear stress from blood flow, particularly in smaller arteries.47,48
NO and cerebrovascular autoregulation
(A) NOS effect: Cerebral autoregulation involves complex interactions between neurogenic and endothelial factors with the goal to match cerebral blood flow (CBF) to metabolic demand. The role of endogenous NO in the maintenance of CBF has been investigated using indirect approaches such as by blocking NOS with pharmacologic inhibitors. Two commonly used inhibitors of NOS are l-arginine methyl ester (L-NAME) and l-monomethyl l-arginine, which are analogs of l-arginine and interfere with the active site of the enzymes. NOS inhibitors have several serious limitations, including their interference of cytochrome C, their ability to induce apoptosis, as well as the ability for the de novo production of NO.49,50
Under normal conditions, NOS inhibition reduces CBF.51,52 After brain hypoxia/ischemia and reperfusion, both nNOS and eNOS participate in the modulation of CBF. In Table 1a, we summarize data from key animal studies that show the role of NOS under normal and various abnormal conditions. Basal microvascular tone is controlled by NO produced by nNOS localized in nerve endings in close proximity to the vascular system. While nNOS is involved in the regulation of cerebral vascular tone and blood flow, particularly in response to hypoxia and hypotension, eNOS regulates flow-mediated vasodilatation.
After hypoxia, NO content rises as a result of increasing calcium concentrations. In the acute phase of ischemia, NO is produced by the activation of nNOS, while later microglia-related eNOS or iNOS (neuro-inflammation) also contribute to the production of NO.53,54 In a model of transient focal cerebral ischemia in rats, the effect of the low versus high doses and timing of administration of L-NAME was studied. Interestingly, mild decrease in the ability of the brain to produce NO is neuroprotective following brief ischemia, as well as in stroke.55,56 In acute ischemia, NO is a major mediator of deviation of CB via collaterals to areas of brain that have been subjected to ischemia.
The severity of hypoxemia might also affect the role of NO as a mediator of CBF. Experiments in rats using microspheres noted that CBF increased after a drop in PaO2 to 33 mmHg (severe hypoxemia) or to 45–60 mmHg (moderate hypoxemia). The increase in CBF in the severely hypoxic group was attenuated when NOS was blocked with l-NAME.51,57 Despite conflicting results, partial blockade of the NOS system is neuroprotective, while complete inhibition is destructive.58,59,60,61
(B) iNO effect: In Table 1b, key studies and their associated methods in various animal models are presented. Of note, iNO did not significantly affect the CBF in a ventilated sheep model when measured by microsphere technique.62 On the other hand, a dose-dependent increase in CBF was observed in a swine model under physiologic conditions without affecting systemic blood pressure. CBF in the latter study was assessed with NIRS and indicator dilution techniques.63 Given the differences in methods, animal models and developmental stages, the effect of iNO on the distal versus proximal cerebral vessels needs to be investigated further.
Initiation of 20 p.p.m. of iNO is associated with a significant and time-related increase in brain NO as measured with continuous monitoring via a voltametric technique with carbon fiber electrodes.64 Investigators noted that if iNO was applied during the ischemic phase, it was protective by increasing collateral arterial recruitment, increasing blood flow to the ischemic brain and decreasing oxidative injury as measured by nitrotyrosine-positive brain cells. However, if iNO was applied during reperfusion, the effects were detrimental as shown by increasing infarct size and blood flow. In this experiment, 20 p.p.m. of iNO was able to overcome NOS inhibition with L-NAME.65 In mice, iNO applied in 30% oxygen/70% air mixture formed nitric oxide carriers in the blood that distributed throughout the body. After experimental cerebral ischemia induced by transient middle cerebral artery occlusion, iNO was associated with dilated arterioles, increased collateral blood flow, and reduced ischemic brain damage, improving the neurological outcome.66 NOS inhibitors impair cerebrovascular autoregulation during moderate hypotension in rats. This phenomenon was observed in the cerebrum, cerebellar cortex, and basal ganglia.67 NO also attenuates the normal oscillations of the diameter of the cerebral arterioles, a reaction opposite to that observed in the mesenteric arteries.68
