Abstract
Background
Increasing evidence suggests that postnatal overfeeding induces childhood obesity, which is strongly associated with metabolic syndrome. Insulin resistance is a risk factor for metabolic syndrome. MicroRNA-221 (miR-221) is involved in the development of obesity and has been reported to negatively regulate insulin sensitivity. However, the underlying mechanism remains unclear.
Methods
Rats raised in small litters (SLs, three pups/dam, n = 10) and normal litters (NLs, 10 pups/dam, n = 10) were used to model early postnatal overfeeding and act as controls, respectively. miR-221 and proteins related to the phosphoinositide 3-kinases (PI3K)/protein kinase B (AKT) pathway were assessed in the liver.
Results
Early postnatal overfeeding significantly increased body weight, visceral fat index, blood glucose, serum triglycerides, and the homeostasis model assessment of insulin resistance at 9 weeks. Real-time polymerase chain reaction (PCR) and western blot analysis revealed that postnatal overfeeding induced insulin receptor and insulin receptor substrate 2 expression, but decreased PI3K and AKT phosphorylation in the liver. Quantitative real-time PCR showed that hepatic miR-221 was significantly overexpressed in the SL group.
Conclusions
These results indicate that postnatal overfeeding induces hepatic miR-221 overexpression and impairs the PI3K/AKT signal pathway, which may cause insulin resistance.
IMPACT
-
We first report postnatal overfeeding induces hepatic miR-221 expression.
-
Postnatal overfeeding impairs PI3K/AKT pathway in the liver of adult rats.
-
Postnatal overfeeding induces obesity and high blood glucose.
-
Avoidance of overfeeding during early postnatal life may prevent obesity and T2DM.
Similar content being viewed by others
Introduction
Obesity and related metabolic disorders have emerged as serious global health problems.1 In the United States, the prevalence of obesity among adults and youth has increased significantly from 1999 to 2000 through 2013 to 2014, and the age-adjusted prevalence of obesity in 2013 to 2014 was 35% among males and 40.4% among females.2 Obesity is associated with increased prevalence of type 2 diabetes mellitus (T2DM), and according to data from the National Health and Nutrition Examination Survey 2007 to 2010, the T2DM prevalence was 18.5% for adults who were obese and 5.4% for normal-weight adults in the United States.3 T2DM is characterized by insulin resistance and/or abnormal insulin secretion, which leads to high serum glucose levels.4
After animals or human beings eat, the insulin produced by β-cells passes into the bloodstream and activates the insulin receptor (INSR) on the surface of the liver cell membrane.5 Activated INSR phosphorylates the tyrosine site of INSR substrate-1/2 (IRS-1/2; the insulin metabolism effects in the liver are mainly due to IRS-2), then activates phosphoinositide 3-kinases (PI3Ks). Activated PI3K can catalyze PIP2 (4,5-2 phosphatidyl inositol phosphate) and generate PIP3, which acts as the second messenger to activate protein kinase B (AKT).5 The PI3K/AKT pathway plays a central role in cellular physiology by mediating growth factor signaling during organismal growth and critical cellular processes, such as glucose homeostasis, lipid metabolism, protein synthesis, and cell proliferation and survival.6 Liver, skeletal muscle, and adipose tissue are three major insulin-targeted tissues involved in glucose utilization, while the liver is the only organ that acutely responds to insulin by reducing glucose levels.7 The PI3K/AKT pathway in the liver has also been reported to regulate sterol regulatory element binding protein-1c expression to mediate triglyceride (TG) synthesis.8 Therefore, impairment of the PI3K/AKT pathway in the liver could cause TG and glucose metabolism disorder.
