Abstract
Background
Despite improved health, shorter stature is common in cystic fibrosis (CF). We aimed to describe height velocity (HV) and contribution of height-related genetic variants to height (HT) in CF.
Methods
HV cohort: standard deviation scores (-Z) for HT, mid-parental height-adjusted HT (MPAH), and HV were generated using our Pediatric Center’s CF Foundation registry data. HV-Z was compared with population means at each age (5–17 y), the relationship of HV-Z with HT-Z assessed, and HT-Z compared with MPAH-Z. GRS cohort: HT genetic risk-Z (HT-GRS-Z) were determined for pancreatic exocrine sufficient (PS) and insufficient (PI) youth and adults from our CF center and their relationships with HT-Z assessed.
Results
HV cohort: average HV-Z was normal across ages in our cohort but was 1.5× lower (p < 0.01) for each SD decrease in HT-Z. MPAH-Z was lower than HT-Z (p < 0.001). GRS cohort: HT-GRS-Z more strongly correlated with HT-Z and better explained height variance in PS (rho = 0.42; R2= 0.25) vs. PI (rho = 0.27; R2 = 0.11).
Conclusions
Despite shorter stature compared with peers and mid-parental height, youth with CF generally have normal linear growth in mid- and late childhood. PI tempered the heritability of height. These results suggest that, in CF, final height is determined early in life in CF and genetic potential is attenuated by other factors.
Impact
-
Children with CF remain shorter than their healthy peers despite advances in care.
-
Our study demonstrates that children with CF have persistent shorter stature from an early age and fail to reach their genetic potential despite height velocities comparable to those of average maturing healthy peers and similar enrichment in known height increasing single-nucleotide polymorphisms (SNPs).
-
Genetic risk scores better explained variability in pancreatic sufficient than in pancreatic insufficient individuals, suggesting that other modifying factors are in play for pancreatic insufficient individuals with CF.
-
Given the CF Foundation’s recommendation to target not only normal body mass index, but normal height percentiles as well, this study adds valuable insight to this discussion.
Similar content being viewed by others
Introduction
Because improved nutritional status correlates with better pulmonary function and increased survival,1 the Cystic Fibrosis Foundation (CFF) recommends optimizing nutritional status and linear growth.2 Resolution of linear growth deficits in youth with CF were expected to accompany (1) initiation of pancreatic enzyme replacement therapy to address malabsorption and (2) normalization of weight in children. However, CFF registry data indicate that even with improved weight, CF patients remain shorter than the population average.3 The extent to which this shorter stature is programmed from early life and/or reflects ongoing compromised growth remains unclear.
Linear growth can be quantified using height velocity (HV), an important indicator of pediatric health. Age, sex, genetic potential, pubertal status, and other environmental factors can all influence HV. Several studies have suggested that HV is attenuated in children with CF;4,5 however, these studies were largely performed in the 1980s and 1990s4,5 and used data from the 1960s and 1970s.6 Few studies have used more recently acquired data.7 Many studies focused on growth during adolescence and implicated compromised pubertal growth as the likely cause for the shorter adult stature observed in CF.4,5,6,7,8,9 However, these studies were unable to easily quantify growth velocity. Recently, HV reference data were developed based upon longitudinal data in a contemporary population of healthy youth enrolled in the Bone Mineral Density in Childhood Study (BMDCS).10 These reference data have the advantage of expressing HV in percentiles and Z scores and therefore facilitate comparison of norms with specific groups such as children with CF.
In addition, height is a strongly heritable trait and genome-wide association studies (GWAS) have identified up to 3290 signals for height in adults.11,12,13 However, the extent to which variants at these loci also contribute to height in CF has not been tested. Understanding the contribution of broad-sense heritability to height in CF might be important in parsing the relative contributions of genes and environment and developing interventions aimed at maximizing growth.
The primary aims of our study were to (1) describe longitudinal growth in a cohort of patients with CF, born in the late 1990s and 2000s, by generating HV Z scores (HV-Z), (2) determine the occurrence of lower HV (HV-Z < 0), and (3) examine the relationship of HV-Z with age. Second, we examined the contribution of mid-parental height to stature in this same cohort. Finally, in a separate cohort, we examined the relationship of a previously validated height genetic risk score (GRS) with height Z scores (HT-Z) in youth and adults with CF.
