Abstract
Background
The earliest onset of puberty had shifted downward, which may be due to the role of early growth and development factors in childhood.
Methods
All of 1575 Kindergarten Two (K2) children from Anhui province, China were followed up to elementary school. Girls (n = 342) with available data on AR and breast development were included for this analysis. Polygenic risk score (PRS) was computed based on 17 single nucleotide polymorphisms for early puberty. Accelerate failure time (AFT) model was used to describe thelarche timing by early AR among girls with different polygenic susceptibility.
Results
After adjustment for perinatal anthropometric, household income, parental education and prepuberty BMI-Z score, puberty started 4.12-month earlier in early AR girls compared with normal AR girls (TR: 0.96; 95% CI: 0.95, 0.98, p < 0.001). Furthermore, this puberty-accelerating effect was observed among girls with high (6.06-month earlier, TR: 0.94; 95% CI: 0.90, 0.99) and moderate PRS (4.20-month earlier, TR: 0.96; 95% CI: 0.93, 0.98). No similar results were observed in the low PRS groups (TR: 1.00; 95% CI: 0.96, 1.04).
Conclusions
Girls with early AR displayed younger age at thelarche; however, this accelerating effect was only observed among those with genetic susceptibility to early puberty.
Impact
-
Early AR plays a more important role in predicting earlier thelarche among girls with high and moderate PRS.
-
This study combined with the hot topics of pubertal-related polygenic risk score (PRS) for pubertal timing to examine the longitudinal association between early AR with accelerated pubertal onset.
-
Our results mean that accelerating growth in the early childhood years after birth might forecast early puberty only among girls with genetic predisposition to early puberty. Prevention strategies and management options should be emphasized to target early childhood to address secular trend for early puberty observed in the past decades in China.
Similar content being viewed by others
Introduction
The childhood–adolescent–adulthood transition is an important milestone in human development, of which puberty is a pivotal phase in the life-course whose characteristics can affect adulthood health outcomes (e.g. type 2 diabetes, cardiovascular mortality).1 The secular trend toward earlier menarche seemed to cease for the last 30−40 years in industrialized countries. However, the puberty initiation of girls, marked by the first visible signs of breast development, has shown an advanced trend.2,3 To some degree, a decline in the age of pubertal onset is a maladaptive response to the present obesity epidemic,2,4 or reflects the potential influence of genetic, nutritional, the role of early growth and development factors in childhood.5,6 Consequently, modifiable factors, especially early life growth factors, remain to be well documented.
As one of the most important indicators of early growth, adiposity rebound (AR) is defined as the time in which body mass index (BMI) rebounds immediately after it reaches its nadir, observed between ages 5 and 7.7 Early AR is conventionally considered to have a close relationship with obesity, but there are novel ideas into early AR that have implied an acceleration of pubertal growth and development in the twenty-first century compared with in the past.8,9,10 Williams and Dickson8 proposed that the timing of AR might be an early indicator of physical maturity rather than obesity, which was supported by a case-control study showing that early AR could be observed in children with premature adrenarche.9 One study from German et al.10 further demonstrated that age at which thelarche and menarche occurred correlated positively with the timing of AR. However, these studies on AR and pubertal development have mainly focused on children from high-income countries, with limited evidence from the rest of the populations, and specifically evaluated for puberty development, which warrants further clarity.
In the last 10 years, genome-wide association studies (GWAS) have successfully identified some genomic loci related to age at menarche (AAM) and other markers of pubertal development (thelarche or pubarche) in females,7,11,12,13 which have transformed our understanding of genetic contributions to this complicated trait. The current study aims to test for interaction between polygenic risk and early AR in the prediction of thelarche timing in a sample of Chinese girls followed from preschool.
