Abstract
Background
Diagnosis of bacterial meningitis (BM) is challenging in newborn infants. Presently, biomarkers of BM have limited diagnostic accuracy. Analysis of cerebrospinal fluid (CSF) metabolites may be a useful diagnostic tool in BM.
Methods
In a nested case–control study, we examined >400 metabolites in CSF of uninfected infants and infants with culture-confirmed BM using gas and liquid chromatography mass spectrometry. Preterm and full-term infants in a Level III or IV Neonatal Intensive Care Unit were prospectively enrolled when evaluated for serious bacterial infection.
Results
Over 200 CSF metabolites significantly differed in uninfected infants and infants with BM. Using machine learning, we found that as few as 6 metabolites distinguished infants with BM from uninfected infants in this pilot cohort. Further analysis demonstrated three metabolites associated with Group B Streptococcal meningitis.
Conclusions
We report the first comprehensive metabolic analysis of CSF in infants with BM. In our pilot cohort, we derived a metabolic signature that predicted the presence or absence of BM, irrespective of gestational age, postnatal age, sex, race and ethnicity, presence of neurosurgical hardware, white blood cell count in CSF, and red blood cell contamination in CSF. Metabolic analysis may aid diagnosis of BM and facilitate clinical decision-making in infants.
Impact
-
In a pilot cohort, metabolites in cerebrospinal fluid distinguished infants with bacterial meningitis from uninfected infants.
-
We report the first comprehensive metabolic analysis of cerebrospinal fluid in infants with bacterial meningitis.
-
Our findings may be used to improve diagnosis of bacterial meningitis and to offer mechanistic insights into the pathophysiology of bacterial meningitis in infants.
Similar content being viewed by others
Introduction
Bacterial meningitis (BM) is a devastating illness affecting 0.1–0.4 neonates per 1000 live births, with a higher incidence in preterm and chronically hospitalized infants.1,2 Of previously healthy infants presenting to care with fever in the first and second month of life, 1.2% and 0.4%, respectively, will be diagnosed with BM.3 Up to 10–20% of infants die, and 20–50% of survivors develop seizures, cognitive deficiencies, motor abnormalities, and hearing and visual impairments.4 Despite declines in mortality, morbidity has not improved in decades.5 The impact of neonatal BM is even greater in resource-limited settings worldwide, where mortality estimates are in excess of 50–60%, with a third of survivors exhibiting neurodevelopmental impairment.6
Prompt identification of BM is crucial to institute appropriate antimicrobial therapy and optimize outcomes.7 The gold standard for diagnosis of BM is culture of cerebrospinal fluid (CSF). However, lumbar puncture (LP) is often deferred until after the administration of antibiotics in critically ill infants making culture results unreliable. In these cases, diagnosis of BM hinges on interpretation of CSF white blood cell (WBC) counts and differential, glucose, and protein. LP in infants is technically challenging, and 30% of CSF obtained is contaminated with enough blood to make interpretation of WBC counts difficult or impossible.8 Further, neonatal BM may occur in the presence of normal CSF parameters.9 Multiple alternative diagnostic biomarkers have been proposed, including acute phase reactants and cytokines, but none has yet achieved sufficient diagnostic accuracy to be employed in clinical practice.10,11 As a result, in the absence of interpretable laboratory studies and culture of CSF, infants are often treated conservatively with long courses of intravenous antibiotics. There is a critical need for novel, accurate methods to minimize injury to the infant not only from BM but also from associated interventions, including exposure to unnecessary broad-spectrum antimicrobial agents and prolonged need for central venous catheters.
Mechanisms underlying brain injury in infants with BM are poorly understood, limiting our ability to predict clinical outcomes. Furthermore, no therapies are currently available to protect the developing brain in the setting of BM. Comprehensive metabolic analysis, or metabolomics, has recently emerged as a powerful tool for the identification of novel biomarkers for diagnosis and prediction of disease severity and outcome. Recent investigations have demonstrated the ability of plasma metabolites to predict outcomes in adults with bacterial sepsis.12,13 In addition, limited metabolic analysis of CSF was shown to have some success segregating adults with BM from those with viral meningitis or uninfected subjects.14 However, no investigations of the CSF metabolome in infants with meningitis have been performed to date.15
We hypothesized that measurement of CSF metabolites would aid in diagnosis of BM in infants. In this pilot study, we aimed to identify individual metabolites, as well as metabolic pathways, significantly altered in infants with culture-proven BM compared to uninfected infants. A deeper understanding of the metabolic milieu present in infant CSF has the potential to bolster our diagnostic approach to meningitis and also suggest novel therapies to protect the developing brain from lasting damage due to infection, thus promoting better outcomes.
