Abstract
Background
After birth, breastfeeding is the exclusive source of hormonal signaling between mother and infant. Hospitalized infants often receive donor milk when their own mother’s milk is unavailable.
Methods
The presence of insulin, leptin, cortisol, progesterone, and testosterone was examined in samples from milk bank donors and mothers of preterm infants. We further investigated the effect of Holder pasteurization (HoP) on hormone levels.
Results
Comparing nonpasteurized samples, leptin levels were nearly threefold higher in milk from mothers of preterm infants versus donated milk, and regardless of milk source, leptin levels were significantly decreased by HoP. Insulin concentrations were also decreased by HoP, and among mothers of preterm infants, obesity was associated with significantly higher content of leptin and insulin. While combined use of donor milk and HoP was associated with cortisol levels nearly threefold higher than those in nonpasteurized own mother’s milk, progesterone and testosterone content did not differ by source or pasteurization.
Conclusions
The hormonal composition of breast milk is impacted by HoP and maternal obesity. Compared to nonpasteurized maternal milk, use of pasteurized donor milk dramatically decreases the intake of leptin while increasing the intake of cortisol. Further research is necessary to define optimal breast milk processing practices.
Similar content being viewed by others
Introduction
Breastfeeding provides irreplaceable nutritional and nonnutritional factors that influence the postnatal adaptation and development of the infant. In neonatal intensive care practice, three options are available for enteral nutrition: the infant’s own mother’s milk, banked donor milk, and commercial infant formula. Breastfeeding has numerous protective effects compared to formula. It is associated with lower risks of gastroenteritis, respiratory tract infections, sudden infant death syndrome, asthma, atopic dermatitis, diabetes, obesity, and childhood leukemia.1,2 Of particular importance for the preterm infant, breastfeeding reduces the risk of necrotizing enterocolitis and enhances brain development.3,4 Previous investigators have examined the presence of bioactive factors in mature breast milk,5 but little is known about the hormonal content of milk produced by the mothers of preterm infants.
If maternal milk is not available, the recommended feeding for preterm infants is banked donor milk.6 Previous studies have shown that donor milk reduces necrotizing enterocolitis and other morbidities compared to formula.7 Microbiological safety of donor milk is usually ensured by Holder pasteurization (HoP) in human milk banks.6 Several studies have shown that HoP may decrease the levels of bioactive compounds and hormones in term breast milk.8 Maternal hormonal influence is essential for ideal fetal development, first through transplacental circulation, and after birth, breastfeeding is the infant’s only source of maternal hormones. Preterm birth prematurely terminates transplacental hormone delivery; therefore, in the absence of an alternative source, preterm infants may be exposed more briefly to maternal hormones. Thus, it is critically important to determine the extent to which maternally derived hormones can still be transferred to the preterm infant after birth through breast milk. In our study, we focused on pleiotropic hormones that are known to be present in breast milk produced for term infants, including leptin, insulin, cortisol, progesterone and testosterone.
