Abstract
Background
Neuromonitoring at the bedside is the key to understand the pathophysiological mechanisms of brain injury associated with neonatal encephalopathy. The current practice is to monitor the forehead using a noninvasive cerebral oximetry—it remains unknown to what extent cerebral hemodynamics in other brain regions is different to the frontal region.
Method
A multichannel near-infrared spectroscopy (NIRS) system was used to monitor neonates (n = 14) with fetal acidosis and mild neonatal encephalopathy at four brain regions (the frontal, posterior, left temporal, and right temporal lobes). The data were compared to delineate the regional difference in (1) cerebral hemodynamics and (2) pressure autoregulation. For both analyses, wavelet transform coherence was applied.
Results
We observed frontal–posterior heterogeneity as indicated by significantly lower coherence between these two regions (p = 0.02). Furthermore, areas with regional magnetic resonance imaging (MRI)-detected lesions showed greater hemodynamic variations compared to non-affected areas (p = 0.03), while cerebral autoregulation was not affected and showed no difference.
Conclusion
Cerebral hemodynamics in mild neonatal encephalopathy is heterogeneous across different brain regions, while cerebral autoregulation remains intact. These findings indicate the robustness of the wavelet measure of cerebral autoregulation in this population, but need to be further investigated in the presence of severe injury.
Impact
-
This proof-of-concept study is the first to investigate the regional difference of cerebral hemodynamics and autoregulation in mild neonatal encephalopathy.
-
Study findings confirm that brain functions are complex in the developing neonatal brain and that cerebral hemodynamics are region specific in newborns with frontal–posterior heterogeneity among brain regions probed by multichannel NIRS.
-
Regional MRI lesions were associated with differences across NIRS regional channels among the affected side.
-
Cerebral autoregulation with multichannel NIRS is not affected by regional MRI abnormalities.
Similar content being viewed by others
Introduction
Hypoxic–ischemic encephalopathy (HIE) is a serious public health problem that afflicts millions of newborns worldwide with neurodevelopmental deficits in 50% of survivors,1 including more recent studies in mild encephalopathy. Bedside monitoring of cerebral hemodynamics based on near-infrared spectroscopy (NIRS) can provide important insights into the pathophysiological mechanisms of related brain injury.2 NIRS is a noninvasive technology, well suited for bedside monitoring of regional brain tissue oxygenation and pressure autoregulation resulting from interrupted maternal and/or fetal placental blood flow. The standard positioning of NIRS probes is typically the anterior fronto-parietal in location to best reflect the watershed global cerebral blood injury pattern while avoiding artifacts from adhesion to the hair.
In a recent study,3 we have used the cerebral oximetry to monitor neonates with HIE and validated a novel wavelet coherence analysis to assess the dynamic relationship between spontaneous oscillations in mean arterial pressure (MAP) and cerebral tissue oxygenation saturation (SctO2) and to determine the status of cerebral autoregulation noninvasively. We derived wavelet-based metrics of phase, coherence, and gain for quantitative evaluation of cerebral autoregulation, and discovered that cerebral autoregulation in neonates with HIE was time-scale-dependent in nature and correlated with neurological outcomes at 2 years. An important remaining knowledge gap is to determine whether cerebral hemodynamics at different brain regions correlate or differ from the frontal region, and whether the location has any effect on the cerebral autoregulation of asphyxiated newborns. This step is necessary to test the rigor of the proposed research measurement of cerebral autoregulation.
This current study aims to monitor neonatal cerebral hemodynamics from four brain regions (anterior, posterior, left temporal, and right temporal lobes) with a multichannel NIRS system based on a novel wavelet coherence analysis and identify any regional heterogeneity in cerebral hemodynamics and pressure autoregulation.
Methods
Patients
The study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and informed consent was obtained from parents before enrollment. Inclusion criteria included newborns delivered at ≥36 weeks of gestation with a birth weight of ≥1800 g, metabolic acidosis, and signs of encephalopathy within the first 6 h of birth who were admitted to the neonatal intensive care unit at Parkland Hospital from March 2018 to February 2019.