Methods
Study population and setting
Subjects were recruited from the Neonatal Intensive Care Units (NICUs) at Children’s Hospital of Philadelphia, Hospital of the University of Pennsylvania, and Pennsylvania Hospital. Recruited subjects included infants aged 0–12 months who underwent LP or withdrawal of CSF from indwelling neurosurgical hardware (ventriculo-peritoneal [VP] shunt or Ommaya reservoir) for evaluation of possible meningitis. The Institutional Review Boards at the Children’s Hospital of Philadelphia and the University of Pennsylvania approved this study. Informed consent was obtained from families prior to analysis of CSF samples and data acquisition. From this larger, prospective cohort, we designed a nested case–control study to include infants with BM and uninfected infants matched by gestational age, postnatal age (PNA), sex, and race.
Study definitions
Subjects for whom CSF culture results were positive for a bacterial pathogen and whom clinicians treated with a prolonged course of antibiotics were defined as having BM. Subjects whose CSF samples demonstrated no laboratory evidence of meningitis and whose CSF culture results were negative were defined as uninfected. To ensure CSF culture results of uninfected infants were truly negative, we selected from a large pool of culture-negative samples only those not receiving antibiotics prior to LP. Owing to the critical and unstable nature of many of the infants in our cohort, 12/19 of the infants with BM received antibiotics prior to LP. Six of the 12 had CSF samples obtained within 24 h of receiving antibiotics. The remainder of the BM samples were acquired from 2 to 5 days into antibiotic therapy due to clinical situation or limited sample availability.
Study procedures and sample collection
We obtained an additional aliquot of CSF at the time of LP or shunt/reservoir tap performed owing to suspicion for serious bacterial infection. Alternatively, frozen (−20 °C), unused CSF samples were retrieved from our clinical laboratories following parental consent. CSF samples were coded and stored at −80 °C. Given the exploratory nature of this study, sample size was driven by availability of positive samples for this relatively infrequent condition.
Sample analysis
Specimens deidentified relative to patient information and disease status were shipped on dry ice to Metabolon, Inc. where metabolites were extracted and characterized by semi-quantitative liquid and gas chromatography mass spectrometry (LC/GC-MS).16,17 Volumes of specimens required to perform analysis were 50–100 μL. Compounds were identified using Metabolon’s proprietary visualization and interpretation software, in which MS peaks representing individual compounds in CSF were cross-referenced against a library of >4000 known compounds.16 A vast array of breakdown products of sugars, proteins, fats, cholesterols, and nucleic acids are detected using this technique. After MS peaks were identified, relative quantification of MS peaks was performed using area under the curve (AUC). Subsequent quality control and data curation processes were performed to ensure accurate, consistent identification, as well as to minimize system artifacts, mis-assignments, and background noise. Library matches for each compound were verified for each sample.
Data collection
We abstracted demographic, clinical, and laboratory data including risk factors for infection, culture results, and treatment details from the medical records of enrolled patients. Data were maintained in a coded electronic database.
Statistical analysis
We computed summary statistics for demographic variables, risk factors, and laboratory parameters. Continuous variables were presented as medians and interquartile ranges, while categorical variables were presented as proportions and/or percentages. p Values were calculated with Graphpad Prism 7 using Mann–Whitney U test, Chi-square, or Fisher’s exact test, as applicable.
To compare metabolomics data between desired groups, raw compound data, expressed as relative intensities, were log2-transformed. We then used a linear model with the “limma” package in R18 to determine compounds whose relative intensities significantly differed between groups. Given the risk of false positives with a large data set undergoing multiple comparisons to find differentially abundant metabolites, p values were adjusted for the false discovery rate (FDR).19 Only those compounds whose adjusted p values were <0.05 were considered to significantly differ between study groups.
