Abstract
Background
Potentially, orally administered antibodies specific to enteric pathogens could be administered to infants to prevent diarrheal infections, particularly in developing countries where diarrhea is a major problem. However, to prevent infection, such antibodies would need to resist degradation within the gastrointestinal tract.
Methods
Palivizumab, a recombinant antibody specific to respiratory syncytial virus (RSV), was used in this study as a model for examining the digestion of neutralizing antibodies to enteric pathogens in infants. The survival of this recombinant IgG1 across digestion in 11 infants was assayed via an anti-idiotype ELISA and RSV F protein-specific ELISA. Concentrations were controlled for any dilution or concentration that occurred in the digestive system using mass spectrometry-based quantification of co-administered, orally supplemented, indigestible polyethylene glycol (PEG-28).
Results
Binding activity of Palivizumab IgG1 decreased (26–99%) across each phase of in vivo digestion as measured by both anti-idiotype and RSV F protein-specific ELISAs.
Conclusion
Antibodies generated for passive protection of the infant gastrointestinal tract from pathogens will need to be more resistant to digestion than the model antibody fed to infants in this study, or provided in higher doses to be most effective.
Impact
-
Binding activity of palivizumab IgG1 decreased (26–99%) across each phase of in vivo infant digestion as measured by both anti-idiotype and RSV F protein-specific ELISAs.
-
Palivizumab was likely degraded by proteases and changes in pH introduced in the gut.
-
Antibodies generated for passive protection of the infant gastrointestinal tract from pathogens will need to be more resistant to digestion than the model antibody fed to infants in this study, or provided in higher doses to be most effective.
-
The monoclonal antibody IgG1 tested was not stable across the infant gastrointestinal tract.
-
The observation of palivizumab reduction was unlikely due to dilution in the gastrointestinal tract.
-
The results of this work hint that provision of antibody could be effective in preventing enteric pathogen infection in infants.
-
Orally delivered recombinant antibodies will need to either be dosed at high levels to compensate for digestive losses or be engineered to better resist digestion.
-
Provision of enteric pathogen-specific recombinant antibodies to at-risk infants could provide a new and previously unexplored pathway to reducing the infection in infants.
-
The strategy of enteric recombinant antibodies deserves more investigation throughout medicine as a novel means for treatment of enteric disease targets.
Similar content being viewed by others
Introduction
Among children aged <5 years in developing countries, diarrhea is a leading cause of illness and death.1 The proportion of deaths attributed to diarrhea in this age group is approximately 25.2% in Africa, 10.0% in the Americas, 30.4% in the Eastern Mediterranean, 10.6% in Europe, 11.0% in the Western Pacific, and 31.3% in South-East Asia.2 In developing countries, the major contributors to diarrhea in children <5 years include rotavirus, calicivirus, enteropathogenic and enterotoxigenic Escherichia coli,3 Shigella spp., Vibrio cholerae, and Campylobacter jejuni.4 Breastfeeding protects infants against infectious diarrhea due to maternal antibodies and other bioactive proteins.5 We hypothesized that oral supplementation of enteric pathogen-specific recombinant antibodies could survive digestion and protect against neonatal enteric infection similarly as human milk antibodies. To prevent infection, such antibodies need to resist gastrointestinal digestion to remain biologically active. The extent to which recombinant antibodies might survive across the infant digestive tract, however, remains unknown. Palivizumab (Synagis®), a recombinant humanized monoclonal immunoglobulin G1 (IgG1) that binds to the respiratory syncytial virus (RSV) fusion protein and stops viral replication, is the only Food and Drug Administration (FDA)-approved monoclonal antibody for preventing infections in high-risk infants (albeit for intramuscular injection).6 To examine recombinant antibody digestion across the infant gastrointestinal tract, palivizumab was selected as a model protein. Though palivizumab is respiratory pathogen-specific, our goal is to translate our findings to diarrheal pathogen-specific recombinant antibodies. Palivizumab added to human milk fed to infants was used in this study as a model for neutralizing antibodies against enteric pathogens. Infants who were already admitted to the Doernbecher Children’s Hospital Neonatal Intensive Care Unit that already had an indwelling nasogastric or orogastric feeding tube were recruited to participate in this study. The development of new oral antibody therapy to treat or prevent enteric infections in newborns is critical because infants are more at risk to infectious diseases than children due to their immature immune system.