The developmental origins of the health and disease hypothesis propose that the prenatal period and early neonatal life strongly influence health outcomes throughout life.9,10 It has been reported that rapid weight gain during infancy is associated with childhood obesity and is a major risk factor for cardiovascular disease, non-alcoholic fatty liver disease, and T2DM during adulthood.11 These diseases are closely related to metabolic syndrome. Metabolic syndrome is a clinical condition associated with at least three of the following metabolic risk factors: excess visceral adiposity (abdominal obesity), insulin resistance, hyperglycemia, hypertension, and dyslipidemia (high TGs and low high-density lipoprotein cholesterol). In particular, a strong association between obesity and metabolic syndrome was found in prior studies.12 Some animal experiments have reported that postnatal overfeeding induces obesity, hyperinsulinemia, hyperglycemia, and dyslipidemia,13,14 which are the main manifestations of metabolic syndrome. Neonatal overfeeding induced by small-litter (SL) rearing can induce persistent hyperphagia, obesity, elevated triacylglycerols, and impaired glucose tolerance.15 Therefore, in the current study, we used SL raised rats to induce postnatal overfeeding, which is a well-established animal model, to evaluate the short- and long-lasting consequences of childhood obesity.16
MicroRNAs (miRNAs) are a class of small RNA molecules that do not encode amino acids and typically consist of ~23 nucleotides.17 In animals, miRNAs are highly conserved and their seed regions are usually paired with the 3′-untranslated region (3′-UTR) of messenger RNA (mRNA) to exert their regulatory effects by inhibiting protein translation processes.18 miRNAs are involved in a variety of biological processes, including adipogenesis and fat metabolism.19,20 Differential expression of miRNAs has been reported in the tissues of obese vs. non-obese human individuals.21 Postnatal overfeeding causes adulthood obesity, although the underlying mechanism is unknown. The PI3K/AKT pathway is associated with both glucose and lipid metabolism; therefore, the present study was designed to determine whether the hepatic PI3K/AKT pathway is involved in the development of obesity. Our data show that postnatal overfeeding can lead to hepatic miR-221 overexpression and impairs the PI3K/AKT pathway in adult rats, which causes dysregulated TGs and glucose metabolism.
Materials and methods
Experimental model and blood and tissue collection
Sprague–Dawley rats were housed in a temperature-controlled room (23 ± 2 °C) with 12-h light/dark cycles. The protocol and animal procedures were performed in accordance with the Guide to the Care and Use of Experimental Animals and approved by the Animal Research Committee of Fujian Medical University. Adult virgin female rats were caged with male rats at a ratio of 3:1. During pregnancy and lactation, dams were housed in individual cages and given water and a standard pellet diet ad libitum. The mean size of birth litters used in the study was 8–12 pups/dam. Three days after birth, male pups were randomly distributed among the dams to achieve cross-fostering. At this time, the litter size was adjusted to 10 male pups (normal-litter, NL group, four litters) or reduced to three male pups in order to induce postnatal overfeeding (SL group, 10 litters). At weaning (day 21), the rats of both groups had free access to a standard pellet diet and water. Body weight gain was measured throughout life. Food intake was recorded every week from 21 to 63 days old. Fasting blood glucose (FBG) levels were measured with Yicheng blood glucose test paper (Yicheng JPS-7 glucose meter, China) using tail vein samples. Rats were fasted overnight and killed after anesthesia with pentobarbital sodium (40 mg/kg body weight). NL- and SL-fasted rats were killed when they were either 21 or 63 days old, and we collected blood, liver, and visceral fat. Subsequent analyses were undertaken on 10 rats randomly selected from each group (n = 10).
Blood was drawn from the abdominal aorta, left at room temperature for 30 min, and then centrifuged at 3000 r.p.m. (4 °C, 10 min) to obtain serum. Livers and abdominal fat were separated and weighed, and then immediately frozen with liquid nitrogen and stored at −80 °C for later assays. The liver index (liver weight/body weight × 100%) and the fat index (abdominal fat/body weight × 100%) were calculated. The homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated as follows: FBG (mmol/L) × fasting insulin (FINS; mIU/L)/22.5.
Serum biochemical test
Serum FINS was quantified using an enzyme-linked immunosorbent assay kit (ELISA, Elabscience Biotechnology Co., Ltd., Wuhan, China). Serum TG was quantified using an assay kit (Nanjing Jiancheng, Nanjing, China), according to the manufacturer’s instructions.
Quantitative real-time PCR assay for mRNA expression and miR-221 detection
Total RNA was extracted from the liver using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. The purity and concentration of RNA were determined from OD260/280 readings using a spectrophotometer (NanoDrop ND-1000, Thermo Fisher Scientific, Wilmington, DE, USA). The extracted mRNA was reverse transcribed into complementary DNA (cDNA) with a TAKARA OneStep RT-PCR kit using 1 μg of total RNA and the oligo (dT) primers supplied in the kit. Gene expression was studied using quantitative real-time PCR (qRT-PCR). The procedure was performed using SYBR Green chemistry with a StepOnePlus qRT-PCR detection system (Applied Biosystems). The qRT-PCR program started with 95 °C for 30 s. The 40 cycles of the qRT-PCR program consisted of denaturation at 95 °C for 5 s, annealing at 60 °C for 30 s, and extension at 95 °C for 5 s, followed by one cycle of a final extension at 60 °C for 1 min. Data are shown as fold change (2−△△Ct). Samples were analyzed in triplicate in three independent experiments. All primer sequences are presented in Table 1.