Materials and methods
Study participants and data collection
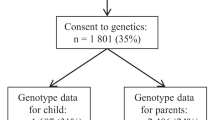
Data were analyzed from an established prospective puberty cohort since September 2010 in Anhui province, China. Children were selected from two kindergartens through clustering convenience sample. Participants were classified as cohort 1 (2010), cohort 2 (2011) and cohort 3 (2012) according to the recruiting year. During the period of kindergarten, physical examination was carried out every 3 months, eight times in total. After entering elementary school, all children were followed up annually with a questionnaire survey, as well as a physical and pubertal development assessment. Parents/caregivers completed the questionnaires when children were in grades 1 and 2. Survey questionnaires were completed by children since grade 3. DNA of each children was extracted and genotyped from buccal cheek swabs in 2017. The final sample of children who had effective data on adiposity rebound, breast Tanner stage and polygenic susceptibility consisted of 342 girls aged 9.25−11.58 years in the last follow-up. Response rates and sample size for the three cohorts are depicted in Fig. 1. Children were excluded from the sample for organic or chronic diseases that could affect puberty or if they were taking oral or inhaled glucocorticoids or human growth hormone, and all the girls enrolled in this analysis did not experience thelarche regression. We secured approval from the Institutional Review Boards at Anhui Medical University (No. 20160112) and then obtained written informed consent from parents and school teachers, as well as child assent.
Measurements
Definition of breast development
Breast Tanner stage was assessed through both observation and palpation every year from Grade 2 to Grade 4 in primary school by two trained female pediatric endocrinologists, which follows the same protocol of China Puberty Collaboration Study.14 Each endocrinologist had more than 90% agreement (same breast stage) with another in breast assessment. Stage of development for breast development was defined as Tanner stage classified between 1 and 5, children progress from a prepubescent (Tanner Stage 1) state to a full sexual maturity (Tanner Stage 5) state.15,16 Onset of breast development (thelarche) was defined as attaining breast Tanner stage 2+.5
Assessment of early adiposity rebound (AR)
As illustrated in Fig. 1, for cohort 1, each child had 12 measurements of height and weight from K2 (eight measurements during K2 and K3) to grade 4 in primary school. For cohorts 2 and 3, 11 and 10 measurements were collected for each child from K2 to grade 3 and from K2 to grade 2, respectively. Age at adiposity rebound was determined via visual inspection of individual growth curves.17 The following criteria were adopted to reduce any subjectivity in the assessment: (1) all consecutive measurements of BMI after the nadir had to show an increase, and (2) any increase in BMI after the nadir had to equal or exceed 0.1 kg/m2.7,17 According to previously published article by our group,18 we defined early AR as age at AR younger than 5.67y, which was the 25th percentile curves of BMI among 1575 children.
Genotyping and SNP selection
DNA was extracted from buccal cheek swabs through PCR-RFLP and real-time PCR and genotyped in the Sequeom Mass Array. The mean concordance rates were 98.8% for the Sequeom Mass Array Targeted Genotyping System.
The current study identified 21 independent genome-wide significant signals that related to early puberty, located at 14 genomic loci (in/near TMEM38B, RXRA, BSX, KDM3B, VGLL3, FUSSEL18, MKL2, LIN28B, RXRG, PRDM13, MCHR2, SEC16B, 3q13.32, RNF144B-ID4). Of those 21 SNPs, 11 SNPs for decreasing age at menarche that reached genome-wide significance were chosen from a joint analysis of 32 GWAS of 87,802 women of European ancestry.19 Ten SNPs were selected that associated with both decreasing age at voice breaking in men and menarche in women from the largest genomic analysis.20 One SNP (rs5932886) has been excluded due to genotyping rate < 10%. Of remaining 20 SNPs, 3 SNPs (rs35327298, rs142058842 and rs4237264) were excluded due to minor allele frequency (MAFs) < 5%, and LIN28B on chromosome 6 (rs314276) which was the first genetic determinant associated with earlier breast development and voice breaking was selected.21 We verified that the loci were not in a significant linkage disequilibrium with each other (r2 < 0.3) by using the SNP server and Han Chinese (CHB) data. Genotype data were imputed based on the CHB HapMap data (phase 2 and phase 3). Details regarding single nucleotide polymorphisms (SNP) included in the PRS had been listed in a published article of our group.22
Each SNP was recorded as 0, 1 and 2 according to the number of effect allele (e.g., if the effect allele is A, then AA = 2, AG = 1, GG = 0). Polygenic risk score (PRS) was calculated from 17 SNPs by using the following formula:
To clarify the effect of polygenic risk and early AR on the breast development of girls, at assessment, puberty PRS was categorized into high risk (>P75), moderate risk (P25−P75) and low risk (<P25) groups.