Methods
Study populations
Patients enrolled in the CFF patient registry at the Children’s Hospital of Philadelphia (CHOP) were included in the Height-Velocity-Z (HV) Cohort. The registry was queried from January 2007 to October 2015. Subjects were excluded if co-occurring, non-CF-related conditions known to affect linear growth were identified during review of the electronic medical record (for example, genetic disorders such as Russell-Silver syndrome owing to uniparental/maternal disomy of chromosome 7 that contains CFTR). The CHOP Institutional Review Board (IRB) granted an exemption for this research.
The Genetic Risk Score (GRS) Cohort included individuals ages ≥2 years treated at the CHOP and Hospital of University of Pennsylvania (Penn) CF Center who were offered participation in a genotyping study. Individuals with known Type 1 Diabetes were excluded. The Penn and CHOP IRBs approved the study, and all participants provided written informed consent and assent (when age appropriate) to participate.
Electronic medical records and clinical characteristics
For the HV cohort, simultaneous heights and weights in consecutive years (±1 months) were extracted from the registry, which contains data from routine office visits. Measurements of height and weight are obtained from calibrated scales and stadiometers by trained Medical Assistants during these visits to the CHOP CF Center. Medical records were reviewed for demographic characteristics, reported maternal and paternal heights, pulmonary function tests, systemic glucocorticoid use, CF-related liver disease status, CF-related diabetes status, use of medications that might affect growth (such as stimulant medications), and other chronic or congenital conditions. For the GRS cohort, demographic characteristics, anthropometric and pulmonary function data were collected from the medical record from 2 years prior to study enrollment to 3 years post enrollment. For both cohorts, CDC height, weight, BMI, and weight-for-length Z scores were calculated based on published standards.14 All height and weight Z scores were within CDC parameters for biologically plausible data.15 For individuals age >20 years, Z scores were calculated based on that of a 20-year-old individual.
Mid-parental adjusted height (MPAH) and HV calculation
For the HV cohort, HV was calculated as the difference in height divided by the difference in age between consecutive annual study visits, and HV-Z were calculated using the method developed by Kelly et al.10 Predicted height based on genetic potential was estimated from parental stature using the method developed by Himes et al.16 and validated in CF.17 In brief, the Himes method predicts the child’s genetic potential for height by adjusting for the influence of taller or shorter than average parents. To calculate a Himes’ adjusted height, we first calculated the mid-parental heights based on parental self-report. We then determined the Himes adjustment value based on the child’s gender, age, height, and mid-parental height, and applied this value to the child’s actual height to obtain the MPAH.16 Z scores for mid-parental adjusted heights (MPAH-Z) were calculated using the CDC 2000 growth curves.14
Determination of height GRS
DNA was isolated from blood or saliva for the GRS cohort and genome-wide SNP genotype was determined on the Ilumina Infinum II OMNI Express plus Exome BeadChip (Illumina, San Diego, CA, USA). PLINK, a free, open-source whole genome association analysis toolset, was utilized to calculate a HT-GRS by summing the height increasing alleles of 3290 biallelic SNPs that were GWAS-implicated with adult height.18,19 The HT-GRS calculation incorporated the individual SNP effect sizes (i.e., weighted) and was converted to a Z score using the mean and standard deviation for the GRS obtained from a dataset of “healthy” children in the BMDCS cohort.20
Statistical analysis
Descriptive statistics are presented as median and interquartile range.
For the HV-cohort, between-group Length-Z at <1 year and HT-Z at 7 years (prepubertal) and in adulthood (post-pubertal, defined as a HV of 0 for >2 years) were compared using a repeated measure mixed model. These ages were chosen as they represent distinct and important time points in linear growth, and also allow for comparison across ages without repeated measures for each subject within each age category. Within each year for ages 5–17 years for males and 5–16 years for females, HV-Z in CF subjects was compared with a population mean HV-Z score of 0 using a Student’s t test. The relationship between HV-Z and HT-Z was explored using a linear random effects model. Finally, MPAH and MPAH-Z were compared with unadjusted height and HT-Z, respectively, at <1 year, 7 years and in adulthood using Wilcoxon signed-rank tests. Adult MPAH-Z was compared between men and women using a Wilcoxon rank-sum test.
