Abstract
Background
The associations of renal, hepatic, and hematologic markers with metabolic risk (MR) have already been shown in adolescents. However, it is still controversial which marker best predicts metabolic changes in youth. The aim of this study was to verify the association of MR with alanine aminotransferase (ALT), aspartate aminotransferase (AST), uric acid, and hemoglobin (Hb) in adolescents.
Methods
We evaluated 1713 Brazilian adolescents aged 10 to 17 years. MR was calculated using a continuous metabolic risk score, including the sum of Z-scores of waist circumference, systolic blood pressure, fasting glucose, high-density lipoproteins, triglycerides, and cardiorespiratory fitness. Cutoff points were set for MR prediction for five metabolic components (ALT, AST, AST/ALT ratio, uric acid, and Hb).
Results
MR was strongly associated with increased uric acid (odds ratio [OR]: 2.50; 95% confidence interval [CI]: 1.74–3.59), ALT (OR: 2.64; 95% CI: 1.63–4.27), and AST levels (OR: 2.53; 95% CI: 1.24–5.18). Uric acid was shown to be the best predictor for MR (sensitivity: 55.79%; specificity: 61.35%; area under the curve: 0.616).
Conclusion
Elevated hepatic, renal, and hematological markers were associated with MR in adolescents, especially ALT, AST, and uric acid levels.
Impact
-
Elevated hepatic, renal, and hematological markers were associated with metabolic risk in adolescents, especially ALT, AST, and uric acid levels.
-
It is still controversial which marker best predicts metabolic changes in adolescents. In addition, association of Hb with metabolic risk is under-studied in this population.
-
It is important to further investigate the relationship between elevated Hb and hepatic markers, since there are key aspects not addressed yet. Our results highlight the importance of creating public health policies aimed to child and adolescent population, to prevention of metabolic disorders from an early age.
Similar content being viewed by others
Introduction
The increase in adolescent obesity rates and short- and long-term complications of obesity emphasize the need for effective prevention and treatment. Adolescence is a critical time period during which to address obesity and its comorbidities, such as metabolic risk (MR) because it is a primary time to develop autonomy, yet adolescents have not reached maturity. For this reason, early identification of diseases or metabolic changes are important to prevent obesity and MR complications, as well as liver and cardiovascular diseases.1,2 Continuous metabolic risk score (cMetS) can be a viable marker to detect MR, since it is a grouping of multiple cardiovascular risk factors that includes low levels of cardiorespiratory fitness (CRF),3 high waist circumference (WC), elevated systolic blood pressure (SBP), elevated triglycerides (TGs), low levels of high-density lipoprotein cholesterol (HDL-c), and elevated fasting glycemia.4
The clinical screening of MR by biological markers is important and allows the prevention and monitoring of health status.5 One set of biological markers that has shown importance for MR includes renal, hepatic, and hematologic markers. For example, in the United States, over the past 20 years, there has been an increase in non-alcoholic fatty liver disease (NAFLD) in almost 11% of adolescents. The presence of NAFLD was independent of body mass index, considering that multiple factors may influence this condition.6 In Chinese, NAFLD was found in 9.3% of children and adolescents, with NAFLD predominating in those who were overweight and obese.7 Thus, examining specific biomarkers related to hepatic, renal, and hematologic factors associated with MR is important.
In this sense, earlier studies in Asian and American territories found metabolic abnormalities through the Z-score of metabolic syndrome (MetS) associated with liver changes in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) enzymes.8,9 In addition, higher Z-score of MetS is related to higher uric acid concentration in adolescents.10,11 Furthermore, Chao et al.12 found associations of hepatic markers with hemoglobin (Hb), in which high concentrations of Hb were associated with cardiovascular dysfunction and an increase in ALT. However, association of Hb with MR is under-studied in adolescents and is unclear which indicator best predicts MR in this population. Therefore, in the present study, we sought to verify the association of MR with liver enzymes, uric acid, and Hb in adolescents.
Methods
The study population consisted of schoolchildren from public and private schools from Santa Cruz do Sul, Rio Grande do Sul, Brazil. A total of 1713 adolescents from 25 schools were surveyed, covering the rural and urban areas (north, south, east, west, and center) of the municipality. This study was based on a study called “School Health – Phase III” (2014/2015), with evaluation of children and adolescents aged 6–17 years, which was approved by the Research Ethics Committee of the University of Santa Cruz do Sul (UNISC) (protocol no. CAAE: 31576714.6.0000.5343; number: 714.216).
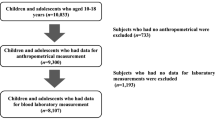
For this investigation, adolescents aged 10–17 years old were included, and parents provided informed consent and students 12 years or older signed the assent. We excluded the students diagnosed with lung diseases, those who were unable to perform the CRF test, those who were not fasting, and those unable to perform the blood collection, as described in Fig. 1.