The stage of development and the duration, as well as the dose of iNO also influence outcomes. Thus, iNO at 40 p.p.m. for 23 h administered 1 h after cardiac arrest and cardiopulmonary resuscitation (CPR) in adult mice prevents water diffusion abnormalities, caspase-3 activation, and cytokine induction in the brain. Deficiency of the α1 subunit of soluble guanyl cyclase prevented the ability of iNO to improve outcomes after CPR.69 In these studies, the application of iNO at higher dose and for a prolonged time during the reperfusion phase was associated with positive effects. Further, using a modified Vannucci model in PN9 mice, high dose iNO (50 p.p.m.) reduced neuronal damage when given during hypoxia,66 while iNO given after hypoxia–ischemia increased ischemic lesions.70 iNO at a higher dose (80 p.p.m.) was associated with increased blood flow and deleterious effects, which could be related to the accumulation of peroxynitrite.65
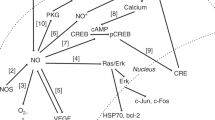
The biochemical effects of iNO that contribute to the maintenance of brain autoregulation are poorly investigated and most of the knowledge is extrapolated from studies that involve the endogenous production of NO as noted above. Central nervous system (CNS) vascular tone is mediated by control of endogenous cellular mechanisms, such as local cGMP concentration. Indeed, cGMP along with other factors, such as prostaglandin E, cyclic AMP, and protein kinase A, can affect the activation of ATP-sensitive (KATP) and voltage-gated (KV) K+ channels, which exist at the cell membrane and, once activated, can cause hyperpolarization of the cell, membrane calcium channel deactivation, decrease in intracellular calcium, and vasodilatation. Similar effects can occur via direct decrease in calcium release from sarco/endoplasmic reticulum Ca2+-ATPase via the protein kinase G. Brain injury and associated loss of autoregulation involves the deactivation of the these K+ channels and iNO modulates their function to be protective in maintaining autoregulation.71 Although the exact mechanisms have not been elucidated, one effect of iNO is to interfere with the production of endothelin-1 (ET-1) and activation of extracellular signal-regulated kinase (ERK) isoform of mitogen-activated protein kinase (MAPK).72 ET-1 is known to impair opening of K+ channels via the release of ROS and phosphorylation of ERK/MAPK, a distal signaling system important in the control of CBF.
In summary, the preclinical studies highlighted above show a benefit in using iNO during the acute phase of brain ischemia in contrast to its use during the reperfusion period, which might exacerbate brain injury.
Role of iNO in neuronal development, myelination, and memory
Endogenously produced NO plays an important role in the development of the CNS.73,74 NO promotes immature oligodendrocyte myelination and in animal models of stroke is neuroprotective,65,75,76,77 possibly by changing the expression patterns of semaphorins, key membrane proteins for axonal growth guidance and migration.75,78 The global inhibition of NOS in rodents significantly decreases myelin density in the corpus callosum and striatum and affects long-term cognitive behavior.75 Gibbs et al.79 showed that NO works to coordinate proliferation and patterning during brain development and endogenous NO production is upregulated after brain damage which results in neurogenesis.80,81
Olivier et al.75 showed that iNO exposure at 20 p.p.m. during the first week of life was associated with significant increase in myelin fiber density at P7 in several white matter areas, including the lateral corpus callosum in the normal neonatal rat. The effect was more pronounced in periventricular white matter and to a lesser degree in the cortex. In P1 animals, brain cGMP was increased within 2 h of initiation of iNO, suggesting that the effects of iNO were observed in the brain. In P3 and P7 animals, the effect was less obvious, suggesting that there is a change in response with age.75 Rodents who were treated with low dose iNO (5 p.p.m.) demonstrated attenuated hyperoxia-induced white matter inflammation, cell death, and enhanced the density of proliferating oligodendrocytes and oligodendroglia maturation. iNO enhanced an early upregulation of P27kip1 and brain-derived growth factor, and iNO-treated animals maintained learning scores to a level similar to that of normoxic controls.82 Phan Duy et al.83 showed that both early (30 h) and late (7 days) administration of iNO (at 20 p.p.m.) caused proliferative effects on progenitor cells on several zones of the subventricular zone, white matter, and cortex. In experiments that included co-labeling of progenitor cells with the proliferative marker BrDu and caspase-3, a marker of apoptosis, iNO prevented apoptosis and these cells had normal survival. Under physiologic conditions, astrocytes as well as pericytes were increased in cortical and white matter areas, changes suggestive of increased angiogenesis. iNO could involve the modulation of erythropoietin (EPO) which was associated with enhancement in the proliferation and migration of the site of injury of subventricular zone (SVZ) neural progenitors.