Perinatal acidemia was determined by umbilical arterial blood gas included a pH of 7.0 or less, a base deficit of 16 mEq/L or greater in umbilical artery blood, or any postnatal blood sample within 1 h of life. In order to establish the diagnosis of encephalopathy, a neurological examination was performed within 6 h of birth according to the National Institute of Child Health and Human Development (NICHD) classification for modified Sarnat staging,4 which assessed (1) the level of consciousness, (2) spontaneous activity, (3) posture, (4) tone, (5) primitive reflexes, and (6) autonomic nervous system. Infants with mild encephalopathy were the subject of this neuromonitoring analysis.
Data collection
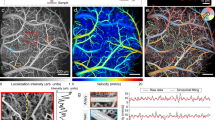
A multichannel NIRS system (CW-6, TechEn Inc., Milford, MA) was used to monitor cerebral hemodynamics continuously at the bedside. This system uses lasers at 690 and 830 nm as light emitters and avalanche photodiode (APD) as detectors. Each laser is modulated to a high frequency (≥6.4 kHz). The detected signals by the APDs are demodulated via high-pass filters to retrieve the light intensity so that the near-infrared signal does not interfere with the ambient room light (60 Hz). As shown in Fig. 1a, four compact NIRS probes were constructed to monitor the neonatal brain at different regions: the frontal (anterior), posterior (posterior), left temporal (left), and right temporal (right) lobes, respectively. Each probe held a single emitter–detector pair (channel); the emitter–detector distance was 2.5 cm. The probes were secured on the head with tapes and elastic bandage. Continuous data acquisition was initiated in a quiet room while the newborn patient was stable. Each data acquisition session lasted for at least 60 min and would be prolonged if the patient had intermittent body movements. The data sampling rate was 25 hHz.
a Schematic setup of multichannel NIRS monitoring. Four emitter–detector pairs (channels) were placed at the frontal (anterior), posterior (posterior), left temporal (left), and right temporal (right) regions, respectively. The anterior channel was adjacent to the standard cerebral oximeter probe and thereby used as a reference channel: anterior–posterior (A–P), anterior–left (A–L), and anterior–right (A–L) pairs. b Analysis of regional difference in cerebral hemodynamics. For each paired comparison shown in a, wavelet transform was applied to characterize coherence in a time–frequency domain, as demonstrated in the right panel. In that panel, the x-axis in that represents time and the y-axis represents scale. The color scale represents the magnitude of squared cross-wavelet coherence R2, which ranges from 0 (no coherence) to 1 (complete coherence). The black line contouring red areas designate areas of significant coherence (p < 0.05). The arrows designate the relative phase between the two paired signals. c Analysis of regional difference in cerebral autoregulation.
For all the newborn patients with an indwelling arterial line, arterial blood pressure was continuously recorded using a CNS monitor (Moberg ICU Solutions, Ambler, PA). The data sampling rate was 1 Hz. Event markers were used to synchronize the CNS monitor with the multichannel NIRS system in the beginning of data collection.
Data processing
All the acquired data were processed and analyzed in MATLAB (MathWorks, Inc.). The channel-wise raw data from CW-6 was inspected retrospectively to remove segments corrupted by large motion artifacts (e.g., yawns, cries). The remaining motion-free data were used to calculate relative concentrations of oxygenated hemoglobin Δ[HbO2] and deoxygenated hemoglobin Δ[Hb] based on the modified Beer–Lambert law.5 In this step, the differential pathlength factor was 6.8 at 690 nm and 5.8 at 830 nm.6 Then, differential hemoglobin concentration, Δ[HbD] = Δ[HbO2] − Δ[Hb], was derived. Previous studies7,8,9 have demonstrated that Δ[HbD] is a valid surrogate for cerebral blood flow in assessment of cerebral autoregulation status. Therefore, we focused on Δ[HbD] changes in the subsequent analyses: the channel-wise Δ[HbD] data were first down-sampled at 1 Hz to reduce the high-frequency components. Then, a second-order polynomial detrending was applied to remove the slow drifts. MAP data were also inspected to remove segments with large motion artifacts (e.g., yawns, cries), followed by a second-order polynomial detrending to remove the slow drifts.