Human leptin promotes cognitive development,9 and leptin supplementation provides neurodevelopmental protection in mice.10 Late preterm and term male infants have lower leptin levels than female infants,11 and limited investigations have shown that leptin levels may be higher in breast-fed versus formula-fed term infants.12 Preterm delivery leads to the early separation from the mother as a source of leptin and predisposes to postnatal leptin deficiency.13
Insulin, an anabolic hormone, promotes the absorption of carbohydrates and increases the blood-to-brain transport of leptin.14 Insulin plays a role in neuronal survival, dendritic arborization, and short- and long-term memory consolidation in the hippocampus.15 Insulin administration can work in concert with endogenous insulin production to modulate blood glucose levels and protect against hyper- and hypoglycemic states.14 In adults with pancreatic insufficiency, oral insulin can be absorbed enough to induce hypoglycemia,16,17 but this has not been demonstrated in preterm infants.18,19 Even with incomplete absorption, enteral insulin can exert local effects, including accelerated intestinal maturation18,19 and improved microbiome diversity.20
Steroid hormones have diverse physiological effects, but their exact roles during early development are not completely understood. Cortisol participates in numerous physiological processes; it contributes to gluconeogenesis, counteracts insulin, and acts as a diuretic.21 Studies suggest that glucocorticoids in milk may influence psychological maturation and growth.22 Progesterone is considered a neurosteroid, as its synthesis in the brain has been documented. Progesterone affects remyelination, plays a role in the development of brain and behavior and suppresses immune responses of the mother to fetal antigens and potentiates T-cell differentiation.23,24 Testosterone is a key hormone in the development of male reproductive tissues. Also, it likely has an important role in the physiology of brain functions.25 An early postnatal androgen elevation, or mini-puberty, contributes to human neurobehavioral sexual differentiation.26 In adults, orally administered progesterone and testosterone are absorbed, especially when given with fats or meals, but their bioavailability is considerably reduced by first pass hepatic metabolism.27,28,29 Given the hormones’ emerging roles in development,30,31 milk-borne progesterone and testosterone could exert biologic effects, especially in the presence of hepatic immaturity, even if they ultimately reach the systemic circulation in relatively low concentrations. The testosterone content within breast milk has not been defined, and progesterone levels in breast milk have only been described for mothers that delivered at term.32
The effects of preterm delivery and specific breast milk processing practices, such as HoP, on the non-nutritive components of breast milk have not been defined. Therefore, our aims were to investigate possible differences in insulin, leptin, cortisol, testosterone, and progesterone concentrations between preterm mother’s own milk and term donor milk and to examine whether HoP affects the levels of these developmentally important hormones in breast milk. We ultimately hypothesized that provision of maternal milk versus pasteurized donor milk significantly alters the hormonal intake of preterm infants.
Materials and methods
Study design and population
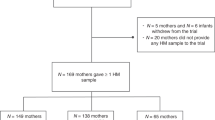
We recruited 26 mothers who gave birth to preterm infants who were hospitalized in the neonatal intensive care unit of the University of Iowa Stead Family Children’s Hospital. To avoid sampling colostrum or transitional milk, we collected samples from mothers who were able to continuously breastfeed and whose infants were 3−5 weeks old. Mothers of preterm infants pumped the milk samples in the neonatal intensive care unit to polypropylene tubes, after which the samples were stored at −20 °C until they were thawed for analysis. The donor samples (n = 31) were collected from mothers who delivered at term. The donor mothers expressed their milk manually or by pump at their homes and later donated the samples to the Mother’s Milk Bank of Iowa (Iowa City, IA, USA). The donors from the milk bank were selected from among those who had met the criteria to donate and were screened and tested based on the protocol of the Human Milk Banking Association of North America. The study was approved by the institutional review board of the University of Iowa and the Mother’s Milk Bank of Iowa. The preterm mothers and milk donors consented to the use of their milk samples for this study.
Sample preparation and analysis
The milk samples were sonicated to disrupt milk fat globules and allow proteins to enter the aqueous phase. One aliquot was pasteurized in a preheated water bath at 62.5 °C for 30 min. Then, the pasteurized and nonpasteurized aliquots were centrifuged at 15,000 × g at 4 °C. Consistent with recommendations from prior investigations, the fat layer was discarded, and the skim milk was subjected to analysis.20,33 Milliplex MAP Kit assays were performed to investigate the levels of insulin, leptin, cortisol, progesterone, and testosterone in preterm and donor breast milk. The tests were performed in duplicate. The amounts of leptin and insulin were determined by customized magnetic bead assay (HMHEMAG-34K) (END Millipore Corporation, Billerica, MA, USA). The levels of cortisol, progesterone and testosterone were measured with a separate customized array (MSHMAG-21K) (END Millipore Corporation, Billerica, MA, USA). To measure the leptin and the insulin concentrations, 25 µl of standards and quality controls and 50 µl of milk samples were added to a 96-well plate. After 18 h incubation with mixed beads at 4 °C, we washed the plates then added 50 µl detection antibody followed by 50 µl Streptavidin-Phycoerythrin to each well. In the case of cortisol, progesterone, and testosterone, sample extraction was accomplished using a Savant SpeedVac concentrator (ThermoFisher Scientific, Waltham, MA, USA), and 50 µl of milk was transferred to a 96-well plate and incubated for 18 h with 25 µl HRP conjugate and 25 µl prepared Bead solution. After incubation with secondary antibody and Streptavidin-Phycoerythrin, we added 100 µl of sheath fluid to the wells. We ran the plates on FLEXMAP 3DTM with xPONENT software (Luminex Corp, Austin, TX, USA). Based on prior publications, we anticipated the following minimum concentrations in pg/ml: 320 for insulin,34 270 for leptin,35 290 for cortisol,36 860 for progesterone,32 and no data were available for testosterone. For our chosen assays, the lower limits of quantification (LLOQ) in pg/ml were 44 for insulin, 21 for leptin, 85 for cortisol, 70 for progesterone, and 40 for testosterone. The intraassay coefficients of variation were less than 10%. The interassay coefficients were 8.5% for insulin, 9.3% for leptin, 8.2% for cortisol, 6.4% for progesterone, and 5.2% for testosterone.
