Abstract
Background
Appearance of sleep cycling has been associated with good outcome in term and preterm infants, but the normal time of its appearance has not been determined. The objectives of this study were, to correlate the time of sleep cycling appearance and the length of quiet sleep in neonates with different degrees of mild perinatal stress.
Methods
Three groups of term infants recorded with aEEG after birth were studied: infants delivered by planned cesarean section (group 1), infants with mild perinatal stress (group 2) and infants with mild neonatal encephalopathy (group 3). Groups were correlated with the appearance and length of quiet sleep.
Results
In all, 132 infants were assessed. Quiet sleep appearance differed significantly between groups (p < 0.001). All infants in group 1 developed quiet sleep before the age of 6 h compared to 81% in group 2 and 52% in group 3 (p < 0.001). No differences in the quiet sleep length was found between groups. Belonging to group 3 (p < 0.001) and 1-min Apgar score (p = 0.002) significantly predicted a delay in appearance of the first quiet sleep period. Cesarean delivery significantly predicted an earlier appearance of quiet sleep (p < 0.001).
Conclusions
Appearance of quiet sleep after birth but not its length may be delayed in case of mild perinatal stress.
Similar content being viewed by others
Introduction
Sleep and wake cycles are set by the circadian rhythm center located in the supra chiasmatic nucleus of the ventral hypothalamus and regulated mainly by melatonin.1,2 In neonates, sleep cycles are between two main stages: quiet sleep (QS) and active sleep (AS).3,4 QS is characterized by a regular breathing pattern, lack of eye movements and low variance in heart rate with a concurrent EEG consisting of relatively high amplitude slow wave alternating with lower amplitudes classically termed tracé alternant.5 AS is characterized by an irregular breathing pattern, slow or fast eye movements, face muscle activity and high variance in the heart rate with a concurrent continuous EEG.5 EEG activity during wakefulness and AS is similar. First evidence of sleep cycling emerges at the 20th week of gestation and can be demonstrated in prematurely born infants as soon as the 24th week of gestation.6,7,8 As sleep cycling is an inherent part of cerebral function and maturation, it is hypothesized that its presence is a sign of cerebral well-being.9,10 This hypothesis is supported by studies in both high-risk term10,11,12,13,14,15 and preterm16,17 neonates that found a significant correlation between the timing of the appearance of cycling and better long-term outcome. Nevertheless, though sleep cycling has been detected before and after birth, the timing of its appearance (or reappearance) after delivery in healthy newborns is not known. This information is important as a reference data for past and future studies.
We hypothesized that sleep cycling emerges soon after birth in healthy term neonates and is delayed in the event of neonatal stress around delivery even if not followed by neonatal encephalopathy.
The primary objective of this study was to correlate the time of appearance of electrophysiological QS (as an indicator of sleep cycling) of term neonates to different degrees of perinatal stress that is not followed by moderate or severe hypoxic ischemic encephalopathy (HIE).
Secondary objectives were to time the length of the first QS period and to explore its correlation with the degree of perinatal stress.
Methods
This was a cross-sectional study including both prospectively and retrospectively recruited infants. The study was approved by the local ethics committee in Soroka Medical Center and one of the parents of the prospectively recruited infants signed an informed consent form. Need for consent was waived for the retrospective group by the local ethics committee.
Infants were considered for inclusion in the study if they were born between 37 and 42 completed weeks of gestation, had an aEEG recording that was initiated no later than 4 h of age and did not suffer from HIE grade 2 or 3 according to Sarnat and Sarnat criteria.18 Infants were excluded from the study in case of metabolic or genetic conditions, culture positive early neonatal sepsis, abnormal cerebral imaging, convulsions, therapeutic hypothermia, treatment with sedatives and antiepileptic drugs. Also, in the prospectively recruited group, infants who developed hypoglycemia, respiratory distress or any other condition that necessitated special care were excluded from the study.
According to these criteria, three groups were formed as follows.
Group 1
Prospective group
Healthy term newborns delivered by planned elective cesarean section (CS) due to maternal conditions not influencing the fetus (e.g. repeat CS). Parents of these infants were approached several days before surgery during the preoperative consultation for possible recruitment. Those who agreed to participate in the study were approached again before the planned delivery for final recruitment.
Infants whose parents were approached in the preoperative clinic but were born before their scheduled surgery due to any reason were excluded from the study.