Analysis of regional heterogeneity
To investigate the regional heterogeneity in cerebral hemodynamics and pressure autoregulation, we used the anterior channel of NIRS as a reference, which is the standard location for cerebral oximetry monitoring (e.g., the INVOS™ series). All the other NIRS channels were defined as remote channels and compared with the anterior channel. It resulted in three paired comparisons as shown in Fig. 1a: the anterior–posterior (A–P), anterior–left (A–L), and anterior–right (A–L) pairs. For each pair of channels, two types of analysis were conducted separately:
Cerebral hemodynamics: The regional heterogeneity in cerebral hemodynamics was assessed by computing the coherence between every two paired Δ[HbD] time series directly. In this step, wavelet transform coherence (WTC) was used. Briefly, WTC quantifies the squared cross-wavelet coherence, R2, and relative phase, Δφ, in a time–frequency domain.10 R2 ranges between 0 and 1 and can be conceptualized as a localized correlation coefficient between the two paired signals in the time–frequency domain; Δφ ranges between −π and π and represents the time lag. In this study, the hemodynamic fluctuations measured at different locations were largely synchronous. Thus, we focused on the mean R2 over time as a measure of coherence between the two paired channels.
Cerebral autoregulation: WTC was also used to quantify the coherence between MAP and channel-wise Δ[HbD] that was measured at each of the four regions (Fig. 1a). Similar to our recent study,3 the percentage of significant coherence, P(s), was quantified as the percentage of time during which the MAP → HbD coherence was statistically significant (p < 0.05). Then, an autoregulation index (AI) was further quantified as the mean value of P(s) in a selected wavelet scale range as a measure.3 At last, the AI values from the three remote locations (i.e., the posterior, left, and right channels) were compared with the anterior reference to delineate the regional heterogeneity in cerebral autoregulation.
All the WTC analyses above were conducted in a wavelet scale range of s = 10–300 s. This scale range corresponded to 0.0033–0.1 Hz in frequency domain, a representative band of cerebral autoregulation in the literature.11,12
Neuroimaging assessments
Brain magnetic resonance imaging (MRI) was performed using 3-Tesla MRI (Philips Healthcare Systems, TX) on each HIE survivor on average at 5 ± 1 days of age prior to discharge for evidence of neurological abnormalities and injuries. MRI findings were scored for abnormalities by an experienced pediatric neuroradiologist based on the NICHD classification.13,14 Regional MRI-detected abnormalities, such as bleeding and asymmetric lesions, were described in detail with respect to their locations. When abnormalities were detected under the frontal, posterior, or left/right temporal NIRS sensors, the respective channels were labeled as “affected” to determine the effect of local lesions on the concomitant NIRS recording.
Statistical analysis
Continuous outcome variables were aggregated, via classical test theory, across a selected time period by each subject, and then used the mean coherence estimates for analyzing differences in a given time period measurement of interest between groups. All the paired group comparisons were performed based on the non-parametric Wilcoxon’s rank-sum testing procedure, because of a small sample size. Bonferroni correction was employed to account for statistical significance in multiple comparisons, especially for the case with more than two groups. A p value of <0.05 was considered to be statistically significant.
Results
Patient characteristics
Fourteen neonates met the eligibility criteria and were monitored in the first 24 h after birth following the parental informed consent. The patients had an equal gender distribution (males = 50%) and a gestational age range of 39 (38–41) weeks with an uneventful hospital stay. None of the enrolled infants had evidence of basal ganglia or watershed injury based on pre-discharge MRI obtained at 5 ± 1 days of life. During the actual data collection, all neonates were hemodynamically stable, had normal blood gases, and were not disturbed by feeding or changing. After excluding channels with significant artifacts, a total of 37 channel pairs remained for the subsequent paired comparisons. Six neonates were labeled to have affected NIRS measurements due to the presence of regional MRI abnormalities (1 subdural hemorrhage, 3 posterior occipital intraventricular hemorrhages (IVHs) in the occipital horns, 1 right brainstem lesion, 1 left temporal injury, 1 germinal matrix hemorrhage). All infants had a normal neurological examination upon discharge.