Statistical analysis
For statistical analyses, we utilized GraphPad (GraphPad Software, La Jolla, CA, USA) with normal distributions confirmed by Shapiro−Wilks tests. Analyte levels below the LLOQ were replaced by values equal to the LLOQ divided by the square root of 2, as previously performed.13 Data were analyzed by ANOVA or paired Student’s t test, as appropriate, and the sample size was chosen to provide >80% power to detect moderate effect sizes (Cohen’s d = 0.6) with significance set at p < 0.05.
Results
Maternal age and infant sex did not significantly differ between the two cohorts. As expected, the milk bank donors delivered at later gestational ages and they were further removed from the time of delivery when the samples were collected (Table 1). While additional demographics were not available for the milk bank donors, 24 of the preterm mothers were Caucasian (including two Hispanic), one was African-American, and one multiracial. Mean body mass index (BMI) for the preterm cohort was 29.4 ± 8.3 kg/m2, and 10 (38%) had a BMI > 30. None of the women in the preterm cohort had diabetes mellitus, two (8%) had chronic hypertension, and four (15%) had preeclampsia or HELLP syndrome.
We detected all five hormones of interest, insulin, leptin, cortisol, progesterone, and testosterone in term donor and preterm milk. All samples had insulin levels in excess of the LLOQ. Leptin was quantifiable in all 26 maternal and 28/31 donor samples; cortisol was quantifiable in 23/26 maternal and all 31 donor samples; progesterone was quantifiable in 5/26 maternal and 11/31 donor samples; and testosterone was quantifiable in 13/26 maternal and 24/31 donor samples. While nonpasteurized preterm milk contained threefold more leptin than nonpasteurized term donor milk (p < 0.01), there were no significant differences in insulin, cortisol, progesterone, or testosterone concentrations between nonpasteurized term donor and preterm milk samples (Table 2).
HoP significantly decreased insulin and leptin levels, by 13 and 81% respectively (Table 3). Cortisol, progesterone, and testosterone levels were not significantly influenced by HoP (Table 3). During hospitalization preterm infants receiving human milk are fed their own mother’s milk or pasteurized donor milk. While the insulin, progesterone, and testosterone concentrations do not statistically differ between those two options, the preterm infant’s own mother’s milk contains 16 times as much leptin as donor milk and about one-third as much cortisol as donor milk (Table 4).
Exploring potential contributory or confounding variables, we found no effect of infant gender on milk hormone levels (data not shown). We next examined whether maternal BMI is associated with the hormonal content of preterm breast milk. Cortisol, progesterone, and testosterone were not influenced by maternal BMI. However, the milk samples of obese mothers (BMI 30 or above) had significantly increased insulin and leptin levels compared to samples from mothers without obesity, and those associations persisted after HoP (Fig. 1).
Using two-way ANOVA, breast milk insulin levels (a) and leptin levels (b), determined before (gray bars) and after (black bars) Holder pasteurization, were compared for mothers with or without obesity. *p < 0.05 or **p < 0.001 for raw milk from mothers with BMI < 30 versus BMI 30 or higher; †p < 0.05 or ††p < 0.001 for pasteurized milk from mothers with BMI < 30 versus BMI 30 or higher.