The effects of iNO on the development and organization of neuronal pathways, suggested possible effects on memory. NOS inhibitors suppress spatial memory,84 object recognition,85 and alter the behavior in a variety of animal models.86,87 It is interesting that nNOS appears to be more specific for the development of spatial and working memory,88 social interactions, and fear,89 while eNOS knockout mice exhibit enhanced spatial learning, but increased anxiety-like behaviors.90 Both short- and long-term memory have been studied in a variety of animal models of traumatic brain injury (TBI). Typically, after TBI, there is an inflammatory response with activation of microglia and astrocytes with secondary increases in interleukins followed by cerebral edema, loss of autoregulation, and eventually neuronal loss. It is interesting that there is a relative decrease in brain NO between 5 min to 3 h after TBI, which makes the need of supplementing iNO under these conditions a potential therapeutic intervention. For example, in a study where mice were given mild TBI, exposure to iNO for 4, 8, and 24 h at 10 p.p.m., was associated with improved short-term memory (as measured by Object Recognition Task Assessment) at 1, 3, and 7 days post injury.91 By histology, NO-treated mice had an improved activation of microglia (as assessed by CD45) and astrocytes (by glial fibrillary acidic protein assays), and this effect appeared to improve with the duration of treatment up to 8 h, but not for longer exposures.92 The effects of iNO in modulation of long-term memory are less well described.93,94
These preclinical studies demonstrate that iNO at lower doses improves myelination and neuronal density, as well as associated memory functions.
In summary, in this first of a two-part review, we describe the cellular and molecular mechanisms of NO in the CNS with an effort to determine potential neuroprotective or neurotoxic effects of its inhaled form (iNO), a commonly used medication in the neonatal intensive care unit.
iNO has profound local vasodilatory effects in the lung and understanding the mechanism of transport and unloading of NO by the RBC is important, especially since it affects brain autoregulation, metabolism, and function. Despite the extensive use of iNO as a pulmonary vasodilator, the exact effects in the neonatal brain remain poorly investigated.
NO functions in the CNS like a neurotransmitter with unique and distinguishing features. Some of these features such as the ability for fast production, the multiple sources of production, and its ability to move extremely fast through biological membranes away from its production site, differ from other locally produced neurotransmitters. Despite its short half-life, NO can affect other downstream pathways, modulating their action and hence causing long-lasting biological effects.
The inhaled form of NO contributes to neuroprotection after ischemia in the developing brain depending on the concentrations, stage of development, the timing, and the duration of exposure after the insult. Excessive NO is associated with free radical production and neurotoxicity. The neonatal brain is more susceptible to oxidative stress after hypoxia–ischemia and more sensitive to downstream activation of apoptotic mechanisms. NO can interfere directly at the level of mitochondria and trigger the initiation of apoptosis. Although preclinical studies summarized in this review suggest a benefit in the acute ischemic phase, there are also potential detrimental effects during reperfusion. iNO at low doses improves myelination and neuronal density, as well as associated functions such as memory, while higher doses result in toxicity.
Significant gaps in knowledge, due to lack of studies especially in newborns, include methods capable of measuring brain NO concentrations in real time and their correlation with anatomic sites as well as NOS subtype activity (nNOS, eNOS, or iNOS) and downstream biochemical effects. It is possible that usual doses of inhaled NO might be neurotoxic for the neonatal brain during both brief and extended periods of time, especially under conditions of oxidative stress and hypoxia–ischemia–reperfusion. In the second part of this review article, we will focus on the known effects of iNO under pathological conditions, with an overall goal to identify areas for future investigation and research.