The machine learning classification algorithm random forests excels at creating decision trees in the setting of many input variables.20 In our case, the decision tree of interest was whether a sample should be classified as BM or uninfected. Given the size of our cohort, random forests have the additional advantage of being less prone to overfitting than other classification algorithms. In order to generate a decision tree, random forests repeatedly “train” on a proportion of individuals in a cohort (20% in our case), then use the remaining proportion of the cohort to “test” the decision tree. While not equivalent to use of an independent cohort to test the validity of a signature of BM, this represents a form of internal validation. It is important to note that a signature of BM was not derived and tested on exactly the same subjects. We took a conservative and unbiased approach to derive a signature of BM with three times repeated tenfold cross-validation and the random forests algorithm using the R package Caret.21 Metabolites were then gradually eliminated from the prediction model with recursive feature elimination to test the performance of progressively more focused metabolic signatures of BM. The R package “random Forest”22 was used to construct a visual model of the prediction algorithm.
Results
Patient characteristics
Subjects included 19 infants with culture-confirmed BM and 19 uninfected infants with no significant differences in gestational age, birth weight, PNA, sex, and race and ethnicity (Table 1). Overall, 58% of the cohort was male, and 68% was preterm. Seven infants with BM and two uninfected infants had bloodstream infections at the time of LP. Nine infants with BM had a VP shunt or Ommaya reservoir in place, compared to only one uninfected infant with indwelling neurosurgical hardware. CSF WBC and protein were significantly elevated, while CSF glucose was significantly decreased, in infants with BM versus uninfected infants. CSF red blood cell (RBC) count was significantly higher in subjects with BM. However, the proportion of subjects in each group with substantial RBC contamination of CSF, defined as ≥500 RBC/mm,3 8 was not significantly different. Most bacterial isolates from CSF were Gram positive (68%) (Supplementary Table S1). The most common pathogens identified in CSF were Streptococcus agalactiae (n = 7) and Staphylococcus aureus (n = 5). Of note, one infant with Staphylococcus epidermidis isolated from CSF was deemed infected by the medical team and received a full course of treatment.
Derivation of a metabolic signature of BM
We first broadly identified and quantified biochemicals in CSF of infants with BM and uninfected infants. MS revealed that 210 of the 422 individual metabolites detected in CSF significantly differed (adjusted p value <0.05) between infants with and without BM (Fig. 1a and Supplementary Table S2). Of the 210 metabolites, 196 were enriched in BM, while 14 were reduced.
a Heat map in which columns represent individual compounds that significantly differ (adjusted p < 0.05) between infants with BM and uninfected infants. Columns highlighted in red represent compounds significantly increased. Columns highlighted in green represent compounds significantly decreased. Rows represent individual subjects with BM (red text) and uninfected infants (black text). Samples are organized by Euclidean clustering based on levels of all significantly different compounds. b Multi-dimensional scaling (MDS) plot of uninfected infants (black dots) and infants with BM (red dots), organized by random forests based on levels of all 422 detected metabolites. Points closer together indicate similarity in the levels of metabolites, while points farther apart indicate dissimilarity. Points in the left hemisphere of the plot are predicted to be uninfected infants. Points in the right hemisphere of the plot are predicted to have BM. c Receiver operating characteristic (ROC) curve for random forests modeling of disease status and tenfold cross-validation with three repeats. Area under the curve (AUC) = 0.94.
We next sought to understand whether detectable metabolites, in aggregate, predicted the presence or absence of BM. We took an unbiased approach using three times repeated tenfold cross-validation and random forests to generate a prediction algorithm using combinations of metabolites. Based on levels of all 422 detected metabolites, random forests segregated infants with BM from uninfected infants with an AUC of 0.94 (Fig. 1b, c).
