Abstract
Background
The role of antiviral prophylaxis to prevent Epstein–Barr virus (EBV) viremia or posttransplant lymphoproliferative disorder in pediatric solid organ transplant recipients is controversial. We examined whether valganciclovir (VAL) prophylaxis for cytomegalovirus infection was associated with EBV viremia following transplantation in EBV-naive children.
Methods
A single-center, retrospective study was conducted of EBV-naive pediatric heart and renal transplant recipients with an EBV-positive donor from January 1996 to April 2017. VAL was tested for association with EBV viremia-free survival in the first 6 months posttransplantation when immunosuppressant exposure is the highest. Survival models evaluated VAL duration, with adjustment for other baseline confounders.
Results
Among the cohort (n = 44), 3 (6.8%) were heart transplants, 25 (56.8%) received VAL, and 22 (50%) developed EBV viremia in the first-year posttransplantation. Mean time-to-viremia was 143 vs. 90 days for the VAL and no-VAL groups, respectively (p = 0.008), in the first 6 months. Only two patients developed viremia while on VAL. Each additional day of VAL was associated with 1.4% increase in viremia-free survival (p < 0.001). Multivariable modeling of VAL with other baseline risk factors did not identify other independent risk factors.
Conclusion
VAL is independently associated with delayed onset of EBV viremia, with prolongation of delay with each additional day of antiviral prophylaxis.
Similar content being viewed by others
Introduction
Epstein–Barr virus (EBV) is a human herpesvirus that infects >90% of adults worldwide.1 Best known for causing infectious mononucleosis, EBV infection is also associated with several malignancies, including posttransplant lymphoproliferative disorder (PTLD).2 Nearly 85% of cases of PTLD in children is the result of EBV-induced proliferation of latently infected B cells.3 EBV-specific immunity, in particular, cytotoxic T lymphocyte responses, is critical for controlling EBV-infected B cells and preventing unchecked lymphoproliferation and frank malignancy.4,5 Since many pediatric organ transplant recipients have not yet been exposed to EBV, they are at high risk of PTLD when infection is acquired from the allograft, particularly while heavily immunosuppressed.6 EBV viremia, defined as detection of viral DNA in blood by PCR, reflects active replicating infection and viral load correlates with the risk of PTLD.7,8,9,10 As such, EBV viral load monitoring in blood is routinely performed posttransplantation to screen for the development of PTLD.
Currently available antiviral drugs, such as the nucleoside analog valganciclovir (VAL),11 which is routinely used posttransplantation to prevent cytomegalovirus (CMV) infection, inhibit lytic EBV replication. However, because antiviral treatment has no effect on the proliferation of latently infected B cells or lymphomas,12 the standard of care when EBV viremia is detected is to reduce immunosuppression to permit natural immunity to develop. This approach increases the risk for allograft rejection13,14and even in the setting of subclinical EBV infection may be associated with chronic allograft injury and loss of transplant function in pediatric kidney transplant recipients.15
The role of antiviral prophylaxis for EBV at-risk transplant recipients remains controversial.16,17,18,19,20,21,22,23,24 During initial EBV infection, lytic replication may play a role in establishment and dissemination of latent B cell infection and thus be susceptible to antiviral suppression. This rationale has been proposed to justify the use of nucleoside analogs to prevent or delay EBV infection early posttransplantation, particularly when the first 6 months immunosuppression is the highest.12 However, existing guidelines do not recommend antiviral prophylaxis for EBV infection,5,25 even in those patients at highest risk for PTLD (EBV donor seropositive, recipient seronegative; “D+/R−”). In this study, we sought to determine first whether VAL prophylaxis delays onset donor-derived primary EBV infection in D+/R− pediatric population in the 6 months post-solid organ transplantation and then whether there was a reduction of overall infection rate in the first year.
Methods
Patients and study design
We performed a single-center, retrospective study of EBV-naive pediatric heart and kidney transplant recipients from January 1996 to April 2017. Patients included in the study were aged <20 years at the time of transplant and EBV D+/R− serological profile and did or did not receive VAL in the first 6 months posttransplant for CMV prophylaxis. Patients with incomplete monitoring data (e.g., missing polymerase chain reaction (PCR) monitoring data; patients with viral PCR test frequency fewer than 6 or 9 tests in the first 6 or 12 months, respectively), human immunodeficiency virus-1 infection, or stem cell transplantation were excluded. The University of British Columbia Clinical Research Ethics Board reviewed and approved the study protocol and waived the requirement for patient consent.
All data collection occurred between August and November 2017. Data analysis took place from November 2017 to January 2018. Patients were identified based on inclusion criteria using the province-wide registry “Patient Records and Outcome Management Information System” that contains laboratory and drug treatment information of all transplant recipients in British Columbia, Canada. Paper and electronic records additionally reviewed to capture baseline characteristics: age, sex, weight, height, organ transplanted, donor source (living vs. deceased), induction immunosuppression, and tacrolimus exposure during the first 3 months posttransplantation. Calcineurin inhibitor 12-h trough levels were collected at monthly intervals for all of the recipients.
Clinical decisions regarding immunosuppressive treatment and antiviral prophylaxis were made individually by the treating physician. Standard care during this period was to provide VAL for 3–6 months if either the donor or recipient were CMV seropositive but not to administer VAL for EBV prophylaxis when it was not indicated for CMV (CMV D−/R−). VAL dose is calculated according to pediatric recommendation as follows: dose (mg) = 7 × BSA × CrCl, where BSA is body surface area (m2) and CrCl is the creatinine clearance estimated by the Schwartz equation (ml/min/1.73 m2). Antiviral prophylaxis data included start and end dates, dosage, and route of administration. All recipients were followed for at least 12 months posttransplant.