Body mass index
During annual visits, height and weight were examined by trained and certified staff members. Height was measured using a portable stadiometer to the nearest 0.1 cm and weight with electronic scale (Tanita TI1618) to the nearest 0.1 kg. BMI was calculated as weight (in kg) divided by height (in m) squared, which has been converted into BMI-for-age Z score. Children with BMI-Z score between +1SD and +2SD, and >+2SD were defined as overweight and obesity, respectively.23
Covariates
Detailed information on birthweight, infancy feeding mode (exclusive breastfeeding, mixed feeding and formula feeding), gestational age, delivery mode of children was reported by their parents in K2. Birthweight was converted to Z score (SDS) for sex and gestational age according to the population reference. Preterm was defined as children who were born with gestation age before 37 completed weeks.24 Delivery mode included vaginal and cesarean section.
Parental education was derived from the items of parental reports, including four levels as “junior school or below”, “high school”, “vocational college” and “bachelor or postgraduate”. Household monthly income was collected with a question from parents: “How much does your family’s monthly income?” with six response alternatives: “<5000 yuan per month ($755)”, “5000–10,000 yuan ($755–$1511)”, “10,000–15,000 yuan per month ($1511–$2266)”, “15,000–20,000 yuan per month ($2266–$3021)”, “20,000–30,000 yuan per month ($3021–$4532)” and “>30,000 yuan per month ($4532)”.
Statistical analysis
Demographics from our puberty cohort are presented as frequency (percentage) if categorical in the last three follow-ups. We modeled early AR, puberty-related polygenic risk and pubertal onset, assessing timing of breast stage 2 or higher (B2+), throughout the approximate 8 years of follow-up. Weibull distribution accelerated failure time (AFT) models, derived by Carroll,25 were used to obtain hazard ratios (HR), time ratios (TR) and their 95% confidence intervals (CI) for the association between AR and PRS with the age at thelarche, adjusting for birthweight, infancy feeding, gestational age, delivery mode, household monthly income and parental education, as well as prepuberty BMI percentile. A TR of >1.0 generally indicates a longer time to pubertal onset, while a TR of <1.0 indicates earlier timing of pubertal development compared to the reference group. AFT models are fit for interval-censored data (i.e., onset of puberty occurring at an unknown time between two observations), in addition to the more typical right-censoring. For girls who attained B2+ during follow-up visits, the interval was defined as the period from the last exam visit consistently at stage 1 to the first visit where the girl was observed to be consistently at B2+. All analyses were conducted using SAS 9.4 and STATA 14.0.
Results
The final analysis included 342 girls aged (10.34 ± 0.56) years and had average BMI (18.65 ± 3.35) kg/m2. The percentage of girls with overweight and obesity was 26.02% (89/342) and 18.13% (62/342), respectively. The majority (84.21%, 288/342) of girls attained breast stage 2+ (B2+) (Table 1).
As illustrated in Table 2, the proportion of girls who had attained B2+ during the analysis varied by age, AR, PRS and BMI-Z score. Median ages of B2+ varied by different tertiles of PRS. The age at thelarche among the high-risk group (8.33y) was earlier than that of the low-risk group (8.67y, HR = 2.32, 95% CI: 1.45, 3.51, p < 0.001), but not observed in the group of moderate risk (8.58 years old, HR = 1.30, 95% CI: 0.86, 1.34, p = 0.529).
Girls with early AR matured (reached B2) at a younger age (8.50 years old) than those with normal AR (8.58 years old) (HR = 1.72, 95% CI: 1.30, 2.29, p < 0.001). Girls with BMI-Z score ≥ +1SD were progressively more likely to have attained B2+ at a younger age than those <+1SD (p trend < 0.05).
After adjustment for birthweight-Z score, BMI-Z score, infant feeding, gestational age, delivery mode, household monthly income, parental education, girls who had high PRS risk were, on average, 5.20-month (TR: 0.95; 95% CI: 0.92, 0.97) earlier likely to attain B2+ than those girls who had low PRS (p trend < 0.001). Similarly, girls experiencing early AR were more likely to have earlier timing of thelarche compared with normal AR (4.12-month, TR: 0.96; 95% CI: 0.95, 0.98, p trend < 0.001) (Table 2).
As noted in Fig. 2, we examined the cumulative B2+ prevalence by the tertiles of PRS; girls with early AR matured earlier than those normal AR in the group of high PRS (TR: 0.94; 95% CI: 0.90, 0.99) and moderate PRS (TR: 0.96; 95% CI: 0.93, 0.98). In other words, girls with early AR were progressively 6.06-month and 4.20-month earlier reaching B2 than those with normal AR, respectively, in the high and moderate PRS groups. But not found in the low PRS groups (TR: 1.00; 95% CI: 0.96, 1.04).