The evaluations were carried out at the UNISC. For CRF measurement, students completed a 6-min run/walk test on outdoor athletic track, and the distance covered was obtained in meters.13 For WC evaluation, the subjects remained in the orthostatic position, with the use of a tape measure positioned horizontally, surrounding the individual at the waist level at the midpoint between the rib and the iliac crest.14 BP was determined from the measurement with the students seated student after resting for 5 min. A sphygmomanometer and stethoscope were used on the right arm with a cuff suitable for the brachial perimeter of the student, according to the parameters of the VII Brazilian Hypertension Guidelines.15
For biochemical analysis, specialized personnel performed the blood draws to obtain HDL-c, TG, ALT, AST, uric acid, and glucose. Serum samples were evaluated on automated equipment Miura 200 (I.S.E., Rome, Italy) using commercial kits (DiaSys Diagnostic Systems, Germany) and following the manufacturer’s recommendations. For hepatic enzymes, the reference values of Lira et al.16 were considered (ALT >30 U/L and AST >37 U/L). For AST/ALT ratio, <1 indicates NAFLD presence. Uric acid was determined by a photometric enzymatic method and values were classified as normal and hyperuricemia (>5.5 mg/dL).17 For the evaluation of Hb levels, a whole blood sample was used in the automated equipment Sysmex XS-800i, following the manufacturer’s instructions. The cutoff point for the definition of high Hb was according to age and sex, using reference values for adolescents divided into quartiles, being considered elevated when values >p75, for boys (9–10 years: >13.17 g/dL; 11–12 years: >13.80 g/dL; 13–14 years: 14.40 g/dL; 15–17 years: 15.00 g/dL) and girls (9–10 years: >13.30 g/dL; 11–12 years: >13.47 g/dL; 13–14 years: 13.50 g/dL; 15–17 years: 13.40 g/dL).
MR was calculated using a cMetS by the sum of the Z-score of the following parameters: WC, SBP, glucose, HDL-c, TG, and CRF, as described by Reuter et al.4 Due to the inverse relationship with MR, the HDL-c and CRF Z-score was multiplied by −1. We split the population into four age groups and constructed Z-scores by age group and sex. High values of cMetS indicate an undesirable metabolic risk profile. The cutoff points for MR presence were established at 3.40 for males and 3.61 for females (1.3 SD for boys and 1.4 SD for girls).
General characteristics, such as age, sex, ethnicity, socioeconomic level, region, and zone of residence, were evaluated by self-reported questionnaire. Tanner18 definition was used for maturational stage evaluation, considering five stages: prepubertal (stage I), initial development (stage II), continuous maturation (stages III and IV), and matured (stage V). Adolescents were instructed to fill the image corresponding to his current stage, considering genital (for boys), breast development (for girls), and pubic hair (for both). This evaluation was performed in a suitable room, individually.
Data analysis was performed in the Statistical Package for Social Sciences (SPSS), version 23.0 (IBM, Armonk, NY, USA). The categorical data were expressed in absolute and relative frequency. Analysis of variance (ANOVA) was applied to compare the biochemical variables among students with and without MR. Linear regression was performed to evaluate the association between cMetS with AST, ALT, uric acid, and Hb. Logistic regression was used to evaluate the association between the outcome (MR presence) and the categorical independent variables (AST, ALT, uric acid, Hb). Data were expressed as odds ratio (OR) and 95% confidence interval (CI). All models were adjusted for sex, age, ethnicity, and maturational stage. The cutoff points for MR, using biochemical parameters, were established through sensitivity and specificity analysis, using the receiver operator characteristic curve. For all the statistical tests used, significant differences were considered for p < 0.05.
Results
Table 1 shows the sample characteristics. Altered uric acid was present in 14.7% of the adolescents, and values suggestive for NAFLD were observed in 14.4%. The presence of MR was found in 13.6%, equally distributed between boys and girls (n = 92 for boys and n = 141 for girls; p = 0.166; data not shown).
Adolescents with MR, compared to those adolescents without MR, presented higher levels of uric acid (4.76 versus 4.22 mg/dL; p < 0.001), as well as high levels of ALT (19.05 versus 16.69 U/L; p = 0.021) and Hb (13.48 versus 13.20 g/dL; p = 0.006) (Table 2).
When analyzing the continuous variables, a positive relationship of cMetS with uric acid (β: 0.25 SD; p < 0.001), ALT (β: 0.10 SD; p < 0.001) and AST (β: 0.05 SD; p = 0.042) in adolescents was observed (Table 3).