References
Alderton, W. K., Cooper, C. E. & Knowles, R. G. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 357, 593–615 (2001).
Abman, S. H. & Kinsella, J. P. Inhaled nitric oxide for persistent pulmonary hypertension of the newborn: the physiology matters! Pediatrics 96, 1153–1155 (1995).
Roberts, J. D., Polaner, D. M., Lang, P. & Zapol, W. M. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 340, 818–819 (1992).
Kinsella, J. P. et al. Clinical responses to prolonged treatment of persistent pulmonary hypertension of the newborn with low doses of inhaled nitric oxide. J. Pediatr. 123, 103–108 (1993).
Kinsella, J. P., Neish, S. R., Shaffer, E. & Abman, S. H. Low-dose inhalation nitric oxide in persistent pulmonary hypertension of the newborn. Lancet 340, 819–820 (1992).
Roberts, J. D.Jr. The Inhaled Nitric Oxide Study Group et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N. Engl. J. Med. 336, 605–610 (1997).
Lotze, A.Survanta in Term Infants Study Group et al. Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. J. Pediatr. 132, 40–47 (1998).
Pedoto, A. et al. Treatment of septic shock in rats with nitric oxide synthase inhibitors and inhaled nitric oxide. Crit. Care Med. 26, 2021–2028 (1998).
Barrington, K. J. et al. The hemodynamic effects of inhaled nitric oxide and endogenous nitric oxide synthesis blockade in newborn piglets during infusion of heat-killed group B streptococci. Crit. Care Med. 28, 800–808 (2000).
Ishihara, S. et al. Inhaled nitric oxide prevents left ventricular impairment during endotoxemia. J. Appl. Physiol. (1985) 85, 2018–2024 (1998).
Hataishi, R. et al. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 291, H379–H384 (2006).
Fox-Robichaud, A. et al. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J. Clin. Invest. 101, 2497–2505 (1998).
Troncy, E., Francoeur, M. & Blaise, G. Nitric oxide (NO)/nitrogen dioxide (NO2) scavengers. Br. J. Anaesth. 80, 697–698 (1998).
Head, C. A. et al. Low concentrations of nitric oxide increase oxygen affinity of sickle erythrocytes in vitro and in vivo. J. Clin. Invest. 100, 1193–1198 (1997).
Ng, E. S. et al. Enhanced S-nitroso-albumin formation from inhaled NO during ischemia/reperfusion. Circ. Res. 94, 559–565 (2004).
Werner, J. et al. Differing roles of nitric oxide in the pathogenesis of acute edematous versus necrotizing pancreatitis. Surgery 121, 23–30 (1997).
Beghetti, M. et al. Inhaled NO inhibits platelet aggregation and elevates plasma but not intraplatelet cGMP in healthy human volunteers. Am. J. Physiol. Heart Circ. Physiol. 285, H637–H642 (2003).
Gessler, P. et al. A new side effect of inhaled nitric oxide in neonates and infants with pulmonary hypertension: functional impairment of the neutrophil respiratory burst. Intens. Care Med. 22, 252–258 (1996).
Gries, A. et al. Randomized, placebo-controlled, blinded and cross-matched study on the antiplatelet effect of inhaled nitric oxide in healthy volunteers. Thromb. Haemost. 83, 309–315 (2000).
Baud, O. et al. Nitric oxide-induced cell death in developing oligodendrocytes is associated with mitochondrial dysfunction and apoptosis-inducing factor translocation. Eur. J. Neurosci. 20, 1713–1726 (2004).
Li, J. et al. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc. Natl Acad. Sci. USA 102, 9936–9941 (2005).
Iadecola, C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 20, 132–139 (1997).
Jia, L., Bonaventura, C., Bonaventura, J. & Stamler, J. S. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380, 221–226 (1996).
Stamler, J. S. et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276, 2034–2037 (1997).
Vallance, P. et al. Direct measurement of nitric oxide in human beings. Lancet 346, 153–154 (1995).
Lancaster, J. R. Jr. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc. Natl Acad. Sci. USA 91, 8137–8141 (1994).