For miR-221 isolation and detection, a microRNA cDNA Synthesis Reaction System and Real-Time Quantitative PCR Reaction System were used according to the manufacturer’s instructions (Mir-X™ miRNA First-Strand Synthesis and SYBR® qRT-PCR User Manual, Takara Bio Company, USA). The prepared qRT-PCR reaction solution (except for the cDNA template) was mixed and then distributed into a 96-well plate. The cDNA template of the qRT-PCR reaction was added, and the amplification conditions were set. The amplification reaction was carried out using a QuantStudioTM 3&5 Real-Time PCR System with U6 as a control. All reactions were performed in triplicate. The primer sequences for miRNA-221 and U6 are listed in Table 2.
Western blot
Cell lysates from livers were prepared in 1.5-mL microtubes on ice in 1 mL of lysis buffer. Lysates were solubilized by continuous stirring for 1 h at 4 °C and were then centrifuged for 30 min at 14,000 × g. Tissue lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and proteins were transferred onto polyvinylidene fluoride (PVDF) membranes, blocked with 5% skimmed milk in Tris-buffered saline containing 0.1% Tween-20 for 1 h, and then incubated with primary antibodies for 16 h at 4 °C (anti-INSR, -IRS-2, -PI3K, -AKT, -p-PI3K, -p-AKT, and -β-actin; Proteintech, IL, USA). Membranes were washed three times in Tris-buffered saline containing 0.1% Tween-20 and then incubated with anti-mouse or anti-rabbit IgG antibodies for 1 h. Immunoreactive bands were visualized using an ECL commercial kit (Amersham Pharmacia Biotech, Little Chalfont, UK).
Statistical analysis
All values are expressed as the mean ± standard error of the mean (SEM). Multiple comparisons were analyzed by one-way analysis of variance combined with the post hoc Fisher’s least significance difference test. A value of p < 0.05 was considered to be statistically significant between the means of the two groups. Statistical analysis was performed using the SPSS 20.0 software (SPSS Inc., Chicago, IL).
Results
Postnatal overfeeding in rats induces adulthood obesity
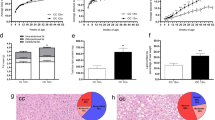
To investigate the effect of postnatal overfeeding on body weight, we adjusted litter sizes on postnatal day 3 to either 10 male pups/dam (NL) or three male pups/dam (SL). NL and SL pups had the same average body weight at the time of litter adjustment, but SL pups gained weight more rapidly and were significantly heavier at weaning (at 3 weeks) and into adulthood (at 9 weeks, Fig. 1a). The naso-anal length of SL rats was significantly larger than that of NL rats at 9 weeks (Fig. 1b). Visceral fat measurements from the SL group were significantly heavier than those of the NL group at 3 and 9 weeks (Fig. 1c, d). Food intake in the SL group was slightly higher than in the NL group, but this was not statistically different from 3 weeks to adulthood at 9 weeks (Supplementary Fig. S1).
Postnatal overfeeding increases body weight (a), naso-anal length (b), visceral fat (c), and visceral fat index (visceral fat weight/body weight, d). Rats at postnatal day 3 were adjusted to 10 male pups/litter for the normal-litter (NL) group and 3 male pups/litter for the small-litter (SL) group, to induce postnatal overfeeding. Values are expressed as mean ± SEM. *Mean value is significantly different from NL group at P < 0.05; n = 10.
Postnatal overfeeding increases HOMA-IR, serum and hepatic TG, and changes liver morphology
A commonly used estimate of insulin resistance is the HOMA-IR, which is proportional to the production of plasma glucose and insulin levels in fasted animals.22 We found that HOMA-IR was significantly higher in the SL group compared to the NL group at 9 weeks, indicating that insulin resistance occurred in the SL group (Fig. 2c). Serum TG was also measured at 3 and 9 weeks, and results show that serum and hepatic TGs in the SL group were significantly higher than in the NL group (Fig. 2d, g). Representative liver morphology was compared between the two groups, and the results show postnatal overfeeding increased liver size and changed the liver color from dark black to brown yellow (Fig. 2e). The liver index was higher in the SL group than in the NL group at 9 weeks (Fig. 2f).