Discussion
We studied the levels of relevant hormones in preterm and term donor breast milk and assessed the effect of HoP. Utilizing the latest technology, we were able to detect the biologically active forms of the hormones and demonstrate the capacity to quantify key hormones with emerging roles in neurologic development and metabolic adaptation. Leptin, cortisol, progesterone, and testosterone may be absorbed from the gastrointestinal tract and, through the circulation, affect organ development and postnatal adaptation. The investigated hormones can also act directly in the gastrointestinal tract, influencing the composition of the microbiome, which affects development through the microbiome−gut−brain axis. Presumably, the protective factors of breast milk both influence the composition of gut microbiota and contribute to maturation of gut-associated lymphatic tissue.20,37 Furthermore, these hormones can act like growth factors, influencing the differentiation of gut epithelia.38
Leptin is a pleiotropic hormone with multifunctional effects during human development. Its early neurotrophic effect on the hypothalamus has been previously described.39 Milk leptin correlates positively with infant serum leptin, which correlates with infant BMI and weight.12 Leptin is not present in formula, but it is present in breast milk, and breast-fed infants have elevated plasma leptin levels.12 Our findings confirmed previous investigations, which have shown that leptin is highly affected by HoP.8 Likewise, the leptin levels we measured in preterm milk are very similar to those reported by both Bielicki and Eilers.35,40 In Bielicki’s study, term colostrum had higher leptin levels than preterm colostrum, but leptin levels quickly decreased in term but not preterm milk, such that term and preterm levels were similar when the final samples were collected at 6 weeks.35 In Eiler’s investigations, preterm and term milk leptin content did not differ, but they again noted a longitudinal decrease specifically among the term cohort.40 Compared to the prior investigations, the lower leptin levels we detected in mature term milk may reflect the much longer postpartum interval that preceded milk collection in our study, designed to reflect the nutritional options typically available for preterm infants.
The intact form of leptin is transported from blood into the parenchymal compartment of the brain. Several factors, such as decreased breast milk intake, male sex, preterm birth and intrauterine growth restriction increase the risk of perinatal leptin deficiency.41 Because preterm infants are often born prior to the third-trimester leptin surge, they are at the greatest risk of neonatal leptin deficiency.42 Previous studies suggest that leptin replacement influences feeding behavior and endocrine functions,43 and the prolonged hypoleptinemia observed in preterm infants may lead to increased susceptibility to diet-induced obesity.44 Even if leptin is incompletely absorbed following enteral administration, gut epithelial cell proliferation can be enhanced by exogenous leptin.45 Our findings are consistent with previous studies reporting that obese mothers have elevated insulin and leptin levels in their breast milk,40,46 and leptin levels are decreased in mature milk donated for use in preterm infants.46
Given that insulin is actively transported into human milk and is protected from degradation, it presumably plays a functional or developmental role in the infant.47 In preclinical models, Koldovský and coworkers showed that intact insulin could retain biological activity when ingested, cross from breast milk into the bloodstream, and decrease blood glucose levels.47,48 Epithelial cell insulin receptors exist in the intestine of both piglets and calves, and insulin has been suggested to play a role in influencing growth and development of the small intestine.38 Shehadeh et al.34 recruited nondiabetic mothers of preterm or term infants, and consistent with our data, they noted no effect of gestational age on breast milk insulin levels. Our results revealing increased insulin levels in the breast milk of women with increased BMI identifies a potential etiology for the development of hypoglycemia in infants of obese mothers,49 but limited bioavailability may minimize the impact oral insulin has on the blood glucose levels of most infants.18,19 Like leptin, insulin is a peptide hormone, and it was not surprising that HoP significantly decreased insulin levels. Utilizing a similar donor milk bank population and the same HoP process, Ley et al.50 also noted a significant postpasteurization reduction in insulin (46% decrease). The smaller effect size we detected (13% decrease) could reflect differences in sample processing (e.g., sonication) or method of detection (electrochemiluminescence versus microbead array).