Groups 2 and 3
Retrospective groups
Infants for these groups were recruited from our local aEEG database where all recordings of infants are kept since December 1995. Files of all infants that had an aEEG recording until January 2014 due to suspected perinatal asphyxia at birth defined as an acute perinatal event (e.g. cord prolapse, placental abruption) or suspected fetal distress (by the fetal heart rate monitor) together with low Apgar score (less than 5 at 5 min) or low cord pH (equal or less than 7.1) or prolonged resuscitation at birth were screened for the study according to the above inclusion and exclusion criteria. Using the infants’ clinical data, two groups were created as follows.
Group 2
Infants that did not develop any sign of HIE.
Group 3
Infants who developed signs of HIE grade 1 (and were discharged home with that diagnosis).
aEEG monitoring
aEEG monitoring was performed with either the analog Cerebral Function Monitor (CFM) 5022 (Lectromed, Hertfordshire UK) until mid-2004 (20 infants from groups 2 and 3) or with the digital CFM 6000 (Olympic Medicals/Natus, Pleasanton, CA) thereafter (81 infants from all groups). Both devices use the same algorithm developed by Maynard and Prior,19 where one EEG channel is recorded from two parietal electrodes. Briefly, the EEG signal is amplified, filtered below 2 Hz and above 15 Hz, rectified, smoothened and compressed to 6 cm per hour and displayed on semi logarithmic scale.
aEEG monitoring in group 1 was initiated as soon as clinically possible following delivery but no later than 3 h of age in a dark and quiet place in the neonatal department. In group 1 monitoring lasted until the infant was transferred for care with his mother (not earlier than 6 h of age). In groups 2 and 3, the clinical routine is to monitor infants for at least 24 h, and if clinically possible to extend monitoring until the first quiet sleep is apparent (if it did not appear earlier).
Sleep cycling on aEEG
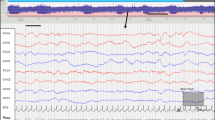
The beginning of the cycle was defined as the appearance of the first wave of QS in the aEEG recording (i.e. a clear widening of the aEEG band, mostly at its minimal amplitude, usually reaching a lower border of around 5 microvolts, creating a sinosuidal pattern of the tracing and lasting more than 5 min during a cycle of at least 20 min)6. The end of the QS period was defined when the aEEG amplitude returned to the pre-QS amplitude (Fig. 1). These time points were marked separately by two of the authors (R.A. and E.S.) who were blinded to the clinical status of the infants. In case of disagreement a consensus was reached. Time to quiet sleep was calculated from the time of birth. This timepoint was chosen as the pattern of widening of the aEEG is a prominent pattern that is readily discernable.
Recording from an infant born following a planned cesarean section. Upper pane: 3 h of amplitude integrated EEG recording: Alternating strip between a wide band (5−25 micV) (corresponding to the quiet sleep period in the neonate) in the beginning and the end and a narrower band (10–25 micV) (corresponding to the active sleep or awake period in the neonate). Lower pane: 15 s of the original EEG extracted from the period of the dashed line between the two arrowheads. Discontinuous EEG of the Tracé-Alternant type, typical of the neonatal quiet sleep period
Data collection
The following clinical data were collected: time of birth, gestational age at birth, gender, ethnicity, peri-delivery problems, presentation at birth, mode of delivery, anesthetic mode and medications prior to CS (prospective group), first umbilical cord pH, first arterial pH (retrospective groups), first and fifth minute APGAR scores, birth weight and head circumference.
Data analysis
Data were analyzed using the Statistical Package for Social Sciences (IBM SPSS version 21). In all analyses, alpha level was set to 0.05 (two sided). Pearson’s Chi square, one-way ANOVA and Kruskal−Wallis tests were used as appropriate. Dunn test was used for post-hoc analysis. Kaplan−Meier estimator was used to demonstrate the proportion of infants along time that developed quiet sleep aEEG activity. Stepwise forward regression was used for multivariable analysis (enter criteria 0.15, stay criteria 0.1) using the following variables: group, cesarean delivery, dichotomized 1 and 5 min Apgar scores (Apgar 1 less than 5 and Apgar 5 less than 7), cord pH, sex, ethnicity, birth weight, gestational age and head circumference. The dependent variable, “time to quiet sleep”, was transformed to its natural logarithm to attain normal distribution. We selected the model with the best AICc (Akaike information criterion corrected).