This study aimed to determine the binding activity of palivizumab IgG1 across infant in vivo digestion. As it is unclear the extent to which in vivo digestion can be replicated by in vitro and ex vivo digestion, we examined the survival of this recombinant antibody in parallel studies via in vitro7 and ex vivo.8
Materials and methods
Sample collection
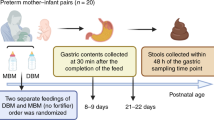
This study was approved by the Institutional Review Board of Oregon Health & Sciences University (OHSU IRB #18274). The use of palivizumab in neonates was given a may-proceed clearance by the United States FDA (IND #140999). Infant inclusion criteria were the following: infants already admitted to Doernbecher Children’s Hospital Neonatal Intensive Care Unit, greater than 34 weeks corrected gestational age, an indwelling nasogastric or orogastric feeding tube, and tolerating full enteral feeding volumes. Infants were excluded from the study if they had anatomic or functional gastrointestinal disorders that would impact protein digestion, were medically unstable, were non-viable, or had disorders that would be expected to affect normal digestion. After obtaining informed consent from parents, a tube was placed into the distal duodenum or proximal jejunum through a nare, with the position of the tube port confirmed by abdominal X-ray. Only those infants with the side port of the post-pyloric tube at or beyond the third part of the duodenum to the proximal jejunum were eligible to have small intestinal fluid samples obtained. Gastric, intestinal, and stool samples were collected from 11 infant pairs (infant demographics are shown in Table 1 and Supplementary Table S1). Feeds of either human milk or formula were prepared with palivizumab and PEG-28 (see concentrations in Supplementary Table S1). PEG is not absorbed nor digested within the gastrointestinal tract and is generally recognized as safe by the FDA.9,10 Feeds were delivered via naso- or orogastric tubes over 30 min or less. From the same feed, a volume (0.5–2 mL) of each infant’s gastric contents was collected 1 h after the initiation of feeding in a 3-mL syringe from the feeding tube via suction, placed into sterile vials on ice, and then frozen. Samples were collected from the nasojejunal/duodenal tube via gravity flow as the digesta passed the collection tube port into sterile vials on ice, and then frozen. Stool samples were collected from diapers into sterile containers. Gastric, intestinal, and stool samples were stored at −80 °C. All samples were transported to Oregon State University on dry ice and stored at −80 °C upon arrival. Demographic information for each participant was extracted from the medical record and securely stored electronically.
Anti-idiotype palivizumab enzyme-linked immunosorbent assay (ELISA)
Binding activity of palivizumab in all samples was determined via ELISA as described by Demers-Mathieu et al.11 with some modifications. Samples were diluted with 10% of human AB serum (Corning, Manassas, VA, USA) in PBST at 200× and 400× for milk, gastric, and intestinal samples, and 1× and 2× for stool samples. The concentration of palivizumab measured by ELISA from each feed was denoted as 100% stability, and the percentage stability from gastric, intestinal, and stool was calculated based on the measured concentration in each sample compared with the concentration in the feed.
RSV F protein-specific IgG ELISA
The RSV F protein-specific IgG concentrations of samples were quantified by ELISA as described by Lueangsakulthai et al.8 with modifications. Samples were diluted with 1% bovine serum albumin (Thermo Scientific, Waltham, MA, USA) in PBST at 400× and 800× for milk, gastric, and intestinal samples, and 1× and 2× for stool samples. The concentration of palivizumab measured by ELISA from each feed was denoted as 100% stability and percent stability within each sample was calculated as described previously.