Glucocorticoids are known to be present in breast milk and absorbed from the gastrointestinal tract.51 Previous studies have similarly shown reduced mature breast milk cortisol levels for mothers that delivered preterm versus term infants,52 although no difference has been detected within transitional milk produced within a week of term or preterm delivery.53 Glucocorticoids play a crucial role in maintaining the delicate hormonal equilibrium that controls metabolism in mammals.54 Cortisol exposure via human milk may provide protection against childhood obesity, and early glucocorticoid exposure may be a regulator of infant metabolism.55 The reduced cortisol levels we detected in fresh milk from mothers of preterm infants versus HoP milk from mothers of term infants are consistent with the results of van der Voorn et al.,52 and we show that the choice of nutrition has implications for infant cortisol intake. As a steroid, cortisol is a relatively thermostable, and previous investigations have confirmed a lack of denaturation following HoP.36
After delivery, maternal progesterone levels rapidly decrease, helping to trigger milk production.32 Preterm infants are born at a time they would typically be exposed to relatively high progesterone concentrations; therefore, postnatal progesterone intake may be particularly important to the preterm infant, and progesterone absorption from the gastrointestinal tract is known to occur.56 Unfortunately, the levels of progesterone that we measured in milk from preterm mothers were similar to the low levels measured in mature milk,32 suggesting targeting collection at an even shorter interval from delivery may be needed to increase progesterone delivery via breast milk.32
Testosterone is a neuromodulator and immunomodulator capable of reversing environmentally induced memory deficits.57 Mini-puberty, the postnatal elevation of testosterone in male infants,58 has been associated with sex-typed behavior at 14 months.59 Little is known about the presence of testosterone in breast milk, and our data are the first to demonstrate similar content in term and preterm breast milk.
Neonatal intensive care units are moving away from the use of formula for preterm infants, and our investigations comparing hormone levels in mother’s own milk to HoP donor milk are novel. Our study does have some limitations. For each mother of a preterm infant, we had a milk sample at only one point in time, which precluded analysis of postpartum changes in milk hormone levels. Given the expected postpartum decline in maternal serum progesterone, longitudinal changes in milk progesterone content could warrant further investigation. While not close to statistical significance, in part due to low levels of progesterone seen in both cohorts and variability in our preterm cohort, we did note higher progesterone levels in the milk of preterm mothers that had a shorter postpartum interval than the milk bank donors. We did not have access to data about the mother’s socioeconomic status, which might have an impact on breast milk hormone concentrations. We also were unable to obtain detailed information about milk bank donors, including BMI. Finally, although each of the hormones were detectable, both progesterone and testosterone were often present in levels below the lower limit of quantification. Removal of the fat layer prior to analysis may have reduced recovery of fat-soluble compounds, including testosterone and progesterone, but the assay we utilized has not been validated for nonaqueous samples. Future studies, potentially with spiked samples and alternative methodologies, could assess differential recovery of hormones in the fat and aqueous layers of milk.
The bioassays applied in this study are the preferred method to measure biologically active hormone levels in breast milk and assess the effects of milk preparation methods, like HoP. We have shown significant differences in the hormonal composition of preterm mother’s own milk and term donor milk and confirmed that the leptin and insulin content of a preterm mother’s own milk is increased with obesity. HoP dramatically decreases the levels of leptin, further magnifying the reduction in leptin content of donor breast milk feedings. Given the decrease in leptin concentration following HoP and the absence of leptin in commercial formulas, targeted leptin administration should be investigated for preterm infants that are not receiving their mother’s own milk.
For preterm infants, breast milk is the exclusive source of maternal hormones, and these infants are the main recipients of pasteurized donor human milk. We detected remarkable differences in the concentration of leptin and cortisol between milk produced by the mothers of preterm infants and pasteurized term donor milk. It is not established that preterm milk levels are ideal or that relatively high levels of leptin and low levels of cortisol are preferred. We have previously demonstrated profound leptin deficiency in preterm infants, with plasma levels often falling below the lowest limits of detection (41 pg/ml) at a time when leptin is believed to have an important role in evolving neuromaturation.13 Cortisol levels of preterm infants are variable, but generally exceed 10,000 pg/ml.60 Those plasma ratios are approximated by the content of donor but not preterm milk. Studies in monkeys and humans have correlated lower milk cortisol levels with increased impulsiveness and social withdrawal,61 an outcome reminiscent of the behavioral phenotype described for premature infants.62 Our findings highlight important differences in the delivery of leptin and cortisol to premature infants based on the source of milk with potential implications for breast milk processing and feeding guidelines.