Results
Between May 2013 and January 2015, of 174 parents that were approached for recruitment to the prospective cohort, 65 consented to participate in the study. Eleven parents withdrew consent and 23 were excluded due to various reasons (delivery before planned CS (10), vaginal delivery (5), perinatal complications (2), pregnancy complications (1), gestational age less than 37 weeks (2), not available (1), simultaneous study births (1), aEEG artifacts (1)). Eventually, 31 infants had an aEEG recording suitable for analysis.
For the retrospective groups, 169 suitable infants were found in our database, 68 were excluded due to: antiepileptic drugs or sedatives (15), hypothermia treatment (8), ventilation (3), other significant conditions (pulmonary hypertension, cardiac defects, etc.) (32) and aEEG artifacts (10). Overall, 101 infants’ recording were suitable for analysis, 68 for group 2 and 33 for group 3.
Comparison between groups of basic clinical and demographic characteristics
The main differences between the three groups relate to their gestational age (p < 0.001), ethnicity (group 1 consisted of Jewish infants only, p < 0.001), umbilical cord pH and Apgar score at 1 and 5 min (group 1 vs. group 2 and vs. group 3, p < 0.001) (Table 1).
Comparison between groups of aEEG characteristics
aEEG recording was initiated in infants from group 1 significantly earlier than in group 2 or 3 by about half an hour (p < 0.001), time to quiet sleep appearance from birth differed significantly between the three groups (p < 0.001) with a post-hoc analysis pointing at significant differences between groups 1 and 3 (p < 0.001), between groups 2 and 3 (p = 0.008) and between groups 1 and 2 (p = 0.022) (Table 2 and Fig. 2). All infants in group 1 developed quiet sleep in the first 6 h after birth compared to 81% in group 2 and 52% in group 3 (differences between groups were significant at p level < 0.001). The length infants remained in quiet sleep was slightly less than half an hour without significant differences between groups.
Difference of the time to emergence of quiet sleep was also found to be significantly different between the three groups using the Kaplan−Meier survival analysis (p < 0.001) (Fig. 2).
Sleep cycling predictors
Grouping, cesarean delivery, 1 and 5 min Apgar scores were included in the final multivariable analysis (Table 3). Belonging to group 3 significantly predicted a delay in the appearance of the first quiet sleep period compared to group 2 (p < 0.001) and to a lesser extent compared to group 1 (p = 0.054). Also, a low 1-min Apgar score (and to a lesser extent, 5-min Apgar score) significantly predicted a delay in quiet sleep appearance (p = 0.002). Cesarean delivery significantly predicted an earlier appearance of quiet sleep (p < 0.001). Time to quiet sleep appearance was not found to be significantly different between groups 1 and 2 in this model.
Discussion
We demonstrated in this study that the time of emergence of QS (as a marker of sleep cycling) is correlated to different indicators of stress around delivery (umbilical cord pH and Apgar score) as well as to signs of mild encephalopathy after birth. We also showed that in newborns minimally stressed around delivery the first QS period appears prior to 6 h after birth. Lastly, the first QS period was timed to a mean of 25 ± 11.1 min irrespective of perinatal stress.
The few studies that assessed the emergence of sleep cycling in term neonates explored its correlation with outcome in infants with HIE. While Ter Horst et al.20 demonstrated a better outcome in a cohort of 30 infants if normal voltage patterns but not cyclicity evolved up to the age of 48 h, Osredkar et al.,14 in a larger group of 171 infants with HIE, demonstrated that good/poor neurodevelopmental outcome was correctly predicted by the emergence of cycling before/after 36 h of age in 82% of their cohort. In their study the median time to emergence of cycling was determined for HIE grade 1 (7 h), grade 2 (33 h) and grade 3 (62 h). Their findings are similar to our finding of a median of 5 h 36 min to the emergence of cycling in HIE grade 1. With the advent of therapeutic hypothermia, sleep cycling emergence was found to be delayed in comparison to the prehypothermia era, but its mere appearance was correlated to better neurodevelopmental outcome.21 Only one study, that we are aware of, assessed sleep architecture in healthy term newborns,22 using conventional 1–2 h EEG recording. In this study ten infants recorded up to 6 h of age demonstrated sleep cycling which is consistent with our findings that all healthy term infants (group 1) initiated cycling before 6 h of age.
Length of quiet sleep has been measured with different modalities.
Mean QS time of 25.6 min was measured in 20 term born infants in their first week of life using a capacitance-type sensor mattress pad23 by Whitney and Thoman and a median of 30 min was measured with aEEG by Osredkar et al.,14 similar to our results for the three groups. Nevertheless, QS duration in our study was more variable, probably reflecting the circumstance that this was the first QS period after sleep cycling was disturbed by the birth process and its accompanying stress.