For the GRS cohort, we first tested the relationship between the HT-GRS-Z and HT-Z using standard Pearson correlation. We then further analyzed this relationship using multiple linear regression, adjusting for age, BMI-Z, and FEV1-Z.21 These analyses were performed separately based on presence or absence of clinically defined pancreatic sufficiency status consisting of pancreatic enzyme use and/or fecal elastase <200 µg/g.22 As individual measures were collected over time, the most recent height measurement was used in the model. For individuals who did not have FEV1 available at that time, the most recent FEV1 measure was used.
All statistical analyses were performed using Stata v15 (StataCorp, College Station, Texas). Z scores were generated for anthropometric data using the “zanthro” command.
Results
Demographic data
HV-Z Cohort (Table 1a): CHOP-specific CFF Registry data were available for 249 subjects, with a total of 1676 simultaneous height and weight measurements available between age 1 week and 21.6 years. The number of measurements for individual subjects ranged between 3 and 10. The cohort was 49% female, and, as is characteristic for populations with CF, the majority were Caucasian (89.6%) and pancreatic insufficient (86%).
GRS cohort (Table 1b): GRS, BMI, height, and FEV1 were available in 328 subjects of median age 22.7 years at the time of the most recent height measurement. The cohort was also 49% female, majority Caucasian (93%) and pancreatic insufficient (86%).
HV-Z cohort
Height–velocity curve and growth pattern
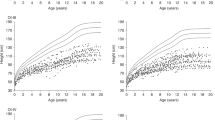
Figure 1 depicts median HV-Z for children with CF (5–17 years). As further detailed in Fig. 2, mean HV at each age interval in our CF youth generally fell between the 25th and 75th percentiles for average maturing children across the available age range until adulthood.10 Across age intervals (5–17 years), 51% had HV-Z < 0 (50th percentile) and 20% had HV-Z < −0.67 (25th percentile), but at no age was HV-Z significantly different from the HV-Z population mean of 0 (50th percentile).
Left: CF males’ mean height velocity (cm/year) at each year plotted against reference curves created using BMDCS data for average maturing healthy children, demonstrating that overall, the CF height velocity z score falls near the 50th percentile. Right: CF females’ mean height velocity (cm/year) at each year plotted against reference curves created using BMDCS data for average maturing healthy children, demonstrating that overall, the CF height velocity z score falls near the 50th percentile.
Growth patterns by age and stature
Length-Z/HT-Z were examined at specific ages (<1 year (n = 24), 7 years (prepubertal, n = 78) and during adulthood (post-pubertal, n = 73)) to assess deviation from mean growth during these periods (Table 2). At age <1 year, median length-Z was −0.74 (IQR: −2; −0.2) for boys (n = 8) and −0.4 (−1.1; −0.1) for girls (n = 16). The median age in this group for boys was 7 months (range 2.6–12 months) and for girls was 4.8 months (range 0.02–12 months). At 7 years, HT−Z was −0.7 (−1.3; 0.1) and −0.6 (−1.1; 0.1), for boys (n = 41) and girls (n = 37), respectively. Adult HT−Z for men (n = 36) and women (n = 37) were −0.7 (−1.6; 0.2) and −0.5 (−0.9; 0.1), respectively. No differences in HT-Z between age categories for either boys (p = 0.1) or girls (p = 0.58) were found.
In an age- and gender-adjusted model, HV-Z was positively associated with HT-Z. For each standard deviation increase in HT-Z, HV-Z increased by 1.5 (p < 0.01). In addition, a sex difference was present with females having a lower HV-Z (beta-coefficient = −0.33; p = 0.01) in this adjusted model (Fig. 3). Moreover, a negative interaction between HT-Z and age was found, such that the positive effect of being taller on HV-Z diminished with increasing age (p < 0.01).