Extraction of PEG-28
All milk, gastric, intestinal, and stool samples were centrifuged at 4000 × g, 4 °C for 30 min. The infranatant including proteins and PEG-28 were collected into new Eppendorf tubes. Centrifuging and collecting the infranatant were performed in duplicate with the same procedure. Thirty microliters of commercial PEG-28 (100 ng/μL; Sigma Aldrich, St. Louis, MO, USA) were prepared as a stock solution of the PEG standard. All samples and the standard underwent identical experimental procedures prior to mass spectroscopy (MS) analysis. One hundred and fifty microliters of chilled ethanol were mixed with 30 μL samples and the standard (stored at −20 °C for 1 h). Samples were placed in a −20 °C freezer for 1 h and centrifuged at 12,000 × g, 4 °C for 20 min. Supernatants containing PEG-28 from samples and standards were collected into new tubes and dried using a SpeedVac (Genevac, Gardiner, NY, USA). To avoid clogging the nano-LC column with intact proteins remaining in samples, trypsin digestion for both samples and the standard was performed. Pellets containing proteins were mixed with 100 μL of 50 mM NH4HCO3 (Thermo Scientific), denatured by reduction with 2 μL of 550 mM dithiothreitol (incubated at 50 °C for 50 min; Promega, Madison, WI, USA) and alkylation with 4 μL of 450 mM iodoacetamide (incubated at room temperature for 1 h in the dark; Sigma Aldrich). Denatured proteins in the pellets were enzymatically digested using trypsin (Promega) with shaking at 300 r.p.m., 37 °C for overnight and combined with each supernatant. Samples and the standard were purified using C18-based solid-phase extraction. The cartridges were preconditioned with 5 mL of ultrapure water, 5 mL of 80% acetonitrile (ACN) with 0.1% trifluoroacetic acid and 5 mL of ultrapure water. Samples were loaded on the cartridges, washed with 5 mL of ultrapure water, and eluted with 5 mL of 80% ACN with 0.1% trifluoroacetic acid. All samples and standards were dried using a SpeedVac. All dried samples (30 μL) and the standard (100 μL) were reconstituted with 3% ACN with 0.1% formic acid. To make a standard curve, the standard was diluted with the reconstitution solvent at 20,000×, 10,000×, 4000×, 2000×, 1000×, 400×, 200×, 100×, 40×, and 20×.
Quantitation of PEG-28 using parallel reaction monitoring (PRM)-based LC/MS
PEG-28 concentration in standards and samples was determined as described by Kim et al.12 with some modifications. For PRM, PEG-28 at m/z 417.933 (z = 3) was selected as a precursor compound in MS/MS analysis. Collision-induced dissociation was used with 35% of normalized collision energy to fragment PEG-28. MS/MS spectra were acquired in the positive-ionization mode over a m/z range of 300–1500 by the Orbitrap at a resolution of 60,000.
MS data processing
Skyline (MacCoss Lab, University of Washington, USA) was used to make a standard curve and calculate the concentrations of PEG-28 in the standards and samples. MS/MS raw data sets generated by PRM were imported into the Skyline and peak areas were extracted using the transition list including the single charge state of total 10 fragment ions: m/z 327.2013 (DP7), 371.2276 (DP8), 459.2800 (DP10), 503.3062 (DP11), 547.3324 (DP12), 591.3586 (DP13), 635.3848 (DP14), 679.4111 (DP15), 723.4373 (DP16), and 767.4635 (DP17). These fragment ions were extracted from raw files using the following parameters in the Skyline: m/z 0.01 in Method match tolerance; DIA in Acquisition method; Orbitrap in a Product mass analyzer; and All ions in Isolation scheme 60,000 at m/z 200 in Resolving power.
Statistical analyses
One-way ANOVA followed by Tukey’s multiple comparison test (GraphPad Prism software, version 8.2.1, La Jolla, CA, USA) was applied to compare the samples from in vivo gastrointestinal digestion. Differences were designated significant at p < 0.05. Pearson’s correlation coefficient test was applied to compare the percentage stability correlation of each sample. The correlation was designated as strong correlation if the coefficient value (r) was between 0.50 and 1, medium correlation if the coefficient value (r) was between 0.30 and 0.49, and low correlation if the coefficient value (r) was below 0.29.