To find the minimum number of compounds whose levels best distinguished infants with BM from uninfected infants, we performed recursive feature elimination with random forests to identify the compounds most important to prediction of BM status or uninfected status. A “signature,” consisting of nine of the most important metabolites, best segregated infants with BM and uninfected infants with an AUC of 0.97 (Fig. 2a). These nine metabolites included proline, N6-acetyllysine, taurine, cytidine, 2-hydroxyglutarate (2-HG), ornithine, thymine, glutamate, and α-ketoglutarate (Fig. 2b). Of note, we found that a signature of BM consisting only of the top six metabolites was similarly effective in classifying infants with BM and uninfected infants (AUC = 0.97, Fig. 2a).
a Each point represents an individual AUC value (y-axis) of a predictive model using a progressively larger number of metabolites (x-axis). The highest AUC of 0.97 is seen using either the top 6 or top 9 metabolites to distinguish infants with BM from uninfected infants. b Standard box and whisker plots showing relative levels of the nine compounds composing the optimal signature to distinguish uninfected infants from those with BM. Individual p values are: proline (p = 0.000003); N6-AL, N6-acetyllysine (p = 0.000008); taurine (p = 0.00001); cytidine (p = 0.000001); 2-HG, 2-hydroxygluatarate (p = 0.0002); ornithine (p = 0.0002); thymine (p = 0.000007); glutamate (p = 0.000003); and αKG, α-ketoglutarate (p = 0.000007).
Metabolic signature of BM is independent of GA, PMA, CSF WBC, CSF RBC, and the presence of neurosurgical hardware
We next assessed for metabolites that distinguished preterm and term infants with BM, but we found no metabolites that significantly differed between these two groups (Supplementary Table S3). We also tested whether metabolites in BM differed on the basis of PNA and found that no metabolites significantly differed between infants older or younger than 30 days PNA with BM (Supplementary Table S4). Further, no compounds significantly differed between infants with BM and CSF WBC counts ≤100 versus those with CSF WBC counts >100 (Supplementary Table S5). Likewise, no compounds significantly differed between infants with BM and CSF RBC counts <500 versus those with CSF RBC counts >500 (Supplementary Table S6). Finally, no compounds significantly differed between infants with BM who did or did not have indwelling neurosurgical hardware (Supplementary Table S7, Supplementary Fig. S1).
A secondary signature associated with GBS meningitis
As Streptococcus agalactiae (Group B Streptococcus (GBS)) was the most common pathogen in our cohort, we sought to determine whether CSF metabolites in GBS BM differed from CSF metabolites in BM caused by other organisms. Three compounds were significantly altered in GBS BM compared to all other BM: α-hydroxyisocaproic acid (HICA), 2-hydroxy-3-methylvaleric acid (HMVA), and sucrose (Fig. 3). Shown both with boxplots (Fig. 3a) and graphically (Fig. 3b), GBS BM was associated with relatively higher levels of HICA and HMVA and relatively lower levels of sucrose. Non-GBS BM, on the other hand, was characterized by relatively lower levels of HICA and HMVA and relatively higher levels of sucrose. As this represented only a subset of our cohort of 19 infants, we do not depict the diagnostic value of these data with receiver operating characteristic curves. Of note, no metabolites significantly differed between Gram-positive BM versus Gram-negative BM (Supplementary Table S8).
a Standard box and whisker plots showing relative levels of the three individual compounds whose levels significantly differ between infants with GBS BM and infants with non-GBS BM. Individual adjusted p values are: α-hydroxyisocaproic acid (HICA, p = 0.02), 2-hydroxy-3-methylvaleric acid (HMVA, p = 0.02), and sucrose (p = 0.03). b Three-dimensional plot shows clustering of infants with GBS BM and non-GBS BM based on levels of HICA, HMVA, and sucrose. Blue dots represent individuals with GBS BM, and red dots represent individuals with non-GBS BM.
Evidence for dysregulation of key metabolic pathways in BM
We next investigated whether metabolites distinguishing infants with BM from uninfected infants belonged to common metabolic pathways. Using all 210 metabolites that significantly differed between infants with BM and uninfected infants, we performed pathway analysis with MetaboAnalyst.23 Several metabolic pathways were significantly over-represented in BM (Fig. 4). Among the significantly over-represented pathways, alanine, aspartate, and glutamate metabolism demonstrated the highest impact score, signifying that key members of this pathway were those dysregulated in BM.