References
Victora, C. G. et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet 387, 475–490 (2016).
Ortega-García, J. A. et al. Full breastfeeding and obesity in children: a prospective study from birth to 6 years. Child Obes. 14, 327–337 (2018).
Lucas, A. & Cole, T. J. Breast milk and neonatal necrotising enterocolitis. Lancet 336, 1519–1523 (1990).
Lechner, B. E. & Vohr, B. R. Neurodevelopmental outcomes of preterm infants fed human milk: a systematic review. Clin. Perinatol. 44, 69–83 (2017).
Vass, R. A. et al. Distribution of bioactive factors in human milk samples. Int. Breastfeed. J. 14, 9 (2019).
American Academy of Pediatrics Committee on Nutrition, Section on Breastfeeding, and Committee on Fetus and Newborn. Donor human milk for the high-risk infant: preparation, safety, and usage options in the United States. Pediatrics 139, e20163440 (2017).
Quigley, M., Embleton, N. D. & McGuire, W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst. Rev. 6, CD002971 (2018).
Escuder-Vieco, D., Espinosa-Martos, I., Rodríguez, J. M., Fernández, L. & Pallás-Alonso, C. R. Effect of HTST and Holder pasteurization on the concentration of immunoglobulins, growth factors, and hormones in donor human milk. Front. Immunol. 9, 2222 (2018).
Meyer, L. R., Zhu, V., Miller, A. & Roghair, R. D. Growth restriction, leptin, and the programming of adult behavior in mice. Behav. Brain Res. 275, 131–135 (2014).
Erkonen, G. E. et al. Neonatal leptin administration alters regional brain volumes and blocks neonatal growth restriction-induced behavioral and cardiovascular dysfunction in male mice. Pediatr. Res. 69, 406–412 (2011).
Ertl, T. et al. Postnatal changes of leptin levels in full-term and preterm neonates: their relation to intrauterine growth, gender and testosterone. Biol. Neonate 75, 167–176 (1999).
Savino, F. et al. Mother and infant body mass index, breast milk leptin and their serum leptin values. Nutrients 8, pii383 (2016).
Steinbrekera, B. et al. Origins of neonatal leptin deficiency in preterm infants. Pediatr. Res. 85, 1016–1023 (2019).
Kastin, A. J. & Akerstrom, V. Glucose and insulin increase the transport of leptin through the blood-brain barrier in normal mice but not in streptozotocin-diabetic mice. Neuroendocrinology 73, 237–242 (2001).
Werner, H. & LeRoith, D. Insulin and insulin-like growth factor receptors in the brain: physiological and pathological aspects. Eur. Neuropsychopharmacol. 24, 1947–1953 (2014).
Crane, C. W. & Luntz, G. R. Absorption of insulin from the human small intestine. Diabetes 17, 625–627 (1968).
Balsam, M. J., Holtzapple, P. G., Kaye, R. & Sewell, E. M. Intestinal absorption of insulin in patients with fibrocystic disease. J. Pediatr. 79, 1011–1014 (1971).
Shulman, R. J. Effect of enteral administration of insulin on intestinal development and feeding tolerance in preterm infants: a pilot study. Arch. Dis. Child Fetal Neonatal Ed. 86, F131–F133 (2002).
Shamir, R. et al. Oral insulin supplementation in paediatric short bowel disease: a pilot observational study. J. Pediatr. Gastroenterol. Nutr. 49, 108–111 (2009).
Lemas, D. J. et al. Alterations in human milk leptin and insulin are associated with early changes in the infant intestinal microbiome. Am. J. Clin. Nutr. 103, 1291–1300 (2016).
McKay, L. I. & Cidlowski, J. A. in Holland-Frei Cancer Medicine 6th edn (eds Kure, D. W. et al.) 213−214 (Decker, Hamilton, Ontario, 2003).
Hinde, K. et al. Cortisol in mother’s milk across lactation reflects maternal life history and predicts infant temperament. Behav. Ecol. 26, 269–281 (2015).
Schumacher, M. et al. Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth Horm. IGF Res. 14(Suppl A), S18–S33 (2004).