Regional heterogeneity across four brain regions
Figure 2a shows the cross-channel Δ[HbD] coherence from a single patient illustrating cerebral hemodynamic heterogeneity in each of the three pairs across the four brain regions (i.e., the anterior, posterior, left temporal, and right temporal). The signals from the four regions were overall synchronous as indicated by the in-phase coherence among all pairs seen in the yellow area, but specific coherence within each time-scale spectrogram was low among the entire cohort as reflected in Fig. 2b. Among the three pairs, the A–P pair had clearly lowest coherence, especially at the physiologically important scale range of 200–300 s where cerebral hemodynamics and autoregulation predominate. This observation was further confirmed by the mean coherence within this specific scale range, which is shown in Fig. 2c: the A–P pair had significantly lower mean coherence than the A–L pair (p = 0.02) and A–R pair (p = 0.02), indicating significantly increased frontal–posterior heterogeneity.
a Individual example of cerebral hemodynamic cross-channel coherence: anterior–posterior (A–P); anterior–left temporal (A–L); anterior–right temporal (A–R). b Grand-averaged coherence spectrum (solid lines: mean; shaded areas: standard error). The A–P pair had lower coherence than the other two pairs, indicating greater frontal–posterior heterogeneity. c With mean coherence in wavelet scale range of s = 200–300 s, the Wilcoxon test’s with Bonferroni correction suggests that A–P pairs had the lowest mean coherence score (p = 0.02).
The four brain regions also showed remarkable heterogeneity in measurements of cerebral autoregulation, as demonstrated in Fig. 3a. At the group level (Fig. 3b), the autoregulatory index AI values from the posterior, left temple, and right temple regions were poorly correlated with the anterior reference (R = 0.27, 0.30, and 0.51, respectively). Together, these observations suggested the regional heterogeneity in measurements of cerebral autoregulation in this cohort.
a Individual example of cerebral autoregulation measured at the anterior, posterior, left temporal, and right temple regions. b Paired comparisons of autoregulation index showing anterior references correlation with the posterior (R = 0.27), left temporal (R = 0.30), and the right temporal (R = 0.51).
Heterogeneity related to MRI-detected abnormalities
To determine the effect of brain lesions on regional cerebral hemodynamics and autoregulation, we separately studied all the channel pairs based on whether they are affected by regional MRI-detected abnormalities (e.g., if the left temporal region was abnormal on MRI, the A–L was labeled as “affected” pair). Figure 4 shows a comparison between the affected pairs versus the unaffected pairs in aspect of cerebral hemodynamics. At the group level (Fig. 4b, c), the affected pairs had significantly lower cross-channel Δ[HbD] coherence than the unaffected pairs (p = 0.03), suggesting that the presence of even small regional lesions could affect the measurements of cerebral hemodynamics in situ.
a The cross-channel Δ[HbD] coherence from two individual neonates: one unaffected (normal MRI no lesions under the NIRS sensors) versus one affected (regional MRI area affected under the NIRS sensor). b Grand-averaged hemodynamic coherence spectrum from the unaffected pairs and the affected pairs (solid lines: mean; shaded areas: standard error). c Mean coherence (averaged locally over s = 200–300 s) was used to compare the unaffected pairs and affected pairs. The Wilcoxon’s test suggests statistical significance in the group comparisons between the affected pairs and unaffected pairs (p = 0.03).
In contrast to the NIRS hemodynamics measurements, Fig. 5 shows that there was no significant differences in cerebral autoregulation measures between the regionally affected (n = 6) versus the unaffected channels (n = 31).
a The MAP → Δ[HbD] coherence at an unaffected channel (no MRI abnormality was under the NIRS sensors) and an affected channel (MRI abnormality was observed under the NIRS sensors) from two individual neonates examples. b Grand-averaged coherence spectrum from the unaffected channels and the affected channels (solid lines: mean; shaded areas: standard error). c Mean coherence (averaged globally over time, that is, the spectrum of s = 0–300 s) was used test the difference between from the unaffected channels and the affected channels. The statistical difference between the unaffected and affected channels was found insignificant.