Discussion
Our findings
By utilizing an 8-year longitudinal study that included 10–12 measurement points for the sample of 342 preschool girls, the current endeavor provides more reliable insights into the effects of early adiposity rebound in predicting early onset of puberty while taking into account inherited polygenic susceptibility. The findings indicate that early AR predicts younger age at thelarche in a genetic background-dependent manner. Specifically, for girls with early AR, more than a third of a year and half a year earlier thelarche (4.20-month and 6.06-month) was observed among those with moderate and high genetic predisposition, with less evidence of an accelerated effect of early puberty in low PRS groups.
This result might further support previous researches highlighting the association between age at AR and pubertal maturation (age at menarche).8,9,10,26,27 Williams and Dickson8 first raised an exciting clue about the specific association between age at rebound and age at menarche, which suggests that timing of rebound is an indicator of physical maturity rather than obesity. Support for this opinion can be found in Dunedin Multidisciplinary Health and Development Study27 and Study of Early Child Care and Youth Development (SECCYD).25 Taylor et al.27 found that average age at menarche of girls with early AR was 0.1-year and 1.9-year earlier than girls with average and late AR groups. German et al.10 identified the age of pubarche−thelarche−gonadarche−menarche as a function of early BMI and AR, indicating that early thelarche and menarche were correlated positively with the age of occurrence of AR. Moreover, Hochberg clarified that the deviation from a decreasing to an increasing BMI (the deflection of age at AR) parallels adrenarche and the deceleration of growth during the transition from childhood to juvenility, suggesting that the AR and adrenarche are some manifestations of the childhood–adolescent transition,23 which was confirmed by a recent research from Marakaki et al.9 indicating that early AR was observed in children with premature adrenarche.
Although some previous studies have indicated the association between early AR and younger age at puberty development, however, these studies have mainly focused on subjective self-reported pubertal indicators, including the age at menarche—a relatively late milestone of pubertal development. In this study, we used the prospectively collected indicator, Tanner staging for breast, as the objective early marker of puberty onset. In addition, the Weibull accelerated failure time model was used to predict the timing of thelarche.
Currently, it is not clear whether the relationship between early AR and accelerated thelarche is simply an association or implies that AR marks a critical period for the pubertal development.28 Some researchers proposed AR as a mechanistic factor that controls the maturational tempo or a measurement of the amount of stored energy by using dehydroepiandrosterone as a signal to prepare the brain for puberty during the transition of childhood–juvenility.10,27,28 The main changes in body composition that occur during AR may be due to rapid accumulation of excessive adipose tissue.27,29,30 Girls with early rebound deposit an extra 0.3–0.9 kg of fat annually, resulting in much higher percentage body fat values by age 7.31 Another small Dunedin study of girls using dual-energy X-ray absorptiometry revealed that girls with earlier AR gain extra fat and not extra lean tissue.27 Similarly, our results showed that girls with early AR had higher BMI than those with normal AR during follow-up.
The mechanisms involved in the association between early AR and accelerated thelarche may be directly and/or indirectly attributed to leptin. A study of 296 children from the Japanese community found that earlier AR was correlated with higher leptin levels at 12 years of age, regardless of gender, suggesting that the age at AR is an important indicator predicting future leptin levels.30 Leptin is an endocrine peptide that is synthesized and released by adipose tissue, which has emerged as one of the major factors allowing the reproductive system to sense the magnitude of energy reserves.32 It is known that leptin, as a communication pathway from the size of the adipose reserve to the hypothalamus,33 has the effect to facilitate GnRH secretion, a kisspeptin expression during peri-puberty.34,35 Emerging evidence from some GWAS studies indicate that there is overlap between the identified loci for puberty and adiposity (e.g. FTO, SEC16B and LIN28B).12,13,33 Hereby, we have conjectured that the role of early AR in predicting accelerated thelarche might be played through some of those specific genes. This may also explain the null association observed among girls with low polygenic susceptibility for early puberty in our study.