Line of best fit was derived from the relationship between HV-Z and HT-Z. The relationship for boys is depicted in the upper panel, and for girls in the lower panel. For each standard deviation increase in HT-Z, HV-Z increased by 1.5 (p < 0.01). A sex difference was present with females having a lower HV-Z (beta-coefficient = −0.33; p = 0.01) than males.
Height adjusted for genetic potential
Maternal and paternal heights were slightly higher than the population average (median maternal HT-Z = +0.27 and paternal HT-Z = +0.13) (Table 2). In general, adjusted heights were lower than unadjusted heights (Fig. 4). For example, the median unadjusted heights of adult males with CF were 7 cm shorter than their fathers and median unadjusted adult women heights were 5 cm shorter than their mothers. Greater divergence from genetic potential was noted with MPAH (p < 0.001) with median height differences using this adjustment magnified to 12 cm for men and 7 cm for women (Table 2). In all, 87% of men and 81% of women with CF had adult heights less than the average parental Z score. Adjusted adult HT-Z score reductions were greater for men with CF than for women with CF (p < 0.03, Table 2).
GRS cohort
Height GRS
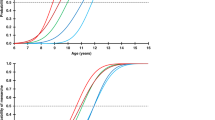
Mean HT-GRS-Z was −0.04. GRS Z score was positively correlated with HT-Z, but the correlation was higher in PS-CF (rho = 0.42) than in PI-CF (rho = 0.27) participants (Fig. 5). In an age, BMI-Z, FEV1-Z and pancreatic status-specific models, every 1 SD increase in HT-GRS-Z was associated with a 0.34 higher HT-Z (p < 0.001, R2 = 0.11; Table 3) in PI-CF. However, for PS-CF subjects, every 1 SD increase in height GRS Z score was associated with a 0.51 higher HT-Z (p < 0.05, R2 = 0.25; Table 3). The variance in HT-Z was less well explained by GRS Z score in PI-CF (R2 = 0.11) compared with PS-CF (R2 = 0.25). In addition, in a combined model, FEV1-Z was positively related to HT-Z in PI-CF (p = 0.02) but not in PS-CF (p = 0.56).
Line of best fit was derived from the age, BMI-Z, FEV1 -Z, and pancreatic status-specific regression models. Height percentiles are provided on the right axis for comparisons. Every 1 SD increase in HT-GRS-Z was associated with a 0.34 higher HT-Z (p < 0.001, R2 = 0.11) in PI-CF. However, for PS-CF subjects, every 1 SD increase in height GRS Z score was associated with a 0.51 higher HT-Z (p < 0.05, R2 = 0.25).
Discussion
Our single-center study demonstrates a clinically relevant height reduction among children and young adults with CF (median HT-Z was −0.7 (24th percentile) for boys and −0.4 (34th percentile) for girls), consistent with previous literature. Upon adjustment for reported parental heights, this reduction is magnified to a Z score of −1.1 (12th percentile) for boys and a Z score of −0.8 (21st percentile) for girls. HV through mid- and late childhood was generally preserved. In addition, individuals with CF and the general population are similarly enriched in height protective genes; GRS Z score was associated with higher HT-Z and the association was stronger in PS-CF even after adjustment for pulmonary function.
Our study confirms that children with CF have below average lengths from a young age, and that height remains persistently low throughout childhood and into adulthood. These data are consistent with prior studies, suggesting that CF patients are short from birth23 and remain short through early childhood, despite early diagnosis with newborn screening and, especially more recently, adequate nutrition.24
The mean HT-GRS Z score of our cohort was close to zero when generated using the BMDCS summarized data and established that height protective genetic variants are similarly represented in CF and the general population. Interestingly, when we stratified our analyses by pancreatic sufficiency status, we found that the GRS Z score association with HT-Z was stronger in individuals with pancreatic sufficient CF. In contrast age, BMI Z score and FEV1 Z score were not associated with HT-Z after adjustment for height GRS in pancreatic sufficient individuals with CF. We believe that BMI Z score did not associate with HT-Z given the overall excellent BMI noted in our center with a median BMI-Z of 0.06. Together, these data suggest that either pancreatic insufficiency in CF or the more general and severe lack of CFTR function associated with PI-CF may significantly modify the association between genetic susceptibility to taller stature and HT-Z in the CF population.