For categorical variables, elevated concentration of all biological markers was associated with MR (Table 4). ALT (OR: 2.64; 95% CI: 1.63–4.27), AST (OR: 2.53; 95% CI: 1.24–5.18), and uric acid (OR: 2.50; 95% CI: 1.74–3.59) showed highest OR.
Cutoff points were set for MR prediction, considering five metabolic components. Uric acid was shown to be the best MR predictor (sensitivity: 55.79%; specificity: 61.35% and AUC: 0.616), establishing cutoff points at 5.64 for males and 4.45 for females. For AST, ALT, AST/ALT, and Hb cutoff points, low specificity and sensitivity were found (Table 5).
Discussion
We found a positive relationship between uric acid levels and cMetS. Adolescents with elevated uric acid levels had a higher prevalence of MR. In addition, adolescents with MR presented higher levels of uric acid when compared to those adolescents with the absence of MR. In Mexican children, a positive relationship between uric acid levels and cMetS has been observed. In China, a 10-year prospective study with adolescents showed that boys with high uric acid levels were at increased risk of hypertension and MetS.19 However, few studies have evaluated the association of uric acid with cMetS in adolescents, especially in Brazil.
The presence of MR in our study was also associated with elevated ALT levels. Adolescents with MR showed the presence of suggestive NAFLD. It is noteworthy that increased uric acid levels contribute to alterations of other metabolic parameters in obese students, especially in association with altered ALT.11 It has been shown that cMetS was a useful marker in the identification of youngsters with high levels of ALT and possible NAFLD, with excess weight being an aggravating factor in cMetS values.8 It seems that altered metabolic parameters (overweight/obesity, elevated WC, and altered SBP, as well elevated levels of uric acid and blood lipids) were strongly associated with hepatic alterations (elevated ALT), which suggests NAFLD.7
For Korean adolescents, sex-adjusted ALT values were a valid marker of possible NAFLD. Many factors may contribute to the relatively high prevalence of suspected NAFLD in schoolchildren, such as lifestyle. In the current study, the relationships of elevated levels of ALT, AST, and AST/ALT with MR factors were independent of anthropometric indices. Therefore, liver enzymes can be considered to contribute to the development of MR factor during childhood and an additional component of MetS.20 Lee and Yang9 found high sensitivity (100%) for ALT, AST, and AST/ALT ratio for predicting MR in Korean girls, with also high specificity, especially for ALT (67.2%) and AST/ALT ratio (61.1%).
When establishing cutoff points for cMetS in adolescents, uric acid presents higher sensitivity and specificity for MR presence, compared to AST, ALT, AST/ALT, and Hb. Low sensitivity and specificity were observed for boys in ALT and Hb, as well for ALT and AST/ALT for both sexes. It seems that uric acid in adolescents is a good predictor for MetS since it has been shown in the current study and also previously.21 Moreover, uric acid was a good predictor of hypertension in a 10 years follow-up study.19
Our study has some limitations that should be mentioned. First, this is a cross-sectional study, which limits the extrapolation of our results. Ideally, the associations should be evaluated in a longitudinal setting. Second, the study was conducted in the southern region of Brazil, and there are important ethnic differences when comparing to other regions of the country. Finally, we used hepatic enzymes to analyze the presence of suggestive NAFLD, and assessing hepatic enzymes with ultrasonography would provide more accurate estimates. Therefore, we emphasize that the conclusions of our study should be interpreted with caution.
The results indicate that elevated hepatic, renal, and hematological markers were associated with MR in adolescents, especially ALT, AST, and uric acid levels. Future work should focus on the development of cutoff points for AST, NAFLD, and Hb and their association with metabolic disorders, obesity, and low levels of CRF, identifying the mechanisms of those components in relation to MR. It is important to further investigate the relationship between elevated Hb and hepatic markers, since there are key aspects not addressed yet. Another important aspect would be to investigate these relationships in various ethnic groups and blood types, to better understand the metabolic disorders and the different characteristics by specific groups. Our results, however, highlight the importance of creating public health policies aimed to child and adolescent population, to prevention of metabolic disorders from an early age.
References
Steinbeck, K. S., Lister, N. B., Gow, M. L. & Baur, L. A. Treatment of adolescent obesity. Nat. Rev. Endocrinol. 14, 331–344 (2018).
Agostinis-Sobrinho, C. A. et al. Muscular fitness and metabolic and inflammatory biomarkers in adolescents: results from LabMed Physical Activity Study. Scand. J. Med. Sci. Sports 27, 1873–1880 (2017).
Andersen, L. B. et al. A new approach to define and diagnose cardiometabolic disorder in children. J. Diabetes Res. 2015, 539835 (2015).