All compounds significantly altered (increased or decreased) between subjects with BM and uninfected infants were subjected to pathway analysis using MetaboAnalyst. All pathways found to be significantly over-represented among metabolites enriched in BM are shown. For each pathway, corresponding p values adjusted for the false discovery rate (FDR) are shown on the right-hand axis. The number of metabolites matched to each pathway is shown in white, embedded within bars depicting the impact score of each pathway. A higher impact score signifies that perturbations in matched compounds are likely to have a greater impact on the performance of the overall pathway.
Discussion
We combined GC/LC-MS and machine learning to comprehensively characterize metabolites in CSF of infants undergoing evaluation for BM. We derived an optimal predictive model, composed of as few as six metabolites, that distinguished infants with BM from uninfected infants in our pilot cohort. Such a focused signature may be useful in future targeted clinical investigations, especially to help guide management in infants with sterile cultures but on antibiotics prior to LP. In secondary analysis of our cohort, three CSF metabolites were associated with BM caused by GBS, a highly prevalent organism among infected neonates and infants in the first months of life.4
Surprisingly, the derived metabolic signature predicted BM equally well in preterm and term infants of varying PNAs. We found no metabolites that significantly differed between preterm and term subjects with BM, despite known differences in the development of the brain and immune system between preterm and term infants.24,25 Also surprising was our finding that no metabolites significantly differed between infants with shunt- or reservoir-associated BM and infants with BM in the absence of indwelling neurosurgical hardware. Altogether, these data support a common pathway for the host response to BM in infants and speak to the potential clinical utility of using metabolites to diagnose BM in a wide range of infants.
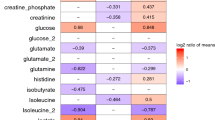
Metabolites in CSF have aided diagnosis of BM for decades, but no individual metabolite or group of metabolites is perfectly sensitive or specific. Lactate and glucose were the first metabolites to be examined in CSF of patients with meningitis.26 A meta-analysis supports that elevated CSF lactate, in particular, is associated with BM in adults and children.27 Underscoring the validity of our approach, we also found significantly elevated lactate and decreased glucose in CSF of infants with BM relative to uninfected infants in our cohort. However, neither lactate nor glucose was among the most important metabolites found to distinguish subjects with BM from uninfected subjects. These data highlight the urgent unmet need for novel tools to diagnose BM in infants, especially in light of the well-known imperfect sensitivity and specificity of traditional laboratory values used to diagnose BM in infants, including CSF glucose.9,28
The compounds that compose the optimal predictive model of BM in our cohort are proline, N6-acetyllysine, taurine, cytidine, 2-HG, ornithine, thymine, glutamate, and α-ketoglutarate. Consistent with our data, others recently found that proline was among the top five amino acids that best segregated children with confirmed tuberculous meningitis from uninfected infants.29 Our findings are also in agreement with prior investigations showing that glutamate and GABA were increased in CSF of children and adults with BM.30,31,32 These data suggest that our metabolic signature may have broader applicability to other patient populations, but this remains to be tested. The data also highlight the power of broad metabolomic analysis, as proline, glutamate, and GABA, either alone or in combination, were not sufficient to predict disease state.30,31,32,33 Only with simultaneous detection of several metabolites not reported in prior investigations could we distinguish subjects with BM from uninfected infants in our cohort.
Many of the most important compounds distinguishing subjects with BM from uninfected infants are intermediates of either glutamate, aspartate, and alanine metabolism or arginine and proline metabolism. These two pathways are intimately linked, both contributing to synthesis of glutamate, the canonical excitatory neurotransmitter in the central nervous system. Several compounds in our signature are intermediates of glutamate metabolism, including glutamate itself. Both ornithine and proline can be metabolized to glutamate through the intermediate pyrroline-5-carboxylate.34 2-HG and its precursor, α-ketoglutarate, are also precursors of glutamate. Alpha-ketoglutarate is consumed, and glutamate is produced, by branched-chain amino acid (BCAA) metabolism, in which leucine is converted to HICA and isoleucine to HMVA. Both HICA and HMVA composed the “secondary” metabolic signature of GBS BM. Altogether, our data support the notion that glutamate dysregulation may play a key role in diagnosis and pathophysiology of BM in infants.