Some previous studies have suggested that discrepancy in final height may be due to diminished and/or delayed pubertal height velocity (PHV) in children with CF.4,5,6,7,8,9 In a large study using the CFF patient registry data and sophisticated statistical analysis, Zhang and colleagues reported impaired pubertal height velocities in children with CF compared with children without CF (PHV < 5th percentile) based on graphs elaborated by Tanner and Davies in 1985.7,10,25,26 The Tanner and Davies curves were generated by overlaying HV data originating largely from the Harpenden study, using a relatively restricted population of European descent, over the cross-sectional US NCHS data and centered on the PHV.27,28 In contrast, the BMDCS HV curves used here employ longitudinal data from a contemporary US population of children and adolescents. These BMDCS-based curves are not centered on age at PHV, but rather on the distribution of age-specific HV values.10 Although this approach limits direct comparison with the Tanner and Davies curves, these newer curves allow for calculation of HV-Z and facilitate comparison of patients with CF to a healthy cohort. Using this novel method, we demonstrated that height velocities for children 5–17 years of age consistently fall within the 25th–75th percentiles of healthy, average maturing children without CF. We further noted that shorter children with CF tended to have slower growth than taller children with CF. This effect is most pronounced in prepubertal children, thereby countering the notion that disparities in height velocities at puberty continue to explain shorter stature in patients with CF despite advances in nutrition. Whether this pattern of stature-dependent growth velocity is also observed in the general population is not known.
The factors determining shorter stature in CF are likely complex, with possible contributions from chronic infection, inflammation, oral glucocorticoid use, as well as potentially altered growth hormone (GH) secretion.29 Notably, in our study’s population, few subjects had significant systemic glucocorticoid exposure. Studies comparing GH secretion across a range of ages are limited, and, whereas linear growth in CF falters antenatally, the role of GH in fetal and infant development is limited.30 Insulin-like growth factor 1 (IGF-1), which—largely independently of fetal pituitary GH–play a prominent role in fetal and neonatal growth and correlate with height later in life, are lower in CF infants from birth compared with controls,29,31,32 Moreover, insulin deficiency results in lower circulating IGF-1 and IGF-binding protein 3 and higher IGF-binding protein-1, suggesting another mechanism of growth impairment in CF across the spectrum of glucose tolerance.33 However, many of these mechanisms would be expected to impair HV throughout childhood, which we did not observe in our study. Differences in insulin secretion and insulin secretory reserve between PI-CF and PS-CF may contribute to the difference in genetic determination of height shown here between these groups.34
Certain limitations of our study are important to note. First, as information was gathered from patient medical records, pubertal status was not formally assessed and our ability to make direct conclusions about growth during puberty is therefore limited. Second, we relied on length and heights obtained at routine medical visits, and their methods of measurement cannot be confirmed. However, studies have suggested that reasonable agreement exists between anthropometric measurements obtained in clinic and those obtained using a research protocol.35 In addition, the number of measurements in the < age 1-year category was limited. Meanwhile, mid-parental height was obtained through parental report and not actually measured. If we consider this self-report approach may overestimate mid-parental height, the calculated MPAH-Z may overstate the deviation from parental height. Finally, the GRS was a weighted estimate using SNPs and effect sizes previously implicated by GWAS of height in adults, which may be not the most relevant in a pediatric cohort. Whether some of these height-related SNPs are involved in CFTR expression is also not known. Although stratification of the GRS and HT-Z provided interesting results with regard to pancreatic status, we acknowledge only a small proportion (14%) of this cohort was pancreatic sufficient.