Gow, A. J. & Stamler, J. S. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature 391, 169–173 (1998).
Hausladen, A., Gow, A. J. & Stamler, J. S. Nitrosative stress: metabolic pathway involving the flavohemoglobin. Proc. Natl Acad. Sci. USA 95, 14100–14105 (1998).
McMahon, T. J. et al. Functional coupling of oxygen binding and vasoactivity in S-nitrosohemoglobin. J. Biol. Chem. 275, 16738–16745 (2000).
Rauhala, P., Lin, A. M. & Chiueh, C. C. Neuroprotection by S-nitrosoglutathione of brain dopamine neurons from oxidative stress. FASEB J. 12, 165–173 (1998).
Konorev, E. A. et al. S-nitrosoglutathione improves functional recovery in the isolated rat heart after cardioplegic ischemic arrest-evidence for a cardioprotective effect of nitric oxide. J. Pharm. Exp. Ther. 274, 200–206 (1995).
Konorev, E. A., Joseph, J., Tarpey, M. M. & Kalyanaraman, B. The mechanism of cardioprotection by S-nitrosoglutathione monoethyl ester in rat isolated heart during cardioplegic ischaemic arrest. Br. J. Pharm. 119, 511–518 (1996).
Rauhala, P., Sziraki, I. & Chiueh, C. C. Peroxidation of brain lipids in vitro: nitric oxide versus hydroxyl radicals. Free Radic. Biol. Med 21, 391–394 (1996).
Rauhala, P. et al. S-nitrosothiols and nitric oxide, but not sodium nitroprusside, protect nigrostriatal dopamine neurons against iron-induced oxidative stress in vivo. Synapse 23, 58–60 (1996).
Gladwin, M. T. et al. Inhaled nitric oxide augments nitric oxide transport on sickle cell hemoglobin without affecting oxygen affinity. J. Clin. Invest. 104, 937–945 (1999).
Funai, E. F., Davidson, A., Seligman, S. P. & Finlay, T. H. S-nitrosohemoglobin in the fetal circulation may represent a cycle for blood pressure regulation. Biochem. Biophys. Res. Commun. 239, 875–877 (1997).
Yonetani, T. Nitric oxide and hemoglobin. Nihon Yakurigaku Zasshi 112, 155–160 (1998).
Inoue, K. et al. Nitrosothiol formation catalyzed by ceruloplasmin. Implication for cytoprotective mechanism in vivo. J. Biol. Chem. 274, 27069–27075 (1999).
Gow, A. J. & Ischiropoulos, H. Nitric oxide chemistry and cellular signaling. J. Cell. Physiol. 187, 277–282 (2001).
Jeffers, A. et al. Hemoglobin mediated nitrite activation of soluble guanylyl cyclase. Comp. Biochem. Physiol. A 142, 130–135 (2005).
Luchsinger, B. P. et al. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc. Natl Acad. Sci. USA 100, 461–466 (2003).
Kondo, T., Dale, G. L. & Beutler, E. Thiol transport from human red blood cells. Methods Enzymol. 252, 72–82 (1995).
Salhany, J. M. & Gaines, E. D. Steady state kinetics of erythrocyte anion exchange. Evidence for site–site interactions. J. Biol. Chem. 256, 11080–11085 (1981).
Toda, N., Ayajiki, K., Enokibori, M. & Okamura, T. Monkey cerebral arterial relaxation caused by hypercapnic acidosis and hypertonic bicarbonate. Am. J. Physiol. 265, H929–H933 (1993).
Wang, Q., Pelligrino, D. A., Koenig, H. M. & Albrecht, R. F. The role of endothelium and nitric oxide in rat pial arteriolar dilatory responses to CO2 in vivo. J. Cereb. Blood Flow. Metab. 14, 944–951 (1994).
Iadecola, C., Pelligrino, D. A., Moskowitz, M. A. & Lassen, N. A. Nitric oxide synthase inhibition and cerebrovascular regulation. J. Cereb. Blood Flow. Metab. 14, 175–192 (1994).
Gaw, A. J. & Bevan, J. A. Flow-induced relaxation of the rabbit middle cerebral artery is composed of both endothelium-dependent and -independent components. Stroke 24, 105–109 (1993). discussion 109–110.