Discussion
This proof-of-concept study is the first to investigate the regional difference of cerebral hemodynamics and autoregulation in mild neonatal encephalopathy using the wavelet metrics. We found significant frontal–posterior heterogeneity among four brain regions probed by multichannel NIRS, indicating the need to monitor the neonatal brain at multiple regions. MRI-detected regional abnormalities at the site of the monitoring probe were found to account for discrepancies in the measurements, but did not affect the cerebral autoregulation.
Several studies on different patient populations have similarly reported important regional differences of cerebral hemodynamics and/or pressure autoregulation. Lemmers and van Bel15 reported that up to 10% differences in SctO2 are detected between the left and right hemispheres in preterm infants, especially when arterial oxygen saturation was unstable. Papademetriou et al.16 reported cerebral oxygenation rScO2 asymmetry to be independently associated with abnormal outcomes after a perinatal ischemic stroke. Wagenaar et al.17 used a multichannel NIRS system to monitor infants during extracorporeal membrane oxygenation (ECMO), and found significant autoregulation differences between the left and right hemispheres. The authors suggested that the right hemisphere was more susceptible to the disruption of cerebral autoregulation due to the cannulation of great blood vessels on that side. Asymmetry of NIRS oxygen saturation has been reported during cardiac surgeries mostly in relations to complications during aortic or venous cannulations.18,19,20 Unlike the above surgical and ECMO patients, neonates in this study were diagnosed with mild encephalopathy, had an uneventful course, and did not undergo any surgical procedure. In that regard, Chiron et al.21 reported in healthy infants a right brain dominance to be associated with a different oxygenation pattern in the two hemispheres. Thus, our study findings support that regional difference of cerebral hemodynamics and autoregulation might be a common phenomenon in newborns, even in the absence of global MRI injuries or basal ganglia, and watershed infarction patterns that are typical of severe HIE. Interestingly, the highest discrepancies were observed in the posterior occipital probe. This might be partly a reflection of the longer distance between this probe and the frontal reference. Another important contributing factor is that the temporal–occipital regions in the developing newborn have been reported to have a higher perfusion and metabolism when compared to adults.20,21,22 These regions therefore could be more vulnerable to any hemodynamic and autoregulation impairments. This heterogeneity needs to be taken into account when comparing neonatal studies with probes placed in different locations. Of note, in this study we focused on Δ[HbD] rather than SctO2 to ensure optimal representation of frequency band (0.0033–0.1 Hz) where cerebral autoregulation occurs and validate the robustness of the wavelet metric.
The current study also examined the effect of any regional lesions, as identified on pre-discharge MRI, on the measurements of hemodynamics and pressure autoregulation in the absence of confounding global injury. Clearly, our study findings support that the areas affected by regional abnormalities in MRI indeed have altered cerebral hemodynamics, resulting in significantly lower coherence compared to non-affected regions.
It is intriguing that cerebral autoregulation measurements were robust and showed no differences between the affected regions versus unaffected regions. This indicates that despite the observed regional NIRS heterogeneity, the subtle MRI affected areas not involving the basal ganglia or watershed patterns of severe asphyxia do not influence cerebral autoregulation. Findings reflect the intact physiological adaptive mechanisms and differ from studies showing impaired autoregulation in association with severe MRI patterns of brain injury.3,9
Autoregulation of cerebral blood flow is very critical to achieve constant cerebral flow over a broad range of cerebral perfusion pressures (CPPs), which is from 25 to 50 mmHg in newborns.22 The control of cerebral blood flow is complex and requires involvement of every component of the neurovascular unit to maintain constant flow in rapid response to changes in CPPs and to a variety of cerebral-metabolic activities.