From our findings and others who described a delay of sleep cycling onset after perinatal stress, it seems plausible that stress induces secretion of various substances (hormones, excitatory neurotransmitters and cytokines) that delay the onset of quiet sleep through increased arousal in the supra chiasmatic nucleus of the ventral hypothalamus.1 This could also explain the association between CS and earlier appearance of quiet sleep.
The main limitation of the study is related to the tool we used to assess sleep states, as we were unable to include behavioral observations, recordings of heart rate and respiratory rate for assessing sleep states, but, as the widening of aEEG trace during quiet sleep is a clearly apparent pattern, we believe that this is an appropriate proxy for the initiation of quiet sleep. Another limitation relates to the mixture of data derived from prospective and retrospective cohorts that were monitored during different periods. Also, the time of initiation of recording was slightly different between the two cohorts, but as cycling appeared earlier in group 1 as compared to the two other groups, we don’t think that this issue affected the results. Though it can be argued that we may have missed the first quiet sleep cycle in some cases, we believe that this was unlikely as infants in all groups were cared for by the medical personnel from birth until electrodes were applied; moreover, in many infant’s recordings in groups 2 and 3, the initial recording was depressed. Within each of the groups the following weaknesses should also be pointed out.
In the prospective cohort, we had an ethnical homogenously selected group born via CS. A selection bias may have skewed the results but trying to approach families from Arab-Bedouin origin was not successful due to language barriers when explaining a study on healthy babies. Also, approaching families expecting normal vaginal delivery was problematic as it was not possible to know when the delivery will take place. The predominance of CS in this group could also explain its effect as a significant predictor of quiet sleep. Lastly, we considered the appearance of the first quiet sleep period as a proxy for the establishment of sleep−wake cycling, but, especially in this specific group, due to obvious reason, we could not increase the monitoring time as the infants had to be sent to their mothers. It should be stated though, that in the retrospective groups, the first quiet sleep period was followed by subsequent cycles as well as in the prospective group when monitoring time was longer.
The inherent weakness in groups 2 and 3 are their retrospective nature, and though a protocol exists in the unit requiring assessment of cerebral activity with aEEG as soon as possible after delivery in high-risk infants, not all infants fitting the protocol were recorded soon after delivery as some of them were not thought to have gone through severe asphyxia at birth. Thus, less severely affected newborns may not have been recorded which might have shown that there is no difference in the appearance of QS between groups 1 and 2.
Another difference between the groups is the environment of the monitoring. Thus, QS in groups 2 and 3 may have been delayed by the relatively noisiest NICU environment they were cared in. This difference may explain the small nonsignificant difference between the time it took groups 1 and 2 to reach QS. Also, though groups 2 and 3 were cared for in the same environment, they were clinically different, and group 3 may have been disturbed more frequently for evaluation and this difference may account in part for the variance in quiet sleep appearance.
Lastly, it might be argued that infants with analog recordings should not be included in the study. But as far as we know, the main problem with the analog device was an artifact of a raised baseline of the tracing which does not influence the cycling pattern.
The strength of this study lies in the well-defined groups that set apart three different stress levels undergone by neonates and the relatively large size of the cohorts with a very early initiation of aEEG recording.
Conclusions
Quiet sleep in healthy newborn babies with minimal delivery-related stress appears before 6 h of age, suggesting minimal disruption of sleep cycling after delivery. Also, it seems from our study that mild perinatal stress may cause a delay in the timing of sleep cycling appearance after birth but not in its length. Our study closes a gap in data on sleep cycling phenomenon after birth.
References
Han, K. S., Kim, L. & Shim, I. Stress and sleep disorder. Exp. Neurobiol. 21, 141–150 (2012).
Seron-Ferre, M., Torres-Farfan, C., Forcelledo, M. L. & Valenzuela, G. J. The development of circadian rhythms in the fetus and neonate. Semin. Perinatol. 25, 363–370 (2001).
Thoman, E. B. Sleeping and waking states in infants: a functional perspective. Neurosci. Biobehav. Rev. 14, 93–107 (1990).
Mirmiran, M. The function of fetal/neonatal rapid eye movement sleep. Behavioural Brain Res. 69, 13–22 (1995).
Shany, E. & Berger, I. Neonatal electroencephalography: review of a practical approach. J. Child Neurol. 26, 341–355 (2011).