Faraci, F. M. Role of endothelium-derived relaxing factor in cerebral circulation: large arteries vs. microcirculation. Am. J. Physiol. 261, H1038–H1042 (1991).
Peterson, D. A., Peterson, D. C., Archer, S. & Weir, E. K. The non specificity of specific nitric oxide synthase inhibitors. Biochem. Biophys. Res. Commun. 187, 797–801 (1992).
Hecker, M. et al. Endothelial cells metabolize NG-monomethyl-l-arginine to l-citrulline and subsequently to l-arginine. Biochem. Biophys. Res. Commun. 167, 1037–1043 (1990).
Buchanan, J. E. & Phillis, J. W. The role of nitric oxide in the regulation of cerebral blood flow. Brain Res. 610, 248–255 (1993).
Sandor, P., Komjati, K., Reivich, M. & Nyary, I. Major role of nitric oxide in the mediation of regional CO2 responsiveness of the cerebral and spinal cord vessels of the cat. J. Cereb. Blood Flow. Metab. 14, 49–58 (1994).
Huang, Z. et al. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science 265, 1883–1885 (1994).
Ashwal, S., Cole, D. J., Osborne, T. N. & Pearce, W. J. Dual effects of l-NAME during transient focal cerebral ischemia in spontaneously hypertensive rats. Am. J. Physiol. 267, H276–H284 (1994).
Margaill, I., Allix, M., Boulu, R. G. & Plotkine, M. Dose- and time-dependence of l-NAME neuroprotection in transient focal cerebral ischaemia in rats. Br. J. Pharm. 120, 160–163 (1997).
Ashwal, S., Cole, D. J., Osborne, T. N. & Pearce, W. J. Low dose l-NAME reduces infarct volume in the rat MCAO/reperfusion model. J. Neurosurg. Anesthesiol. 5, 241–249 (1993).
Pelligrino, D. A., Koenig, H. M. & Albrecht, R. F. Nitric oxide synthesis and regional cerebral blood flow responses to hypercapnia and hypoxia in the rat. J. Cereb. Blood Flow. Metab. 13, 80–87 (1993).
Wainwright, M. S., Grundhoefer, D., Sharma, S. & Black, S. M. A nitric oxide donor reduces brain injury and enhances recovery of cerebral blood flow after hypoxia–ischemia in the newborn rat. Neurosci. Lett. 415, 124–129 (2007).
Trifiletti, R. R. Neuroprotective effects of NG-nitro-l-arginine in focal stroke in the 7-day old rat. Eur. J. Pharm. 218, 197–198 (1992).
Bonnin, P. et al. Dual action of NO synthases on blood flow and infarct volume consecutive to neonatal focal cerebral ischemia. Exp. Neurol. 236, 50–57 (2012).
Shapira, S., Kadar, T. & Weissman, B. A. Dose-dependent effect of nitric oxide synthase inhibition following transient forebrain ischemia in gerbils. Brain Res. 668, 80–84 (1994).
Rosenberg, A. A., Kinsella, J. P. & Abman, S. H. Cerebral hemodynamics and distribution of left ventricular output during inhalation of nitric oxide. Crit. Care Med. 23, 1391–1397 (1995).
Kuebler, W. M. et al. Inhaled nitric oxide induces cerebrovascular effects in anesthetized pigs. Neurosci. Lett. 348, 85–88 (2003).
Rivot, J. P. et al. Nitric oxide (NO): in vivo electrochemical monitoring in the dorsal horn of the spinal cord of the rat. Brain Res. 773, 66–75 (1997).
Charriaut-Marlangue, C. et al. Inhaled nitric oxide reduces brain damage by collateral recruitment in a neonatal stroke model. Stroke 43, 3078–3084 (2012).
Terpolilli, N. A. et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ. Res. 110, 727–738 (2012).
Tanaka, K. et al. Inhibition of nitric oxide synthesis impairs autoregulation of local cerebral blood flow in the rat. Neuroreport 4, 267–270 (1993).