Cerebral blood flow increases with postnatal age in parallel with increased cerebral/metabolic demands in the growing brain. Coupling of brain function and blood flow is important and can be impaired during transition from fetal to neonatal life.23,24,25 Loss of autoregulation in preterm infants was also significantly associated with increased severe IVH and periventricular leukomalacia,7,26 and with mortality in preterm infants.27,28
Autoregulation indices have been well studied in asphyxiated infants undergoing hypothermia therapy and were found to correlate with outcomes.3,9 The fetal circulatory response to asphyxia is a rapid centralization and autoregulation of blood flow in favor of the vital organs: brain, heart, and adrenals, at the expense of peripheral organs.
Our study finding suggests that the cerebral autoregulation in the first day of life in infants with mild encephalopathy is unaffected by subtle focal MRI lesions that spare the deep central gray matter. These findings are timely and of great clinical relevance in view of the atypical MRI patterns of abnormalities more recently described in up to 50% of infants with mild encephalopathy2,3 with a critical lack of understanding of their implications on cerebral physiology and outcomes.
In this paper, we first address a robust wavelet methodology to measure the cerebral hemodynamics and autoregulation observed with mild encephalopathy and its associated MRI lesions. The ongoing follow-up study planned at 24 months will provide the necessary correlation of these observations with neurodevelopmental outcomes.
Strengths of the study include prospective measurements in the absence of motion artifacts, and the maintenance of stable oxygenation, ventilation, and blood pressure parameters during the recording. Infants were maintained midline and no medications were administered during the regional recording, ensuring the rigor of the methodology. Another major strength was the use of the wavelet analysis methodology, which allows to accurately measure similarities across nonstationary time series applicable within a large spectrum of frequencies, including physiologically important very low frequency.3 This approach enabled a comprehensive determination of the multiscale nature of the regional effects of the underlying oscillations in cerebral hemodynamics.
A limitation of this proof-of-concept study design is the small sample size and the lack of long-term neurodevelopmental evaluations, which are still ongoing to determine the clinical impact of the observed regional differences.
Future studies are needed to compare findings between infants with severe global injury and to detect the effect of hypothermia therapy. Since the reperfusion phenomenon associated secondary energy failure can last from hours to days, we recognize that these findings might be different in infants with more severe HIE where autoregulatory disturbances might be detected beyond the first day of life.
Since the multichannel NIRS monitoring and the pre-discharge MRI to identify local brain lesions were not done simultaneously, we could not determine whether the lesions had already developed at the time of NIRS monitoring. Another limitation of the study design is the lack of simultaneous pulse oximetry, which precludes calculation of fractional tissue oxygen extraction.
In conclusion, study findings confirm that in the developing neonatal brain, in general, brain functions are complex, and that in newborns with mild encephalopathy, cerebral hemodynamics are region specific. The underlying differences across brain regions need to be further studied in comparison to infants with more severe injury as they could account for differences in treatment responses and prognosis.
References
Chalak, L. F. Best practice guidelines on management of mild neonatal encephalopathy: Is it really mild? Early Hum Dev. 120, 74 (2018).
Greisen, G. Is near-infrared spectroscopy living up to its promises? Semin. Fetal Neonatal Med. 11, 498–502 (2006).
Tian, F. et al. Wavelet coherence analysis of dynamic cerebral autoregulation in neonatal hypoxic–ischemic encephalopathy. Neuroimaging Clin. 11, 124–132 (2016).
Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol. 33, 696–705 (1976).
Kopsas, L., Herman, P. & Eke, A. The modified Beer–Lambert law revisited. Philos. Med. Biol. 51, N91–N98 (2006).
Essenpreis, M. et al. Spectral dependence of temporal point spread functions in human tissues. Appl. Opt. 32, 418–425 (1993).
Tsuji, M. et al. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics 106, 625–632 (2000).
Soul, J. S. et al. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 61, 467–473 (2007).
Govindan, R. B. et al. Cerebral pressure passivity in newborns with encephalopathy undergoing therapeutic hypothermia. Front. Hum. Neurosci. 8, 266 (2014).
Grinsted, A., Moore, J. C. & Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Proc. Geophys. 11, 561–566 (2004).