Fasting blood glucose (a); fasting insulin (b); HOMA-IR index = FBG (fasting blood glucose) (mmol/L) × FINS (fasting insulin) (mIU/L)/22.5) (c); serum TG level (d); representative liver morphology (e); liver index (liver weight/body weight × 100%) (g); hepatic TG concentration (f). NL normal-litter, 10 male pups/litter; SL small-litter group, 3 male pups/litter. Values are expressed as mean ± SEM. *Mean value is significantly different from the NL group at P < 0.05; n = 10.
mRNA and protein expression of molecules in the PI3K/AKT pathway
We next examined the mRNA and protein expression of molecules related to the PI3K/AKT pathway in the liver. As shown in Fig. 3, compared to the NL group, INSR and IRS-2 mRNA expression were significantly increased in the SL group, but PI3K mRNA expression was suppressed in the SL group at 9 weeks. These results were confirmed by protein expression analyses (Fig. 4a, b). Phosphorylation of PI3K and AKT was decreased in the SL group. Normally, PI3K expression is positively regulated by IRS-2;5 however, in the present study, increased IRS-2 in the SL group failed to activate PI3K expression, indicating impairment of the PI3K/AKT pathway.
Hepatic protein expression was measured by western blot at 9 weeks. Representative blots are shown (a); β-actin content was used to control for loading and results are expressed relative to the control (b). NL normal-litter, 10 male pups/litter; SL small-litter group, 3 male pups/litter. Values are expressed as mean ± SEM. *Mean value is significantly different from NL group at P < 0.05; n = 3.
qRT-PCR analysis of miRNAs in the liver from NL and SL groups
miRNAs regulate gene expression post-transcriptionally by directly binding to the 3′-UTR of target mRNAs.17 Dysregulation of miRNAs has been implicated in the pathogenesis of various diseases, including obesity and diabetes.23 Since miR-221 is involved in the development of obesity24 and has been reported to negatively regulate insulin sensitivity;25 moreover, in a previous study, we have demonstrated that miR-221 bound PI3K to suppress its expression;26 therefore, we performed qRT-PCR to examine miR-221 expression. As shown in Fig. 5b, miR-221 expression was significantly upregulated in the SL group at both 3 and 9 weeks, indicating that miR-221 may bind to PI3K mRNA to inhibit its expression (Fig. 5).
Discussion
Clinical studies have shown that being overweight during critical developmental periods of neonatal life is associated with adult obesity.27 Additionally, several studies using animal models have demonstrated that overfeeding in the first few days of life leads to long-term obesity and induces cardiovascular dysfunction by oxidative stress.28,29 Moreover, maternal diet-induced obesity during the suckling period programs offspring to display an obese phenotype.30 Some studies have shown that formula feeding is a risk factor for being overweight in later life.31,32 Breast-fed infants, compared to those given formula feeds, have lower daily calorie intakes and a 40% decreased risk of developing T2DM.33 In rats, early postnatal overfeeding caused by manipulations to produce SLs has been shown to cause persistent heavier weight, hyperinsulinemia, and insulin resistance.34 In the present study, our data show that postnatal overfeeding induced by SL size leads to obesity, insulin resistance, and glucose and lipid metabolism disorder. This overweight/obese phenotype has been extensively investigated. However, molecular mechanisms underlying the cause of TG and glucose metabolism disorder are poorly appreciated. To the best of our knowledge, no study has yet investigated the effect of postnatal overfeeding on the PI3K/AKT pathway. In this study, we focused on the consequences of overfeeding during early postnatal life on miRNA expression and the PI3K/AKT pathway in the liver.
INSR and its substrate play important roles in the regulation of glucose homeostasis.35 Activated INSR and IRS-2 induce the action of insulin by stimulating the PI3K-AKT pathway and subsequently promoting glucose uptake. In the current study, we observed an increase in INSR and IRS-2 expression, but their stimulation failed to induce activation of PI3K, which may account for the inhibition of miRNAs. MiRNAs represent a class of small non-coding pieces of RNA in eukaryotes. Various miRNAs are encoded by nuclear DNA and are involved in the translation process as repressors of gene expression by RNA silencing and regulation of post-transcriptional processes.36 They can interact and pair with their complementary sequences of RNA; thus, silencing them by either degradation into separate segments, shortening of their poly A tail, and RNA destabilization, or by decreasing the translation of RNA into proteins.17 It has been reported that miRNAs are involved in insulin secretion, β-cell differentiation, glucolipid metabolism, and many other diabetes-related processes.37 Several studies have reported that miRNAs contribute to the progression of T2DM.38 We found that the overexpression of hepatic miR-221 was accomplished by impairment of the PI3K/AKT pathway.