Dirnagl, U., Lindauer, U. & Villringer, A. Nitric oxide synthase blockade enhances vasomotion in the cerebral microcirculation of anesthetized rats. Microvasc. Res. 45, 318–323 (1993).
Minamishima, S. et al. Inhaled nitric oxide improves outcomes after successful cardiopulmonary resuscitation in mice. Circulation 124, 1645–1653 (2011).
Joriot-Chekaf, S. et al. Evaluation of inhaled.NO in a model of rat neonate brain injury caused by hypoxia–ischaemia. Injury 41, 517–521 (2010).
Armstead, W. M. Cerebral blood flow autoregulation and dysautoregulation. Anesthesiol. Clin. 34, 465–477 (2016).
Pastor, P., Curvello, V., Hekierski, H. & Armstead, W. M. Inhaled nitric oxide protects cerebral autoregulation through prevention of impairment of ATP and calcium sensitive K channel mediated cerebrovasodilation after traumatic brain injury. Brain Res. 1711, 1–6 (2019).
Gao, Y. & Raj, J. U. Regulation of the pulmonary circulation in the fetus and newborn. Physiol. Rev. 90, 1291–1335 (2010).
Steinert, J. R., Chernova, T. & Forsythe, I. D. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist 16, 435–452 (2010).
Olivier, P. et al. Nitric oxide plays a key role in myelination in the developing brain. J. Neuropathol. Exp. Neurol. 69, 828–837 (2010).
Pansiot, J. et al. Neuroprotective effect of inhaled nitric oxide on excitotoxic-induced brain damage in neonatal rat. PLoS ONE 5, e10916 (2010).
Matthews, P. M., Edison, P., Geraghty, O. C. & Johnson, M. R. The emerging agenda of stratified medicine in neurology. Nat. Rev. Neurol. 10, 15–26 (2014).
Castellani, V., De Angelis, E., Kenwrick, S. & Rougon, G. Cis and trans interactions of L1 with neuropilin-1 control axonal responses to semaphorin 3A. EMBO J. 21, 6348–6357 (2002).
Gibbs, S. M. Regulation of neuronal proliferation and differentiation by nitric oxide. Mol. Neurobiol. 27, 107–120 (2003).
Ciani, E. et al. Proliferation of cerebellar precursor cells is negatively regulated by nitric oxide in newborn rat. J. Cell Sci. 119, 3161–3170 (2006).
Torroglosa, A. et al. Nitric oxide decreases subventricular zone stem cell proliferation by inhibition of epidermal growth factor receptor and phosphoinositide-3-kinase/Akt pathway. Stem Cells 25, 88–97 (2007).
Pham, H. et al. Inhaled NO prevents hyperoxia-induced white matter damage in neonatal rats. Exp. Neurol. 252, 114–123 (2014).
Phan Duy, A. et al. Nitric oxide pathway and proliferation of neural progenitors in the neonatal rat. Dev. Neurosci. 37, 417–427 (2015).
Bohme, G. A. et al. Possible involvement of nitric oxide in long-term potentiation. Eur. J. Pharm. 199, 379–381 (1991).
Cobb, B. L. et al. Chronic administration of l-NAME in drinking water alters working memory in rats. Brain Res. Bull. 38, 203–207 (1995).
Qiang, M. et al. Nitric oxide is involved in the formation of learning and memory in rats: studies using passive avoidance response and Morris water maze task. Behav. Pharm. 8, 183–187 (1997).
Majlessi, N., Choopani, S., Bozorgmehr, T. & Azizi, Z. Involvement of hippocampal nitric oxide in spatial learning in the rat. Neurobiol. Learn. Mem. 90, 413–419 (2008).
Holscher, C., McGlinchey, L., Anwyl, R. & Rowan, M. J. 7-Nitro indazole, a selective neuronal nitric oxide synthase inhibitor in vivo, impairs spatial learning in the rat. Learn. Mem. 2, 267–278 (1996).
Kelley, J. B., Balda, M. A., Anderson, K. L. & Itzhak, Y. Impairments in fear conditioning in mice lacking the nNOS gene. Learn. Mem. 16, 371–378 (2009).