Zhang, R. et al. Transfer function analysis of dynamic cerebral autoregulation in humans. Am. J. Physiol. 274, H233–H241 (1998).
Zhang, R. et al. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 106, 1814–1820 (2002).
Chalak, L. F. et al. Neurodevelopmental outcomes after hypothermia therapy in the era of Bayley-III. J Perinatol 34, 629–633 (2014).
Rollins, N. et al. Predictive value of neonatal MRI showing no or minor degrees of brain injury after hypothermia. Pediatr. Neurol. 50, 447–451 (2014).
Lemmers, P. M. & van Bel, F. Left-to-right differences of regional cerebral oxygen saturation and oxygen extraction in preterm infants during the first days of life. Pediatr. Res. 65, 226–230 (2009).
Papademetriou, M. D. et al. Multichannel near infrared spectroscopy indicates regional variations in cerebral autoregulation in infants supported on extracorporeal membrane oxygenation. J. Biomed. Opt. 17, 067008 (2012).
Wagenaar, N. et al. Brain activity and cerebral oxygenation after perinatal arterial ischemic stroke. Stroke 50, 2668–2676 (2019).
Bar-Yosef, S., Sanders, E. G. & Grocott, H. P. Asymmetric cerebral near-infrared oximetric measurements during cardiac surgery. J. Cardiothorac. Vasc. Anesth. 17, 773–774 (2003).
Janelle, G. M. et al. Unilateral cerebral oxygen desaturation during emergent repair of a DeBakey type 1 aortic dissection: potential aversion of a major catastrophe. Anesthesiology 96, 1263–1265 (2002).
Sakamoto, T. et al. Cerebral ischemia caused by obstructed superior vena cava cannula is detected by near-infrared spectroscopy. J. Cardiothorac. Vasc. Anesth. 18, 293–303 (2004).
Chiron, C. et al. The right brain hemisphere is dominant in human infants. Brain 120, 1057–1065 (1997).
Volpe, J. J. Neurology of the newborn. Major Probl. Clin. Pediatr. 22, 1–648 (1981).
Richardson, B. S. et al. Webster regional blood flow change in the lamb during the perinatal period. Am. J. Obstet. Gynecol. 160, 919–925 (1989).
Kozberg, M. G. & Hillman, E. M. Neurovascular coupling develops alongside neural circuits in the postnatal brain. Neurogenesis (Austin) 3, e1244439 (2016).
Hendrikx, D. et al. Measurement of neurovascular coupling in neonates. Front. Physiol. 10, 65 (2019).
Pryds, O., Greisen, G. & Johansen, K. H. Indomethacin and cerebral blood flow in premature infants treated for patent ductus arteriosus. Eur. J. Pediatr. 147, 315–316 (1988).
Wong, F. Y. et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics 121, e604–e611 (2008).
Ouyang, M. et al. Heterogeneous increases of regional cerebral blood flow during preterm brain development: preliminary assessment with pseudo-continuous arterial spin labeled perfusion MRI. Neuroimaging 147, 233–242 (2017).
Acknowledgements
We thank all the families of subjects for their consent of participation. This study was supported by National Institutes of Health (NIH) Grant: 5R01NS102617-03 (to L.C.).
Author information
Authors and Affiliations
Contributions
F.T. performed the experiments, developed data analysis algorithms, analyzed the data, and prepared the manuscript. P.S. and S.K. assisted the subject recruitment, experimental preparation, and data acquisition. Y.D. participated in data acquisition and result interpretation. Y.L. performed statistical imputation and interpretation, H.L. and R.Z. discussed and interpreted the results, and revised the manuscript. L.C. initiated and supervised the study, interpreted the results, and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
This study was approved by IRB and consent was obtained.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tian, F., Sepulveda, P., Kota, S. et al. Regional heterogeneity of cerebral hemodynamics in mild neonatal encephalopathy measured with multichannel near-infrared spectroscopy. Pediatr Res 89, 882–888 (2021). https://doi.org/10.1038/s41390-020-0992-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0992-5