Glutamate acts at many postsynaptic receptors, including the AMPA and N-methyl-d-aspartate (NMDA) receptors. Perturbations in glutamate signaling can be caused by bacterial-derived products and have been shown in animal models of BM to cause profound neurotoxicity, which may be reversed by NMDA receptor antagonism.35 Further, in a neonatal rat model of BM, nonspecific inhibition of excitatory amino acid receptors, including glutamate receptors, conferred protection against cortical and hippocampal injury.36 GABA and 2-HG have also been shown to signal through the NMDA receptor and may contribute to neonatal neuronal excitotoxicity.37,38 These data support the notion that direct or indirect blockade of potentially unbridled glutamate receptor activation in the setting of BM may limit brain injury in the setting of BM in infants. Basic investigations into the metabolic changes we uncovered in the setting of BM may lead to adjunctive therapies to antibiotics to minimize lifelong brain injury to infants with BM. Although many of the infants in our cohort study had multiple comorbidities related to prematurity or congenital malformations, future studies will also be directed at understanding a role for metabolites in predicting outcomes related to BM.
It is noteworthy that brains of infants with hypoxic–ischemic encephalopathy (HIE) also exhibit well-characterized elevations in glutamate levels, and many lines of evidence support a neurotoxic effect of this excess glutamate in the pathogenesis of brain injury in HIE.39,40,41 Interestingly, glutamate was found to be reduced by magnetic resonance spectroscopy in the brains of infants with HIE during the period of therapeutic hypothermia, relative to the period after rewarming.42 In the same study, levels of taurine were elevated during cooling. Taurine, another one of the top nine compounds we found that composed the optimal predictive model of BM, has been implicated as a counter-regulatory compound to glutamate and may serve as a neuroprotectant in the setting of brain injury.43 These data support that different insults to the neonatal brain may ultimately lead to similar mechanisms of injury and raise the question of whether therapeutic hypothermia also may be of benefit in BM, as it is in HIE.
This study has several strengths. Using a pilot cohort of infants, we describe a novel metabolic signature associated with BM in infants and a secondary signature associated with GBS BM. In this multi-center study, the population of infants was heterogeneous, and the organisms isolated reflect the prevalent bacterial pathogens in this age group. However, this study also has several limitations. As BM is a rare condition in infants, we recognize that our study is limited by small sample size and in need for prospective validation of metabolites enriched in BM in an independent larger cohort of infants. We also have yet to test the performance of the derived signature of BM in subjects other than infants, in a setting other than the NICU, in a cohort with more Gram-negative organisms represented, and in a body fluid other than CSF. We also acknowledge that the preterm infants in our cohort were of advanced PNA, and thus the metabolic signature derived may more closely reflect that of more mature infants. Finally, as 12/19 of the infants in the BM group received antibiotics prior to LP, it will be important in future studies to validate our findings in a cohort of infants undergoing LP at initial presentation of illness, prior to initiation of antibiotic therapy.
In addition, a large proportion of infants with BM in our study had VP shunts or Ommaya reservoirs, perhaps reflecting the population of infants admitted to our main study site, a Level IV NICU. A local, rather than likely hematogenous, spread of infection from neurosurgical hardware may explain why only a third of the infants with BM in our cohort had concurrent sepsis, as opposed to higher rates of concurrent sepsis previously reported in the neonatal literature.44,45 Nevertheless, we found the metabolic signature detected to be independent of the presence of neurosurgical hardware. The metabolic profile of such brains may well differ at baseline from those not requiring surgery, but this remains to be addressed in future studies.
Conclusions
We developed a metabolic signature in CSF that predicted BM in a heterogeneous pilot cohort of infants. Our findings have the potential to support the future development of a validated clinical tool to rapidly assay for BM with the potential to expedite diagnosis and therapy. Further analysis of metabolites in additional subjects may also provide insights into pathophysiologic mechanisms of brain injury in BM and suggest therapeutic targets to minimize morbidity and mortality in infants.
References
Okike, I. O. et al. Incidence, etiology, and outcome of bacterial meningitis in infants aged <90 days in the United kingdom and Republic of Ireland: prospective, enhanced, national population-based surveillance. Clin. Infect. Dis. 59, e150–e157 (2014).