Study strengths and limitations
Several limitations of the current study warrant consideration. Firstly, although the method for deriving AR in this study was the most commonly used,7,24 multiple useful approaches, e.g. using fractional polynomial multi-level models, have been proposed.33 However, the method we had chosen was the most commonly used, and there is evidence that AR cannot be identified in all children, although the timing of AR was observed in all 342 girls in this analysis. It would be important to further clarify an association between critical period of other early life growth and earlier onset of puberty in children. The second limitation of the study is the younger age of children, lack of observation of thelarche in all girls and lack of boys’ data who had not reached the timing of pubertal development. Therefore, the estimation of age at thelarche used a well-established approach, an accelerated failure time model (specifically the Weibull model), which is an effective approach to estimate interval censoring of pubertal onset during within-interval investigation.5,28 Thirdly, our sample included only Han Chinese girls; thus, the generalizability of our findings to girls of other ethnicities is uncertain. Fourthly, the onset of breast development may regress spontaneously due to the accumulation of adipose tissue among overweight and obese girls. That’s inevitable on over evaluating some girls, who may actually did not present pubertal onset. Therefore, it is important to identify the maturation or puberty on activation of hypothalamic−pituitary−gonadal axis and maturation (hormonal studies, bone age, pelvic ultrasonography, etc.) during the following study. Fifthly, much of the breast development occurs during puberty; specific dietary components or nutrients could be essential environmental factors for thelarche timing. Despite adjusting for potential confounders for early life environment, residual confounding by dietary and nutritional factors cannot be ruled out. Moreover, menarche age of the mothers was not collected, which has been recognized as a potential limitation of the present study. Finally, this analysis only explored the relationship between early AR and onset of puberty among girls. A total of 259 boys in our cohort had an average age of 10.43 years in the last follow-up, 49.4% of them showed testicular development (testicular volume > 3 ml). The association in boys can be more rigorously studied as the ongoing follow-up continues.
Conclusions
Although the meaning of age at adiposity rebound is complicated, an important point is that AR has an effect on adverse health outcomes. The present study demonstrates a potential role of early AR in predicting earlier pubertal timing among girls with high and moderate PRS. It means that accelerating growth in the early childhood years after birth might forecast early puberty only among girls with genetic predisposition to early puberty. Therefore, targeting modifiable factors in earlier life to delay the timing of adiposity rebound is necessary. Prevention strategies and management options should be emphasized to target early childhood to address secular trend for early puberty observed in the past decades in China.
References
Bygdell, M., Kindblom, J. M., Celind, J., Nethander, M. & Ohlsson, C. Childhood BMI is inversely associated with pubertal timing in normal-weight but not overweight boys. Am. J. Clin. Nutr. 108, 1259–1263 (2018).
Gamble, J. Puberty: early starters. Nature 550, S10–S11 (2017).
Willemsen, R. H. & Dunger, D. B. Normal variation in pubertal timing: genetic determinants in relation to growth and adiposity. Endocr. Dev. 29, 17–35 (2016).
Gaskins, A. J. et al. Dairy intake in relation to breast and pubertal development in Chilean girls. Am. J. Clin. Nutr. 105, 1166–1175 (2017).
Biro, F. M. et al. Onset of breast development in a longitudinal cohort. Pediatrics 132, 1019–1027 (2013).
Perry, J. R., Murray, A., Day, F. R. & Ong, K. K. Molecular insights into the aetiology of female reproductive ageing. Nat. Rev. Endocrinol. 11, 725–734 (2015).
Rolland-Cachera, M. F. et al. Adiposity rebound in children: a simple indicator for predicting obesity. Am. J. Clin. Nutr. 39, 129–135 (1984).
Williams, S. & Dickson, N. Early growth, menarche, and adiposity rebound. Lancet 359, 580–581 (2002).
Marakaki, C. et al. Early adiposity rebound and premature adrenarche. J. Pediatr. 186, 72–77 (2017).
German, A., Shmoish, M. & Hochberg, Z. Predicting pubertal development by infantile and childhood height, BMI, and adiposity rebound. Pediatr. Res. 78, 445–450 (2015).
Perry, J. R. et al. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature 514, 92–97 (2014).
Lunetta, K. L. et al. Rare coding variants and X-linked loci associated with age at menarche. Nat. Commun. 6, 7756 (2015).
Day, F. R. et al. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet. 49, 834–841 (2017).