Conclusion
Our data suggest that CF patients are shorter than their peers despite no significant difference in HV over time and similar genetic enrichment in SNPs conferring taller stature. Shorter individuals do have lower HV than taller individuals; however, this contribution of short stature diminishes with age. Taken together, these data argue against diminished pubertal HV as the source short stature in adults with CF. In our genotyped cohort, we conclude that height GRS does describe some of the height variation for individuals with CF, but this effect differs greatly with pancreatic sufficiency status, suggesting that other modifying factors exist for PI-CF individuals. Whether differences in linear growth are related to subtle changes in nutritional status or severity of CFTR mutation is similarly not clear at this time. Further studies assessing impact of novel CFTR modulator therapies may help to address some of these questions.
References
Corey, M., McLaughlin, F. J., Williams, M. & Levison, H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J. Clin. Epidemiol. 41, 583–591 (1988).
Borowitz, D., Baker, R. D. & Stallings, V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 35, 246–259 (2002).
Blackman, S. M. & Tangpricha, V. Endocrine disorders in cystic fibrosis. Pediatr. Clin. North Am. 63, 699–708 (2016).
Landon, C. & Rosenfeld, R. G. Short stature and pubertal delay in male adolescents with cystic fibrosis. Androgen treatment. Am. J. Dis. Child 138, 388–391 (1984).
Davis, P. B. & Byard, P. J. Heterozygotes for cystic fibrosis: models for study of airway and autonomic reactivity. J. Appl. Physiol. 66, 2124–2128 (1989).
Barkhouse, L. B., Fahey, J., Gillespie, C. T. & Cole, D. E. Quantitating the effect of cystic fibrosis on linear growth by mathematical modelling of longitudinal growth curves. Growth Dev. Aging 53, 185–190 (1989).
Zhang, Z., Lindstrom, M. J. & Lai, H. J. Pubertal height velocity and associations with prepubertal and adult heights in cystic fibrosis. J. Pediatr. 163, 376–382 (2013).
Bournez, M., Bellis, G. & Huet, F. Growth during puberty in cystic fibrosis: a retrospective evaluation of a French cohort. Arch. Dis. Child 97, 714–720 (2012).
Zhang, Z., Lindstrom, M. J., Farrell, P. M. & Lai, H. J., Wisconsin Cystic Fibrosis Neonatal Screening G. Pubertal height growth and adult height in cystic fibrosis after newborn screening. Pediatrics 137, e20152907 (2016).
Kelly, A. et al. Age-based reference ranges for annual height velocity in US children. J. Clin. Endocrinol. Metab. 99, 2104–2112 (2014).
Yang, J. et al. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 42, 565 (2010).
Visscher, P. M., Yang, J. & Goddard, M. E. A commentary on ‘common SNPs explain a large proportion of the heritability for human height’ by Yang et al. (2010). Twin Res. Hum. Genet 13, 517–524 (2010).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum. Mol. Genet. 27, 3641–3649 (2018).
Kuczmarski R. J. et al. 2000 CDC growth charts: United States. Adv Data:1–27.
Lawman, H. G. et al. Comparing methods for identifying biologically implausible values in height, weight, and body mass index among youth. Am. J. Epidemiol. 182, 359–365 (2015).
Himes, J. H., Roche, A. F., Thissen, D. & Moore, W. M. Parent-specific adjustments for evaluation of recumbent length and stature of children. Pediatrics 75, 304–313 (1985).
Zhang, Z., Shoff, S. M. & Lai, H. J. Incorporating genetic potential when evaluating stature in children with cystic fibrosis. J. Cyst. Fibros. 9, 135–142 (2010).
Purcell S. C., Christopher PLINK [1.9] www.cog-genomics.org/plink/1.9/.
Chang C. C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4:7 (2015).
Zemel, B. S. et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J. Clin. Endocrinol. Metab. 96, 3160–3169 (2011).
Brazzale, D. J., Hall, G. L. & Pretto, J. J. Effects of adopting the new global lung function initiative 2012 reference equations on the interpretation of spirometry. Respiration 86, 183–189 (2013).
Lohr, J. M., Oliver, M. R. & Frulloni, L. Synopsis of recent guidelines on pancreatic exocrine insufficiency. United European. Gastroenterol. J. 1, 79–83 (2013).
Haeusler, G., Frisch, H., Waldhor, T. & Gotz, M. Perspectives of longitudinal growth in cystic fibrosis from birth to adult age. Eur. J. Pediatr. 153, 158–163 (1994).