Lawn, J. E., Cousens, S., Zupan, J. & Lancet Neonatal Survival Steering Team 4 Million neonatal deaths: when? Where? Why?. Lancet 365, 891–900 (2005).
Biondi, E. A. et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life. JAMA Netw. Open 2, e190874–12 (2019).
Mann, K. & Jackson, M. A. Meningitis. Pediatr. Rev. 29, 417–429, quiz 430 (2008).
Libster, R. et al. Long-term outcomes of Group B Streptococcal meningitis. Pediatrics 130, e8–e15 (2012).
Seale, A. C. et al. Neonatal severe bacterial infection impairment estimates in South Asia, sub-Saharan Africa, and Latin America for 2010. Pediatr. Res. 74, 73–85 (2013).
Heath, P. T. & Okike, I. O. Neonatal bacterial meningitis: an update. Paediatr. Child Health 20, 526–530 (2010).
Thomson, J. et al. Cerebrospinal fluid reference values for young infants undergoing lumbar puncture. Pediatrics 141, e20173405 (2018).
Garges, H. P. et al. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics 117, 1094–1100 (2006).
Srinivasan, L., Kilpatrick, L., Shah, S. S., Abbasi, S. & Harris, M. C. Cerebrospinal fluid cytokines in the diagnosis of bacterial meningitis in infants. Pediatr. Res. 80, 566–572 (2016).
Wei, T.-T. et al. Diagnostic accuracy of procalcitonin in bacterial meningitis versus nonbacterial meningitis: a systematic review and meta-analysis. Medicine 95, e3079 (2016).
Langley, R. J. et al. An integrated clinico-metabolomic model improves prediction of death in sepsis. Sci. Transl. Med. 5, 195ra95–195ra95 (2013).
Rogers, A. J. et al. Metabolomic derangements are associated with mortality in critically ill adult patients. PLoS ONE 9, e87538–7 (2014).
Coen, M., O’Sullivan, M., Bubb, W. A., Kuchel, P. W. & Sorrell, T. Proton nuclear magnetic resonance-based metabonomics for rapid diagnosis of meningitis and ventriculitis. Clin. Infect. Dis. 41, 1582–1590 (2005).
Gordon, S. M., Srinivasan, L. & Harris, M. C. Neonatal meningitis: overcoming challenges in diagnosis, prognosis, and treatment with omics. Front. Pediatr. 5, 139 (2017).
Evans, A. M., DeHaven, C. D., Barrett, T., Mitchell, M. & Milgram, E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal. Chem. 81, 6656–6667 (2009).
Langley, R. J. et al. Integrative ‘omic’ analysis of experimental bacteremia identifies a metabolic signature that distinguishes human sepsis from systemic inflammatory response syndromes. Am. J. Respir. Crit. Care Med. 190, 445–455 (2014).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 57, 289–300 (1995).
Breiman, L. Random forests. Mach. Learn. 45, 5–32 (2001).
Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw. 28, https://doi.org/10.18637/jss.v028.i05 (2008).
Liaw, A. & Wiener, M. Classification and regression by randomForest. R News 2, 18–22 (2002).
Chong, J. & Xia, J. MetaboAnalystR: an R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 34, 4313–4314 (2018).
Melville, J. M. & Moss, T. J. M. The immune consequences of preterm birth. Front. Neurosci. 7, 79 (2013).
Volpe, J. J., Kinney, H. C., Jensen, F. E. & Rosenberg, P. A. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci. 29, 423–440 (2011).
De Sanctis, A. G., Killian, J. A. & Garcia, T. Lactic acid of spinal fluid in meningitis: practical diagnostic and prognostic value. Am. J. Dis. Child. 46, 239–249 (1933).
Huy, N. T. et al. Cerebrospinal fluid lactate concentration to distinguish bacterial from aseptic meningitis: a systemic review and meta-analysis. Crit. Care 14, R240 (2010).
Srinivasan, L., Harris, M. C. & Shah, S. S. Lumbar puncture in the neonate: challenges in decision making and interpretation. Semin. Perinatol. 36, 445–453 (2012).