Stalder, T. et al. Stress-related and basic determinants of hair cortisol in humans: a meta analysis. Psychoneuroendocrinology 77, 261–274 (2017).
Tanner, J. M. Sequence, tempo, and individual variation in the growth and development of boys and girls aged twelve to sixteen. Daedalus 100, 907–930 (1971).
Tanner, J. M. Normal growth and techniques of growth assessment. Clin. Endocrinol. Metab. 15, 411–451 (1986).
González, L. et al. Early adiposity rebound is associated with metabolic risk in 7-year-old children. Int. J. Obes. 38, 1299–1304 (2014).
Sun, Y. et al. [Prospective association between early adiposity rebound and adolescent development in girls]. Zhonghua Yu Fang. Yi Xue Za Zhi 51, 796–800 (2017).
Elks, C. E. et al. Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nat. Genet. 42, 1077–1085 (2010).
Day, F. R. et al. Shared genetic aetiology of puberty timing between sexes and with health-related outcomes. Nat. Commun. 6, 8842 (2015).
Ong, K. K. et al. Genetic variation in LIN28B is associated with the timing of puberty. Nat. Genet. 41, 729–733 (2009).
Sun, Y., Fang, J., Wan, Y., Su, P. & Tao, F. Role of polygenic risk in susceptibility to accelerated pubertal onset following chronic stress exposure. Eur. J. Endocrinol. 181, 129–137 (2019).
Annan, R. A. et al. Relationship between breakfast consumption, BMI status and physical fitness of Ghanaian school-aged children. BMC Nutr. 6, 19 (2020).
Murray, E. et al. Are fetal growth impairment and preterm birth causally related to child attention problems and ADHD? evidence from a comparison between high-income and middle-income cohorts. J. Epidemiol. Community Health 70, 704–709 (2016).
Carroll, K. J. On the use and utility of the Weibull model in the analysis of survival data. Control Clin. Trials 24, 682–701 (2003).
Network NECCR. The effects of infant child care on infant-mother attachment security: results of the NICHD Study of Early Child Care. Child Dev. 68, 860–879 (1997).
Taylor, R. W. et al. Rate of fat gain is faster in girls undergoing early adiposity rebound. Obes. Res. 12, 1228–1230 (2004).
Hochberg, Z. Juvenility in the context of life history theory. Arch. Dis. Child 93, 534–539 (2008).
Rolland-Cachera, M. F. & Péneau, S. Growth trajectories associated with adult obesity. World Rev. Nutr. Diet. 106, 127–134 (2013).
Taylor, R. W. et al. Changes in fat mass and fat-free mass during the adiposity rebound: FLAME study. Int. J. Pediatr. Obes. 6, e243–e251 (2011).
Koyamaa, S., Sairenchib, T. & Arisakaa, O. Early onset of adiposity rebound is associated with higher leptin concentrations in 12-year-old children. ESPE Abstr. 84, P-2-345 (2015).
Valencak, T. G., Osterrieder, A. & Schulz, T. J. Sex matters: the effects of biological sex on adipose tissue biology and energy metabolism. Redox Biol. 12, 806–813 (2017).
Fernandez-Rhodes, L. et al. Association of adiposity genetic variants with menarche timing in 92,105 women of European descent. Am. J. Epidemiol. 78, 451–460 (2013).
Sanchez-Garrido, M. A. & Tena-Sempere, M. Metabolic control of puberty: roles of leptin and kisspeptins. Horm. Behav. 64, 187–194 (2013).
Manfredi-Lozano, M. et al. Defining a novel leptin–melanocortin–kisspeptin pathway involved in the metabolic control of puberty. Mol. Metab. 5, 844–857 (2016).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 81872638).
Author information
Authors and Affiliations
Contributions
Conceptualization and design: Y.S., F.T. Acquisition of data: J.F., C.G., Y.S., Y.W., P.S. Analysis and interpretation of data: J.F., C.G., Y.S., P.S., Y.W., Z.Z. Revising it for intellectual content: Y.S., F.T., P.S., Y.W., Z.Z. Final approval of completed article: All authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
We obtained written informed consent from parents and school teachers, as well as child assent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fang, J., Gong, C., Su, P. et al. Polygenic interactions with adiposity rebound in the prediction of thelarche. Pediatr Res 89, 1026–1031 (2021). https://doi.org/10.1038/s41390-020-1001-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-1001-8