MiR-221 is located on the X chromosome (Xp11.3) and is highly conserved in vertebrates.39 A previous study found that miR-221 is a putative oncogene that has been found to be overexpressed in a number of human tumors.39 Recent studies reported that adipose miR-221 is upregulated in obesity,24 and adipose miR-221 expression is positively correlated with an increased body mass index in the Pima Indian population.40 To our knowledge, this is the first study to report that postnatal overfeeding induces miR-221 overexpression in the liver of adult rats. However, whether miR-221 secreted from the liver affects adipose miR-221 expression and induces obesity remains unknown. Furthermore, our results showing that postnatal overfeeding induces miR-221 overexpression and impairs PI3K/AKT pathway concurrently, begs the question whether miR-221 directly binds to PI3K and suppresses its expression in the liver; further investigations are underway to address this question.
In summary, our data provide evidence that postnatal overfeeding induced by SLs can lead to hepatic miR-221 overexpression and impairment of the PI3K/AKT pathway in adult rats, which causes dysregulated TGs and glucose metabolism. A proposed mechanism is presented in Fig. 6. Our findings suggest that avoidance of overfeeding during early postnatal life appears to be an important and effective means of preventing obesity or T2DM, and that miR-221 may be a new target for the prevention of obesity or T2DM.
References
Jing, L. et al. Cardiac remodeling and dysfunction in childhood obesity: a cardiovascular magnetic resonance study. J. Cardiovasc. Magn. Reson. 18, 28 (2016).
Benjamin, E. J. et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 135, e146–e603 (2017).
Saydah, S. et al. Trends in cardiovascular disease risk factors by obesity level in adults in the United States, NHANES 1999–2010. Obesity (Silver Spring) 22, 1888–1895 (2014).
Zimmet, P., Alberti, K. G. & Shaw, J. Global and societal implications of the diabetes epidemic. Nature 414, 782–787 (2001).
White, M. F. IRS proteins and the common path to diabetes. Am. J. Physiol. Endocrinol. Metab. 283, E413–422 (2002).
Abeyrathna, P. & Su, Y. The critical role of Akt in cardiovascular function. Vasc. Pharmacol. 74, 38–48 (2015).
Whiteman, E. L., Cho, H. & Birnbaum, M. J. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 13, 444–451 (2002).
Li, S. et al. Role of S6K1 in regulation of SREBP1c expression in the liver. Biochem. Biophys. Res. Commun. 412, 197–202 (2011).
Gluckman, P. D., Hanson, M. A., Cooper, C. & Thornburg, K. L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73 (2008).
Gluckman, P. D., Hanson, M. A. & Beedle, A. S. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am. J. Hum. Biol. 19, 1–19 (2007).
Andersen, L. G. et al. Weight and weight gain during early infancy predict childhood obesity: a case–cohort study. Int. J. Obes. (Lond.) 36, 1306–1311 (2012).
Dallmeier, D. et al. Metabolic syndrome and inflammatory biomarkers: a community-based cross-sectional study at the Framingham Heart Study. Diabetol. Metab. Syndr. 4, 28 (2012).
Ji, C. et al. Postnatal overfeeding promotes early onset and exaggeration of high-fat diet-induced nonalcoholic fatty liver disease through disordered hepatic lipid metabolism in rats. J. Nutr. Biochem. 25, 1108–1116 (2014).
Hou, M. et al. Neonatal overfeeding induced by small litter rearing causes altered glucocorticoid metabolism in rats. PLoS ONE 6, e25726 (2011).
Plagemann, A. et al. Perinatal elevation of hypothalamic insulin, acquired malformation of hypothalamic galaninergic neurons, and syndrome x-like alterations in adulthood of neonatally overfed rats. Brain Res. 836, 146–155 (1999).