Hellström-Westas, L., Rosén, I., de Vries, L. S. & Greisen, G. Amplitude-integrated EEG classification and interpretation in preterm and term infants. NeoReviews 7, e76–e87 (2006).
Palmu, K., Kirjavainen, T., Stjerna, S., Salokivi, T. & Vanhatalo, S. Sleep wake cycling in early preterm infants: comparison of polysomnographic recordings with a novel EEG-based index. Clin. Neurophysiol. 124, 1807–1814 (2013).
Scher, M. S., Johnson, M. W. & Holditch-Davis, D. Cyclicity of neonatal sleep behaviors at 25 to 30 weeks’ postconceptional age. Pediatr. Res. 57, 879–882 (2005).
Verma, U. L., Archbald, F., Tejani, N. A. & Handwerker, S. M. Cerebral function monitor in the neonate. I: normal patterns. Dev. Med. Child Neurol. 26, 154–161 (1984).
Thornberg, E. & Thiringer, K. Normal pattern of the cerebral function monitor trace in term and preterm neonates. Acta Paediatrica Scand. 79, 20–25 (1990).
Scher, M. S., Steppe, D. A., Beggarly, M. E., Salerno, D. G. & Banks, D. L. Neonatal EEG-sleep disruption mimicking hypoxic-ischemic encephalopathy after intrapartum asphyxia. Sleep. Med. 3, 411–415 (2002).
Lombroso, C. T. & Matsumiya, Y. Stability in waking-sleep states in neonates as a predictor of long-term neurologic outcome. Pediatrics 76, 52–63 (1985).
van Rooij, L. G. et al. Recovery of amplitude integrated electroencephalographic background patterns within 24 h of perinatal asphyxia. Arch. Dis. Child Fetal Neonatal Ed. 90, F245–F251 (2005).
Osredkar, D. et al. Sleep-wake cycling on amplitude-integrated electroencephalography in term newborns with hypoxic-ischemic encephalopathy. Pediatrics 115, 327–332 (2005).
Scher, M. S., Steppe, D. A. & Banks, D. L. Prediction of lower developmental performances of healthy neonates by neonatal EEG-sleep measures. Pediatr. Neurol. 14, 137–144 (1996).
Hellstrom-Westas, L., Rosen, I. & Svenningsen, N. W. Cerebral function monitoring during the first week of life in extremely small low birthweight (ESLBW) infants. Neuropediatrics 22, 27–32 (1991).
Klebermass, K. et al. Amplitude-integrated EEG pattern predicts further outcome in preterm infants. Pediatr. Res. 70, 102–108 (2011).
Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 33, 696–705 (1976).
Maynard, D., Prior, P. F. & Scott, D. F. Device for continuous monitoring of cerebral activity in resuscitated patients. Br. Med. J. 4, 545–546 (1969).
Ter Horst, H. J. et al. Prognostic significance of amplitude-integrated EEG during the first 72h after birth in severely asphyxiated neonates. Pediatr. Res. 55, 1026–1033 (2004).
Takenouchi, T. et al. Delayed onset of sleep-wake cycling with favorable outcome in hypothermic-treated neonates with encephalopathy. J. Pediatr. 159, 232–237 (2011).
Korotchikova, I. et al. EEG in the healthy term newborn within 12h of birth. Clin. Neurophysiol. 120, 1046–1053 (2009).
Whitney, M. P. & Thoman, E. B. Sleep in premature and fullterm infants from 24-hour home recordings. Infant Behav. Dev. 17, 223–234 (1994).
Acknowledgements
The authors would like to thank Adi Leizarovitz for her active help in recruitment and the families of infants who accepted to participate in this study. This study was part of the MD degree requirements of Dr. Abramsky in Ben Gurion University of the Negev.
Author information
Authors and Affiliations
Contributions
R.A. conceptualized and designed the study, collected and analyzed the data, wrote the first draft of the manuscript and approved its final version. M.S. designed the study, analyzed data, critically reviewed the manuscript and approved its final version. V.N. designed the study, analyzed data, critically reviewed the manuscript and approved its final version. E.S. conceptualized and designed the study, collected and analyzed the data, critically reviewed the manuscript and approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abramsky, R., Stavsky, M., Novack, V. et al. Appearance of sleep cycling after birth in term neonates: an electro-physiologic study. Pediatr Res 87, 711–715 (2020). https://doi.org/10.1038/s41390-019-0560-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0560-z