Mason, S. Cerebrospinal fluid amino acid profiling of pediatric cases with tuberculous meningitis. Front. Neurosci. 11, 534 (2017).
Buryakova, A. V. & Sytinsky, I. A. Amino acid composition of cerebrospinal fluid in acute neuroinfections in children. Arch. Neurol. 32, 28–31 (1975).
Kuroda, H. et al. Cerebrospinal fluid GABA levels in various neurological and psychiatric diseases. J. Neurol. Neurosurg. Psychiatry 45, 257–260 (1982).
Spranger, M. et al. Excess glutamate in the cerebrospinal fluid in bacterial meningitis. J. Neurol. Sci. 143, 126–131 (1996).
Mason, S., Reinecke, C. J. & Solomons, R. Cerebrospinal fluid amino acid profiling of pediatric cases with tuberculous meningitis. Front. Neurosci. 11, 248–248 (2017).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Wippel, C. et al. Bacterial cytolysin during meningitis disrupts the regulation of glutamate in the brain, leading to synaptic damage. PLoS Pathog. 9, e1003380 (2013).
Leib, S. L., Kim, Y. S., Ferriero, D. M. & Täuber, M. G. Neuroprotective effect of excitatory amino acid antagonist kynurenic acid in experimental bacterial meningitis. J. Infect. Dis. 173, 166–171 (1996).
Ben-Ari, Y., Khazipov, R., Leinekugel, X., Caillard, O. & Gaiarsa, J. L. GABAA, NMDA and AMPA receptors: a developmentally regulated ‘ménage à trois’. Trends Neurosci. 20, 523–529 (1997).
Kölker, S. et al. NMDA receptor activation and respiratory chain complex V inhibition contribute to neurodegeneration in d-2-hydroxyglutaric aciduria. Eur. J. Neurosci. 16, 21–28 (2002).
Choi, D. W. & Rothman, S. M. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu. Rev. Neurosci. 13, 171–182 (1990).
Barks, J. D. & Silverstein, F. S. Excitatory amino acids contribute to the pathogenesis of perinatal hypoxic-ischemic brain injury. Brain Pathol. 2, 235–243 (1992).
Pu, Y. et al. Increased detectability of alpha brain glutamate/glutamine in neonatal hypoxic-ischemic encephalopathy. AJNR Am. J. Neuroradiol. 21, 203–212 (2000).
Wisnowski, J. L. et al. The effects of therapeutic hypothermia on cerebral metabolism in neonates with hypoxic-ischemic encephalopathy: an in vivo 1H-MR spectroscopy study. J. Cereb. Blood Flow Metab. 36, 1075–1086 (2016).
Idrissi, El,A. & Trenkner, E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J. Neurosci. 19, 9459–9468 (1999).
Wiswell, T. E., Baumgart, S., Gannon, C. M. & Spitzer, A. R. No lumbar puncture in the evaluation for early neonatal sepsis: will meningitis be missed? Pediatrics 95, 803–806 (1995).
Stoll, B. J. et al. To tap or not to tap: high likelihood of meningitis without sepsis among very low birth weight infants. Pediatrics 113, 1181–1186 (2004).
Acknowledgements
S.M.G. received support for this work from the American Academy of Pediatrics, Children’s Hospital of Philadelphia, and NIH grant T32 AI 118684. L.S. and M.C.H. received support for this work from The Foerderer Awards at the Children’s Hospital of Philadelphia.
Author information
Authors and Affiliations
Contributions
S.M.G., L.S., and M.C.H. designed the study, acquired and analyzed data, and drafted the article. D.M.T., S.R.M., and D.D.F. analyzed data and critically revised the article. M.A.T. and A.H. acquired data. S.A. and J.C.F. acquired data and critically revised the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent statement
Informed consent was obtained from families prior to analysis of infant CSF samples and data acquisition.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gordon, S.M., Srinivasan, L., Taylor, D.M. et al. Derivation of a metabolic signature associated with bacterial meningitis in infants. Pediatr Res 88, 184–191 (2020). https://doi.org/10.1038/s41390-020-0816-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0816-7
This article is cited by
-
Application of metabolomics to neonatal meningitis
Pediatric Research (2020)