Results
Survival of palivizumab IgG1 across in vivo gastrointestinal digestion
The average percentage stability of palivizumab IgG1 was detected by the anti-idiotype and RSV F protein-specific ELISA in milk, gastric, intestinal, and stool samples of 11 infants. The average percentage stability of palivizumab detected by anti-idiotype ELISA across in vivo gastric, intestinal, and stool samples decreased 34.2%, 72.2%, and 100%, respectively (Fig. 1a). The average percentage stability of palivizumab detected by RSV F protein-specific ELISA across in vivo gastric, intestinal, and stool samples decreased 25.8%, 70.9%, and 100%, respectively (Fig. 1b). The percentage stability of palivizumab across in vivo infant gastrointestinal digestion as measured by the anti-idiotype and RSV F protein-specific ELISA were strongly correlated (gastric contents r = 0.65, intestinal contents r = 0.91, and stool r = 0.97). The results demonstrated that palivizumab was degraded across each phase of in vivo gastrointestinal digestion (p < 0.05).
Average percentage stability of palivizumab IgG1 across infant gastrointestinal digestion from a anti-idiotype and b RSV F protein-specific ELISA. Letters a, b, c and d show statistically significant differences between groups (p < 0.05) using one-way ANOVA followed by Tukey’s multiple comparison tests to compare the percentage stability of palivizumab in each sample type compared with the palivizumab concentration in the feed sample for each infant.
PEG-28 concentration quantified via MS
The concentration of PEG-28 in milk, gastric, intestinal, and stool samples of 11 infants was quantified via MS. The average concentration of PEG-28 detected by MS across in vivo feed, gastric, intestinal, and stool samples was 0.16, 0.18, 0.18, and 0.29 ng/μL, respectively (Fig. 2). There were no significant differences in PEG-28 concentration across sample type, indicating no consistent sample type dilution or concentration effect. This finding suggests that the observation of palivizumab reduction was unlikely due to dilution in the gastrointestinal tract.
Survival of palivizumab normalized with PEG-28 across in vivo gastrointestinal digestion
The average percentage stability of palivizumab IgG1 by the anti-idiotype and RSV F protein-specific ELISA normalized with indigestible PEG-28 in milk, gastric, intestinal, and stool samples of 11 infants. The average percentage stability of palivizumab detected by anti-idiotype ELISA across in vivo gastric, intestinal, and stool samples when normalized to PEG-28 concentration decreased 40.5%, 62.2%, and 100%, respectively (Fig. 3a). The average percentage stability of palivizumab detected by RSV F protein-specific ELISA across in vivo gastric, intestinal, and stool samples when normalized to PEG-28 decreased 26.5%, 61.6%, and 100%, respectively (Fig. 3b). The percentage stability of palivizumab across in vivo infant gastrointestinal digestion as measured by the anti-idiotype and RSV F protein-specific ELISA normalized with PEG-28 were strongly correlated (gastric contents r = 0.80, intestinal contents r = 0.93, and stool r = 0.97). The results of the average percentage stability of palivizumab IgG1 detected by both anti-idiotype and RSV F protein-specific ELISA normalized for dilution using the concentration of co-fed PEG-28 demonstrate that palivizumab was degraded after in vivo gastrointestinal digestion.
Average percentage stability of palivizumab IgG1 across infant gastrointestinal digestion from a anti-idiotype and b RSV F protein-specific ELISA normalized with PEG-28 quantified via MS. Letters a, b, c and d show statistically significant differences between groups (p < 0.05) using one-way ANOVA followed by Tukey’s multiple comparison tests to compare the percentage stability of palivizumab in each sample type with the palivizumab concentration in the feed sample for each infant.
Discussion
Enteric infection, whether acute or chronic, is a predominant cause of death in infants in developing countries.13 Additionally, chronic diarrhea is a major cause of growth stunting,14,15 which is strongly correlated with intellectual and psychosocial impairment in survivors.16,17 In the newborn, maternal transplacental-transferred IgG and human milk-derived secretory IgA and IgG enhance infant host defense to prevent infectious disease.13 It has long been recognized that breastfeeding reduces the risk of infectious diseases in infants and young children,18,19,20,21 including diarrheal disease.22,23,24 Given that not all infants receive human milk to enhance their protection against diarrhea, provision of recombinant antibodies as a supplement could help prevent enteric infections. To reduce the incidence, or prevent enteric infection, exogenous antibodies would need to survive across the gastrointestinal tract with remaining biological activity to target and inactivate pathogens.