Arck, P., Hansen, P. J., Mulac Jericevic, B., Piccinni, M. P. & Szekeres-Bartho, J. Progesterone during pregnancy: endocrine-immune cross talk in mammalian species and the role of stress. Am. J. Reprod. Immunol. 58, 268–279 (2007).
Bramen, J. E. et al. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS ONE 7, e33850 (2012).
Bouman, A., Schipper, M., Heineman, M. J. & Faas, M. M. Gender difference in the non-specific and specific immune response in humans. Am. J. Reprod. Immunol. 52, 19–26 (2004).
Hargrove, J. T., Maxson, W. S. & Wentz, A. C. Absorption of oral progesterone is influenced by vehicle and particle size. Am. J. Obstet. Gynecol. 161, 948–951 (1989).
Simon, J. A. et al. The absorption of oral micronized progesterone: the effect of food dose proportionality and comparison with intramuscular progesterone. Fertil. Steril. 60, 26–33 (1993).
Amory, J. K. et al. Oral testosterone with and without concomitant inhibition of 5α-reductase by dutasteride in hypogonadal men for 28 days. J. Urol. 185, 626–632 (2011).
Hu, M. et al. Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proc. Natl. Acad. Sci. USA 112, 14348–14353 (2015).
Wagner, C. K. & Quadros-Mennella, P. Progesterone from maternal circulation binds to progestin receptors in fetal brain. Dev. Neurobiol. 77, 767–774 (2017).
Lu, M. et al. Concentrations of estrogen and progesterone in breast milk and their relationship with the mother’s diet. Food Funct. 8, 3306–3310 (2017).
Aparicio, V. A. et al. Influence of a concurrent exercise training program during pregnancy on colostrum and mature human milk inflammatory markers: findings from the GESTAFIT project. J. Hum. Lact 34, 789–798 (2018).
Shehadeh, N. et al. Insulin in human milk: postpartum changes and effect of gestational age. Arch. Dis. Child Fetal Neonatal Ed. 88, F214–F216 (2003).
Bielicki, J., Huch, R. & von Mandach, U. Time-course of leptin levels in term and preterm human milk. Eur. J. Endocrinol. 151, 271–276 (2004).
van der Voorn, B. et al. Stability of cortisol and cortisone in human breast milk during Holder pasteurization. J. Pediatr. Gastroenterol. Nutr. 65, 658–660 (2017).
Pärnänen, K. et al. Maternal gut and breast milk microbiota affect infant gut antibiotic resistome and mobile genetic elements. Nat. Commun. 9, 3891 (2018).
Georgiev, I. P., Georgieva, T. M., Pfaffl, M., Hammon, H. M. & Blum, J. W. Insulin-like growth factor and insulin receptors in intestinal mucosa of neonatal calves. J. Endocrinol. 176, 121–132 (2003).
Bouret, S. G., Draper, S. J. & Simerly, R. B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 304, 108–110 (2004).
Eilers, E. et al. Leptin determination in colostrum and early human milk from mothers of preterm and term infants. Early Hum. Dev. 87, 415–419 (2011).
Steinbrekera, B. & Roghair, R. Modeling the impact of growth and leptin deficits on the neuronal regulation of blood pressure. J. Endocrinol. 231, R47–R60 (2016).
Hellgren, G., Engström, E., Smith, L. E., Löfqvist, C. & Hellström, A. Effect of preterm birth on postnatal apolipoprotein and adipocytokine profiles. Neonatology 108, 16–22 (2015).
Matochik, J. A. et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J. Clin. Endocrinol. Metab. 90, 2851–2854 (2005).
Attig, L. et al. Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. Int. J. Obes. (Lond.) 32, 1153–1160 (2008).
Hardwick, J. C., Van Den Brink, G. R., Offerhaus, G. J., Van Deventer, S. J. & Peppelenbosch, M. P. Leptin is a growth factor for colonic epithelial cells. Gastroenterology 121, 79–90 (2001).
Chan, D. et al. Adiponectin, leptin and insulin in breast milk: associations with maternal characteristics and infant body composition in the first year of life. Int. J. Obes. (Lond.) 42, 36–43 (2018).