Leung, D. H. et al. Effects of diagnosis by newborn screening for cystic fibrosis on weight and length in the first year of life. JAMA Pediatr. 171, 546–554 (2017).
Tanner J. M., Whitehouse R., Takaishi M.J. Aodic 1966 Standards from birth to maturity for height, weight, height velocity, and weight velocity: British children. I. 41:454 (1965).
Tanner, J. M. & Davies, P. S. Clinical longitudinal standards for height and height velocity for North American children. J. Pediatr. 107, 317–329 (1985).
Tanner, J. M., Whitehouse, R. H., Marubini, E. & Resele, L. F. The adolescent growth spurt of boys and girls of the Harpenden growth study. Ann. Hum. Biol. 3, 109–126 (1976).
Tanner, J. M. & Whitehouse, R. H. Clinical longitudinal standards for height, weight, height velocity, weight velocity, and stages of puberty. Arch. Dis. Child 51, 170–179 (1976).
Wong, S. C. et al. Growth and the growth hormone-insulin like growth factor 1 axis in children with chronic inflammation: current evidence, gaps in knowledge, and future directions. Endocr. Rev. 37, 62–110 (2016).
Hawkes, C. P. & Grimberg, A. Measuring growth hormone and insulin-like growth factor-I in infants: what is normal? Pediatr. Endocrinol. Rev. 11, 126–146 (2013).
Rogan, M. P. et al. Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proc. Natl Acad. Sci. 107, 20571–20575 (2010).
Gohlke, B. C., Fahnenstich, H., Dame, C. & Albers, N. Longitudinal data for intrauterine levels of fetal IGF-I and IGF-II. Horm. Res. 61, 200–204 (2004).
Bizzarri, C. et al. Glucose tolerance affects pubertal growth and final height of children with cystic fibrosis. Pediatr. Pulmonol. 50, 144–149 (2015).
Sheikh, S. et al. Reduced β-cell secretory capacity in pancreatic-insufficient, but not pancreatic-sufficient, cystic fibrosis despite normal glucose tolerance. Diabetes 66, 134–144 (2017).
Bryant M. et al. Agreement between routine and research measurement of infant height and weight. BMJ 100, 4–29 (2015).
Acknowledgements
We would like to thank the subjects with CF for their participation, Russell Localio, PhD of the University of Pennsylvania Department of Biostatistics, Epidemiology and Informatics for assistance with the statistical analyses and Rahul Agarwal for assistance with genetic sample collection. This work was supported by grants from the Cystic Fibrosis Foundation (to A.K., M.R.R., and M.J.K.), Public Health Services Research Grants R01 DK97830 (to A.K. and M.R.R.) and R01 DK085212 (to S.F.A.G.), UL1 TR000003 (Penn and CHOP Clinical & Translational Research Centers) and the Daniel B. Burke Endowed Chair for Diabetes Research (S.F.A.G.). A component of the height GRS findings was presented at the North American Cystic Fibrosis Conference in 2017 as an oral platform presentation. Otherwise, data from this manuscript are not under review or published elsewhere.
Author information
Authors and Affiliations
Contributions
Zofia Zysman-Colman, Marissa J. Kilberg, Victor S. Harrison, Ronald C. Rubenstein and Andrea Kelly—all made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, drafted the article or revised it critically for important intellectual content; and gave final approval of the version to be published. Alessandra Chesi, Struan F.A. Grant, Jonathan Mitchell, Saba Sheikh, Denis Hadjiliadis, Michael R. Rickels, all made substantial contributions to the analysis and interpretation of data, revised the article critically for important intellectual content; and gave final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
The requirement for patient consent was waived by the Children’s Hospital of Philadelphia Institutional Review Board.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zysman-Colman, Z.N., Kilberg, M.J., Harrison, V.S. et al. Genetic potential and height velocity during childhood and adolescence do not fully account for shorter stature in cystic fibrosis. Pediatr Res 89, 653–659 (2021). https://doi.org/10.1038/s41390-020-0940-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0940-4