To date, monoclonal antibodies have not been tested for use in the gastrointestinal tract, nor has there been any report of the fate of any monoclonal in the gastrointestinal tract, including retention of structural integrity or activity. We report the first study of the fate of a recombinant IgG1 in across in vivo digestion as a first step towards investigation of the possibility of enterally dosed monoclonals targeted at enteric pathogens as one method to reduce infant and childhood diarrheal disease.
Palivizumab is a recombinant monoclonal IgG1 that binds to the A antigenic site of RSV F protein, and is FDA approved for use in neonates by intramuscular route to confer passive immunity to RSV in high-risk infants and children.25 We selected palivizumab to examine the extent to which recombinant IgG1 can survive within the infant gastrointestinal tract because of its approved use in infants, its standard monoclonal structure (humanized IgG1), and an assayable in vitro activity. Infants were fed with human milk or formula containing palivizumab and indigestible PEG-28 (used as a marker of dilution/concentration to normalize palivizumab concentration). The use of an in vivo study design is advantageous over an in vitro digestion design, as it better reflects the reality of the extent to which monoclonal antibodies survive within the infant gut.
The percentage stability of palivizumab decreased across each phase of gastrointestinal digestion (feed to gastric to intestinal to stool samples) based on both the anti-idiotype and anti-RSV F protein ELISAs. This finding was consistent whether or not palivizumab concentrations were normalized for dilution/concentration based on PEG-28. Palivizumab was likely degraded by proteases and changes in pH introduced in the gut. Protease-catalyzed primary structure cleavages and pH-induced secondary and tertiary structural changes would be expected to destroy antibody activity if extensive. The observed digestion of palivizumab conformed to that reported in previous studies showing that various monoclonal IgG1 are cleaved in the hinge region after in vitro incubation with pepsin at 37 °C, pH 4.0 (refs. 26,27,28,29,30,31) and trypsin at pH 8.0,29 releasing bivalent fragments F(ab′)2. As detection of palivizumab via the ELISA methods used herein requires the antibody to possess both an intact Fc and Fab segment, cleavage at the hinge region by pepsin and trypsin could cause the observed palivizumab reduction across the infant gastrointestinal tract.
In addition, these findings agree with our previous work indicating that ex vivo gastric and intestinal digestion in infant gastric and intestinal fluids resulted in the degradation of palivizumab IgG1, as well as recombinant palivizumab reformatted as IgA and secretory IgA (sIgA). However, results from the gastric and intestinal ex vivo digestion experiment8 indicated that the naturally occurring RSV F protein-specific IgG and sIgA/IgA were stable. Likewise, a gastric and intestinal in vitro digestion experiment7 demonstrated that the naturally occurring RSV F protein-specific IgG was stable across digestion. These observations provide evidence that the isotype structure or the post-translational modification could influence the survival of a monoclonal antibody across gastrointestinal digestion. Protein engineering to make the structure of recombinant antibodies more similar to naturally occurring antibodies could enhance their resistance to digestion and increase their functionality within the gut. For example, available production techniques that create human-like glycosylation could enhance antibody stability.32,33 This may inform design of future enteral recombinant antibodies.
This study is exploratory and limited by small numbers, as well as the use of a model monoclonal antibody, rather than a recombinant antibody targeted against a diarrheal pathogen. However, a study that does not have these restrictions is not currently feasible. In addition, a comparison of how different factors such as feeds (human milk vs. infant formula) and gestational age affect the extent of palivizumab digestion could not be conducted due to a limited sample size.
Conclusion
Provision of enteric pathogen-specific recombinant antibodies to at-risk infants could provide a new and previously unexplored pathway to reducing diarrhea in the regions where the burden of acute and chronic diarrhea remains significant. Such a strategy, if effective, would be in large part dependent on the production of antibodies at a much lower cost than current methodologies, and would need to fit within larger, multipronged approaches to acute and chronic childhood diarrhea prevention. In addition, the results of this work suggest that to be effective in preventing enteric pathogen infection in infants, orally delivered recombinant antibodies will need to either be dosed at high levels to compensate for digestive losses or be engineered to better resist digestion. Indeed, Sah et al.34 revealed that palivizumab at higher concentration (1000 µg/mL) was more stable in terms of RSV neutralization capacity in gastric and intestinal digestion than that dosed at a lower concentration (60 µg/mL). As infants are exposed to a variety of enteric pathogens, an effective strategy to reduce pathogenic diarrhea would likely require multiple recombinant monoclonal antibodies (one monoclonal antibody per pathogen). Purified human milk antibodies could also be used to protect against enteric pathogens as they are more resistant to digestion and their polyreactive properties allow them to neutralize various pathogenic microbes. The strategy of enteric recombinant antibodies deserves more investigation throughout medicine as a novel means for treatment of enteric disease targets or microbiome “sculpting”; the example of C. difficile disease is a prime example, offering the advantages of specificity and avoidance of non-specific and increasingly ineffective antibiotic-based strategies.