Frisch, C. et al. Superior water maze performance and increase in fear-related behavior in the endothelial nitric oxide synthase-deficient mouse together with monoamine changes in cerebellum and ventral striatum. J. Neurosci. 20, 6694–6700 (2000).
Bevins, R. A. & Besheer, J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study ‘recognition memory’. Nat. Protoc. 1, 1306–1311 (2006).
Liu, P. et al. Inhaled nitric oxide improves short term memory and reduces the inflammatory reaction in a mouse model of mild traumatic brain injury. Brain Res. 1522, 67–75 (2013).
Garthwaite, J. Concepts of neural nitric oxide-mediated transmission. Eur. J. Neurosci. 27, 2783–2802 (2008).
Puzzo, D., Palmeri, A. & Arancio, O. Involvement of the nitric oxide pathway in synaptic dysfunction following amyloid elevation in Alzheimer’s disease. Rev. Neurosci. 17, 497–523 (2006).
Tong, X. K. & Hamel, E. Basal forebrain nitric oxide synthase (NOS)-containing neurons project to microvessels and NOS neurons in the rat neocortex: cellular basis for cortical blood flow regulation. Eur J Neurosci. 12, 2769–2780 (2000).
Bauser-Heaton, H. D. & Bohlen, H. G. Cerebral microvascular dilation during hypotension and decreased oxygen tension: a role for nNOS. Am J Physiol Heart Circ Physiol. 293, H2193–H2201 (2007).
Huang, P. L. et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377, 239–242 (1995).
Topel, I., Stanarius, A. & Wolf, G. Distribution of the endothelial constitutive nitric oxide synthase in the developing rat brain: an immunohistochemical study. Brain Res. 788, 43–48 (1998).
Scotland, R. S. et al. Functional reconstitution of endothelial nitric oxide synthase reveals the importance of serine 1179 in endothelium-dependent vasomotion. Circ Res. 90, 904–910 (2002).
Santizo, R., Baughman, V. L. & Pelligrino, D. A. Relative contributions from neuronal and endothelial nitric oxide synthases to regional cerebral blood flow changes during forebrain ischemia in rats. Neuroreport. 11, 1549–1553 (2000).
McCullough, L. D., Zeng, Z., Blizzard, K. K., Debchoudhury, I. & Hurn, P. D. Ischemic nitric oxide and poly (ADP-ribose) polymerase-1 in cerebral ischemia: male toxicity, female protection. J Cereb Blood Flow Metab. 25, 502–512 (2005).
Terpolilli, N. A., Kim, S. W., Thal, S. C., Kuebler, W. M. & Plesnila, N. Inhaled nitric oxide reduces secondary brain damage after traumatic brain injury in mice. J Cereb Blood Flow Metab. 33, 311–318 (2013).
Pham, H. et al. Inhaled NO protects cerebral white matter in neonatal rats with combined brain and lung injury. Am J Respir Crit Care Med. 185, 897–899 (2012).
Acknowledgements
R.S. holds the William Buchanan Chair in Pediatrics, and L.C. is supported by NIH Grant 1R01NS102617-01.
Author information
Authors and Affiliations
Contributions
D.A. contributed to the concept of the paper, wrote the initial and revised drafts of this manuscript, and approved the final manuscript as submitted; R.S. contributed to the conceptualization of the paper, reviewed and revised the manuscript, and approved the final manuscript as submitted; L.C. contributed to the conceptualization of the paper, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Competing interests
R.S. is on the Scientific Advisory Council of Mallinckrodt Pharmaceuticals and had no role in the development of this review. D.A. and L.C. have no conflicts of interest to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Angelis, D., Savani, R. & Chalak, L. Nitric oxide and the brain. Part 1: Mechanisms of regulation, transport and effects on the developing brain. Pediatr Res 89, 738–745 (2021). https://doi.org/10.1038/s41390-020-1017-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-1017-0
This article is cited by
-
Inhaled nitric oxide suppresses neuroinflammation in experimental ischemic stroke
Journal of Neuroinflammation (2023)
-
Chronic stress but not acute stress decreases the seizure threshold in PTZ-induced seizure in mice: role of inflammatory response and oxidative stress
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)