Reuter, C. P. et al. Cutoff points for continuous metabolic risk score in adolescents from southern Brazil. Am. J. Hum. Biol. 468, e23211 (2019).
Seo, J.-Y. & Kim, J. H. Validation of surrogate markers for metabolic syndrome and cardiometabolic risk factor clustering in children and adolescents: a nationwide population-based study. PLoS ONE 12, e0186050 (2017).
Welsh, J. A., Karpen, S. & Vos, M. B. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J. Pediatr. 162, 496–500 (2013). e1.
Song, P. et al. Prevalence and correlates of suspected nonalcoholic fatty liver disease in Chinese children. Int. J. Environ. Res. Public Health 14, 465 (2017).
Fermin, C. R., Lee, A. M., Filipp, S. L., Gurka, M. J. & DeBoer, M. D. Serum Alanine aminotransferase trends and their relationship with obesity and metabolic syndrome in United States Adolescents, 1999–2014. Metab. Syndr. Relat. Disord. 15, 276–282 (2017).
Lee, K. & Yang, J. H. Which liver enzymes are better indicators of metabolic syndrome in adolescents: the Fifth Korea National Health and Nutrition Examination Survey, 2010. Metab. Syndr. Relat. Disord. 11, 229–235 (2013).
Lee, A. M., Gurka, M. J. & DeBoer, M. D. Correlation of metabolic syndrome severity with cardiovascular health markers in adolescents. Metabolism 69, 87–95 (2017).
Castillo-Durán, C., Sepúlveda, A. C., Espinoza, G. A., Rebollo, G. M. J. & Le Roy, O. C. Hyperuricaemia and metabolic syndrome in obese children and adolescents. Rev. Chil. Pediatr. 87, 18–23 (2016).
Chao, K.-C. et al. Hb and dyslipidaemia as predicting markers of serum alanine aminotransferase elevation in Chinese adolescents. Public Health Nutr. 19, 1067–1073 (2016).
PROESP. Sports Brazil Project https://www.ufrgs.br/proesp/ (2016).
Fernández, J. R., Redden, D. T., Pietrobelli, A. & Allison, D. B. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J. Pediatr. 145, 439–444 (2004).
Malachias, M. et al. 7th Brazilian Guideline of Arterial Hypertension: Chapter 10—Hypertension in children and adolescents. Arq. Bras. Cardiol. 107, 53–63 (2016).
Lira, A. R. F. & Oliveira, F. L. C. Escrivão MAMS, Colugnati FAB, Taddei JAAC. Hepatic steatosis in a school population of overweight and obese adolescents. J. Pediatr. (Rio J.) 86, 45–52 (2010).
Feig, D. I. & Johnson, R. J. Hyperuricemia in childhood primary hypertension. Hypertension 42, 247–252 (2003).
Tanner, J. Growth at Adolescence 2nd edn (Blackwell Scientific, Oxford, 1962).
Sun, H.-L., Pei, D., Lue, K.-H. & Chen, Y.-L. Uric acid levels can predict metabolic syndrome and hypertension in adolescents: a 10-year longitudinal study. PLoS ONE 10, e0143786 (2015).
Mohammadi, F. et al. Association of cardiometabolic risk factors and hepatic enzymes in a national sample of Iranian children and adolescents: the CASPIAN-III study. J. Pediatr. Gastroenterol. Nutr. 58, 463–468 (2014).
Wang, Z.-N. et al. The association between serum uric acid and metabolic syndrome among adolescents in northeast China. Int. J. Clin. Exp. Med. 8, 21122–21129 (2015).
Acknowledgements
This work was supported by the Coordination for the Improvement of Personnel in Higher Education – Brazil (CAPES) (finance code 001).
Author information
Authors and Affiliations
Contributions
S.d.S. participated in study conception and design, acquisition, and interpretation of data, as well drafted and revised the manuscript. C.P.R. contributed to study conception, acquisition, analysis and interpretation of data, and wrote and revised the manuscript. L.B.A., R.A.L., K.A.P., E.D.d.M., A.R.G., and S.I.R.F. assisted with study conception, interpretation of data, and wrote and revised the manuscript. J.D.P.R. conceptualized and designed the study, contributed to acquisition and interpretation of data, and wrote and revised the manuscript. All authors read and approved the final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
All parents or guardians signed a free and informed consent form. In addition, schoolchildren aged 12 years and above signed a consent form.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Souza, S., Reuter, C.P., Andersen, L.B. et al. Metabolic risk associated with liver enzymes, uric acid, and hemoglobin in adolescents. Pediatr Res 88, 945–949 (2020). https://doi.org/10.1038/s41390-020-0832-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0832-7