References
Kotloff, K. L. et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin. Infect. Dis. 55, S232–S245 (2012).
Walker, C. L. F., Aryee, M. J., Boschi-Pinto, C. & Black, R. E. Estimating diarrhea mortality among young children in low and middle income countries. PLoS ONE 7, e29151 (2012).
Lanata, C. F. et al. Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS ONE 8, e72788 (2013).
Walker, R. I. Considerations for development of whole cell bacterial vaccines to prevent diarrheal diseases in children in developing countries. Vaccine 23, 3369–3385 (2005).
Hanson, L. A. Breastfeeding provides passive and likely long-lasting active immunity. Ann. Allergy Asthma Immunol. 81, 523–533 (1998).
Wu, H., Pfarr, D. S., Losonsky, G. A. & Kiener, P. A. Immunoprophylaxis of RSV infection: advancing from RSV-IGIV to palivizumab and motavizumab. Curr. Top. Microbiol. Immunol. 317, 103–123 (2008).
Lueangsakulthai, J., Sah, B. N. P., Scottoline, B. P. & Dallas, D. C. Survival of recombinant monoclonal and naturally-occurring human milk immunoglobulins A and G specific to respiratory syncytial virus F protein across simulated human infant gastrointestinal digestion. J. Funct. Foods 73, 104115 (2020).
Lueangsakulthai, J., Sah, B. N. P., Scottoline, B. P. & Dallas, D. C. Survival of recombinant monoclonal antibodies (IgG, IgA and sIgA) versus naturally-occurring antibodies (IgG and sIgA/IgA) in an ex vivo infant digestion model. Nutrients. 12 (2020).
Pelegri-O’Day, E. M., Lin, E.-W. & Maynard, H. D. Therapeutic protein-polymer conjugates: advancing beyond PEGylation. J. Am. Chem. Soc. 136, 14323–14332 (2014).
Brady, C. E. 3rd et al. Urinary excretion of polyethylene glycol 3350 and sulfate after gut lavage with a polyethylene glycol electrolyte lavage solution. Gastroenterology 90, 1914–1918 (1986).
Demers-Mathieu, V., Lueangsakulthai, J., Qu, Y., Scottoline, B. P. & Dallas, D. C. Binding and neutralizing capacity of respiratory syncytial virus (RSV)-specific recombinant IgG against RSV in human milk, gastric and intestinal fluids from infants. Nutrients 12 (2020).
Kim, B. J., Lueangsakulthai, J., Sah, B. N. P., Scottoline, B. & Dallas, D. C. Quantitative analysis of antibody survival across the infant digestive tract using mass spectrometry with parallel reaction monitoring. Foods 759 (2020).
Kollmann, T. R., Kampmann, B., Mazmanian, S. K., Marchant, A. & Levy, O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity 46, 350–363 (2017).
Arnold, B. F. et al. Cluster-randomised controlled trials of individual and combined water, sanitation, hygiene and nutritional interventions in rural Bangladesh and Kenya: the WASH Benefits study design and rationale. BMJ Open 3, e003476 (2013).
Humphrey, J. H. et al. The sanitation hygiene infant nutrition efficacy (SHINE) trial: rationale, design, and methods. Clin. Infect. Dis. 61, S685–S702 (2015).
Dewey, K. G. & Begum, K. Long-term consequences of stunting in early life. Matern. Child Nutr. 7, 5–18 (2011).