Habbout, A., Li, N., Rochette, L. & Vergely, C. Postnatal overfeeding in rodents by litter size reduction induces major short- and long-term pathophysiological consequences. J. Nutr. 143, 553–562 (2013).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Chekulaeva, M. & Filipowicz, W. Mechanisms of miRNA-mediated post-transcriptional regulation in animal cells. Curr. Opin. Cell Biol. 21, 452–460 (2009).
Sun, L. et al. Mir193b-365 is essential for brown fat differentiation. Nat. Cell Biol. 13, 958–965 (2011).
Zaragosi, L. E. et al. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 12, R64 (2011).
Fu, T. et al. Aberrantly elevated microRNA-34a in obesity attenuates hepatic responses to FGF19 by targeting a membrane coreceptor beta-Klotho. Proc. Natl Acad. Sci. USA 109, 16137–16142 (2012).
Bonora, E. et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23, 57–63 (2000).
Rottiers, V. & Naar, A. M. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell. Biol. 13, 239–250 (2012).
Xie, H., Lim, B. & Lodish, H. F. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes 58, 1050–1057 (2009).
Peng, J. et al. miR-221 negatively regulates inflammation and insulin sensitivity in white adipose tissue by repression of sirtuin-1 (SIRT1). J. Cell Biochem. 119, 6418–6428 (2018).
Huang, F. et al. Palmitic acid induces microRNA-221 expression to decrease glucose uptake in HepG2 cells via the PI3K/AKT/GLUT4 pathway. Biomed. Res. Int. 2019, 8171989 (2019).
Stettler, N. et al. Weight gain in the first week of life and overweight in adulthood: a cohort study of European American subjects fed infant formula. Circulation 111, 1897–1903 (2005).
Velkoska, E., Cole, T. J. & Morris, M. J. Early dietary intervention: long-term effects on blood pressure, brain neuropeptide Y, and adiposity markers. Am. J. Physiol. Endocrinol. Metab. 288, E1236–1243 (2005).
Junior, M. D. F. et al. Postnatal early overfeeding induces cardiovascular dysfunction by oxidative stress in adult male Wistar rats. Life Sci. 226, 173–184 (2019).
Gomes, R. M. et al. Maternal diet-induced obesity during suckling period programs offspring obese phenotype and hypothalamic leptin/insulin resistance. J. Nutr. Biochem. 61, 24–32 (2018).
Ong, K. K. et al. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ 320, 967–971 (2000).
Stettler, N., Zemel, B. S., Kumanyika, S. & Stallings, V. A. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics 109, 194–199 (2002).
Owen, C. G. et al. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am. J. Clin. Nutr. 84, 1043–1054 (2006).
Conceicao, E. P. et al. Oxidative stress programming in a rat model of postnatal early overnutrition—role of insulin resistance. J. Nutr. Biochem. 24, 81–87 (2013).
Evans, J. L., Maddux, B. A. & Goldfine, I. D. The molecular basis for oxidative stress-induced insulin resistance. Antioxid. Redox Signal. 7, 1040–1052 (2005).
Kurtz, C. L. et al. MicroRNA-29 fine-tunes the expression of key FOXA2-activated lipid metabolism genes and is dysregulated in animal models of insulin resistance and diabetes. Diabetes 63, 3141–3148 (2014).
Tang, X., Tang, G. & Ozcan, S. Role of microRNAs in diabetes. Biochim. Biophys. Acta 1779, 697–701 (2008).
Guay, C. et al. Diabetes mellitus, a microRNA-related disease? Transl. Res. 157, 253–264 (2011).
Galardi, S. et al. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J. Biol. Chem. 282, 23716–23724 (2007).
Meerson, A. et al. Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-alpha. Diabetologia 56, 1971–1979 (2013).
Acknowledgements
This work was supported by the Natural Science Foundation of Fujian Province (No. 2017J01810) and the Special Funds for the Development of Local Colleges and Universities Supported by the Central Government (Nos. 1003-03900134 and GW-201601). The funding agencies played no role in the study design, analysis, or writing of this article. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
F.H. designed the study and wrote the manuscript; P.Z. analyzed the experimental data; J.C. and J.W. performed the experiment; W.L. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Huang, F., Zhu, P., Wang, J. et al. Postnatal overfeeding induces hepatic microRNA-221 expression and impairs the PI3K/AKT pathway in adult male rats. Pediatr Res 89, 143–149 (2021). https://doi.org/10.1038/s41390-020-0877-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0877-7