Koldovský, O. Hormonally active peptides in human milk. Acta Paediatr. Suppl. 402, 89–93 (1994).
Mosinger, B., Placer, Z. & Koldovsky, O. Passage of insulin through the wall of the gastro-intestinal tract of the infant rat. Nature 184, 1245–1246 (1959).
Turner, D., Monthé-Drèze, C., Cherkerzian, S., Gregory, K. & Sen, S. Maternal obesity and cesarean section delivery: additional risk factors for neonatal hypoglycemia? J. Perinatol. 39, 1057–1064 (2019).
Ley, S. H., Hanley, A. J., Stone, D. & O’Connor, D. L. Effects of pasteurization on adiponectin and insulin concentrations in donor human milk. Pediatr. Res. 70, 278–281 (2011).
Pundir, S. et al. Maternal influences on the glucocorticoid concentrations of human milk: the STEPS study. Clin. Nutr. 38, 1913–1920 (2019).
van der Voorn, B. et al. Breast-milk cortisol and cortisone concentrations follow the diurnal rhythm of maternal hypothalamus-pituitary-adrenal axis activity. J. Nutr. 146, 2174–2179 (2016).
Groër, M. W., Humenick, S. & Hill, P. D. Characterizations and psychoneuroimmunologic implications of secretory immunoglobulin A and cortisol in preterm and term breast milk. J. Perinat. Neonatal Nurs. 7, 42–51 (1994).
Rose, A. J. & Herzig, S. Metabolic control through glucocorticoid hormones: an update. Mol. Cell Endocrinol. 380, 65–78 (2013).
Hahn-Holbrook, J., Le, T. B., Chung, A., Davis, E. P. & Glynn, L. M. Cortisol in human milk predicts child BMI. Obesity 24, 2471–2474 (2016). 201.
Whitehead, M. I., Townsend, P. T., Gill, D. K., Collins, W. P. & Campbell, S. Absorption and metabolism of oral progesterone. Br. Med. J. 280, 825–827 (1980).
Khalil, R., King, M. A. & Soliman, M. R. Testosterone reverses ethanol-induced deficit in spatial reference memory in castrated rats. Pharmacology 75, 87–92 (2005).
Hines, M. et al. The early postnatal period, mini-puberty, provides a window on the role of testosterone in human neurobehavioral development. Curr. Opin. Neurobiol. 38, 69–73 (2016).
Lamminmäki, A. et al. Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Horm. Behav. 61, 611–616 (2012).
al Saedi, S., Dean, H., Dent, W. & Cronin, C. Reference ranges for serum cortisol and 17-hydroxyprogesterone levels in preterm infants. J. Pediatr. 126, 985–987 (1995).
Dettmer, A. M. et al. Cortisol in neonatal mother’s milk predicts later infant social and cognitive functioning in rhesus monkeys. Child Dev. 89, 525–538 (2018).
Johnson, S. & Marlow, N. Preterm birth and childhood psychiatric disorders. Pediatr. Res. 69, 11–18 (2011).
Acknowledgements
The authors are grateful to Dr. Veronica Peotta Jacobsen for her help in the laboratory work and to the mothers who participated in this study. We thank the support of Jean Drulis and her coworkers from Mother’s Milk Bank of Iowa. This work is supported by the Iowa-Pécs Neonatology Collaborative Research Fund and grant GINOP 2.3.2-15-2016-00021 from the Economic Development and Innovation Operational Program and by the ÚNKP-19-3-I. This work is also supported by the New National Excellence Program of the Ministry for Innovation of the Government of Hungary.
Author information
Authors and Affiliations
Contributions
R.A.V., E.F.B., T.T.C., T.E., and R.D.R. designed the study. R.A.V., M.L.S., K.J.J., and J.R.W. conducted the research. R.A.V., T.T.C., and R.D.R. analyzed the data. R.A.V. wrote the first draft of the manuscript. R.A.V., E.F.B., T.T.C., K.J.J., T.E., and R.D.R. critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vass, R.A., Bell, E.F., Colaizy, T.T. et al. Hormone levels in preterm and donor human milk before and after Holder pasteurization. Pediatr Res 88, 612–617 (2020). https://doi.org/10.1038/s41390-020-0789-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0789-6