Casale, D., Desmond, C. & Richter, L. The association between stunting and psychosocial development among preschool children: a study using the South African Birth to Twenty cohort data. Child Care Health Dev. 40, 900–910 (2014).
American Academy of Pediatrics. Breastfeeding and maternal and infant health outcomes in developed countries. AAP Gd. Rounds 18, 15–16 (2007).
Victoria, C. G. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet 355, 451–455 (2000).
Labbok, M. H., Clark, D. & Goldman, A. S. Breastfeeding: maintaining an irreplaceable immunological resource. Nat. Rev. Immunol. 4, 565–572 (2004).
Lanari, M. et al. Maternal milk protects infants against bronchiolitis during the first year of life. Results from an Italian cohort of newborns. Early Hum. Dev. 89, S51–S57 (2013).
Boone, K. M., Geraghty, S. R. & Keim, S. A. Feeding at the breast and expressed milk feeding: associations with otitis media and diarrhea in infants. J. Pediatr. 174, 118–125 (2016).
Ruiz-Palacios, G. M. et al. Protection of breast-fed infants against Campylobacter diarrhea by antibodies in human milk. J. Pediatr. 116, 707–713 (1990).
Glass, R. I. & Stoll, B. J. The protective effect of human milk against diarrhea: a review of studies from Bangladesh. Acta Paediatr. 78, 131–136 (1989).
Robbie, G. J., Zhao, L., Mondick, J., Losonskym, G. & Roskos, L. K. Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children. Antimicrob. Agents Chemother. 56, 4927–4936 (2012).
Lamoyi, E. & Nisonoff, A. Preparation of F(ab′)2 fragments from mouse IgG of various subclasses. J. Immunol. Methods 56, 235–243 (1983).
Svasti, J. & Milstein, C. The disulphide bridges of a mouse immunoglobulin G1 protein. Biochem. J. 126, 837–850 (1972).
Mason, D. W. & Williams, A. F. The kinetics of antibody binding to membrane antigens in solution and at the cell surface. Biochem. J. 187, 1–20 (1980).
Parham, P. On the fragmentation of monoclonal IgG1, IgG2a, and IgG2b from BALB/c mice. J. Immunol. 131, 2895–2902 (1983).
Mariant, M., Camagna, M., Tarditi, L. & Seccamani, E. A new enzymatic method to obtain high-yield F(ab)2 suitable for clinical use from mouse IgGl. Mol. Immunol. 28, 69–77 (1991).
Rao, P. E. & Kroon, D. J. Orthoclone OKT3. Chemical mechanisms and functional effects of degradation of a therapeutic monoclonal antibody. Pharm. Biotechnol. 5, 135–158 (1993).
Hiatt, A. et al. Glycan variants of a respiratory syncytial virus antibody with enhanced effector function and in vivo efficacy. Proc. Natl Acad. Sci. USA 111, 5992–5997 (2014).
Jefferis, R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 8, 226–234 (2009).
Sah, B. N. P. et al. Partial degradation of recombinant antibody functional activity during infant gastrointestinal digestion: implications for oral antibody supplementation. Front. Nutr. 7 (2020).
Acknowledgements
The authors thank Matt Paluska, Anahi Y. Torres-Pimentel, Jooyoung Yeo, Siana Liti, and Kimberly R. Lane for their assistance with the ELISA experiments. This study was supported by the Bill & Melinda Gates Foundation (OPP1183649). The authors also acknowledge support from the OSU Mass Spectrometry Center in part through instrumentation grant NIH # 1S10OD020111-01 and the Oregon State University Research Office.
Author information
Authors and Affiliations
Contributions
J.L., B.J.K., V.D.-M., B.N.P.S., Y.W., A.O., M.A., A.O'C., B.P.S., and D.C.D conceptualized the study. J.L. and B.J.K designed the experiments, performed data acquisition, and analyzed data. J.L. drafted the article. D.C.D supervised the experiments. All authors revised, adapted, and approved final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Patient consent was required for this basic science study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lueangsakulthai, J., Kim, B.J., Demers-Mathieu, V. et al. Effect of digestion on stability of palivizumab IgG1 in the infant gastrointestinal tract. Pediatr Res 90, 335–340 (2021). https://doi.org/10.1038/s41390-020-01271-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-01271-y