Immunosuppression
Induction immunosuppressive therapy included anti-thymocyte globulin (ATG), interleukin-2 receptor antagonist (basiliximab) with intravenous methylprednisolone. Maintenance immunosuppressive agents included tacrolimus or cyclosporine, mycophenolate mofetil, and prednisone. Target trough levels for cyclosporine were 200–250 ng/ml (days 1–30), 150–200 ng/ml (days 31–60), 100–150 ng/ml (days 61–90), and 80–100 ng/ml thereafter. Target trough levels for tacrolimus were 10–12 ng/ml (days 1–30), 8–10 ng/ml (days 31–60), 6–8 ng/ml (days 61–180), and 4–6 ng/ml thereafter.
Virologic testing
Donor and recipient EBV serostatus were determined at the time of transplantation by VCA IgG positivity (Siemens Centaur) at the BC Centers for Disease Control. EBV viral load was determined by quantitative EBV PCR of plasma samples. DNA was extracted from serum samples via QIAsymphony (Qiagen) using the DSP virus/pathogen mini-kit, with an elution volume of 70 μl. Quantitative PCR for EBV DNA was performed in the BC Children’s Hospital Clinical Laboratory on the ABI 7500 RT platform (Life Technologies) using a previously validated laboratory-developed Taqman(R) assay targeting the EBV BNRF1 gene, which has a limit of detection of 93 IU/ml. Monitoring for EBV viremia was routinely obtained at least monthly intervals or more frequently at the discretion of the transplant physician to identify incident viremia. In the setting of incident viremia, monitoring frequency was typically increased, and depending on clinical symptoms or viral load, viremia was treated by reducing immunosuppression intensity. This treatment was individualized by patient and did not follow a set protocol.
Definitions
The onset of EBV viremia was defined as the first detection of EBV by plasma PCR and was assumed to be donor derived in the first-year posttransplantation. Patients were considered to have received VAL if a minimum of 1 month of prophylaxis was started within the first 2 weeks after transplantation. The days on prophylaxis (DOP) with VAL were counted from the time of transplant to the last day of treatment without EBV viremia or until the onset of EBV viremia. VAL started after onset of viremia was excluded. If VAL was re-started for treatment of CMV viremia or disease after the end of the prophylaxis, it was not included in DOP. The time to viremia (TTV) was counted as the number of days from transplant until the onset of EBV viremia.
Statistical analysis
Statistical analysis was performed using R 3.4.1.26 For the descriptive analysis, continuous variables were presented as median and interquartile range (IQR) or mean ± standard deviation as appropriate; categorical variables were reported as counts and percentages. Time-to-event survival analysis was used to compare the effect of VAL on time to onset of EBV viremia compared with no VAL use. In the first 6 months posttransplant, this was further tested by Cox modeling of DOP with VAL as a predictor of TTV after testing to confirm that the data met the proportional hazards assumption. Given the small number of events, the impact of other baseline characteristics was tested by adding them individually to a baseline model that included only DOP. The effect of VAL on viremia in the first-year posttransplant was also assessed, using an accelerated failure time (AFT) model for time to event, since the proportional hazards assumption appeared to fail in the latter half of the year, implying that the hazard ratio was not constant over time. The log-logistic survival distribution was selected, based on best fit using the Akaike Information Criterion. This fitted model was used to test viremia-free survival and the association with DOP. Regression results are reported in the form of exponentiated regression coefficients, i.e., hazard ratios for the Cox model and acceleration factors in the AFT model, with p values <0.05 deemed to be statistically significant.
Results
Cohort characteristics
A total of 57 patients were identified to be EBV D+/R−, of which 13 were excluded owing to lack of viral load monitoring data to identify onset of EBV viremia. The 13 excluded patients were transplanted between 1996 and 2003, in an era were routine monitoring was not employed and VAL was not available. The baseline demographics of the remaining 44 patients included are summarized in Table 1. This cohort was aged 11.8 ± 6.25 years, 18 (41%) were male, and 3 (6.8%) had heart transplants. The majority of the kidney transplants (59%) were from living donors. More than half (68%) of the patients were at risk of CMV disease posttransplant (CMV D+ and/or R+). All patients received induction immunosuppression with high-dose intravenous methylprednisolone and the majority (91%) also received basiliximab, while the rest received ATG. All patients received maintenance immunosuppressive therapy comprised of prednisone, a calcineurin inhibitor (tacrolimus 98%; cyclosporine 2%), and either sirolimus (2%) or mycophenolate mofetil (98%).
A total of 25 (56.8%) patients received VAL posttransplant, one of whom was low risk for CMV (D−/R−). The median duration of VAL exposure was 172 days (IQR 100, 182) posttransplant and the average oral dosage was 5.3 ± 1.8 mg multiplied by the estimated glomerular filtration rate (eGFR) and BSA daily. Overall, 18 (41%) patients developed EBV viremia in the first 6 months posttransplantation and an additional 4 patients developed EBV viremia between 6 and 12 months posttransplant (50% of all patients). Twelve of the 18 patients (67%) who presented in the first 6 months had not received VAL. Of the two patients receiving VAL developed EBV viremia while on treatment and eight other VAL-treated patients developed viremia after VAL discontinuation. The average dose given to the two patients who developed EBV viremia while on VAL was similar to those who did not develop viremia (5.1 vs. 5.5 mg × eGFR × BSA daily, respectively, p = 0.63).
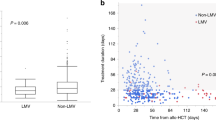
VAL is associated with delayed time to viremia in the first 6 months posttransplantation
Kaplan–Meier survival analysis (Fig. 1) for the first 6 months posttransplant demonstrated a delayed onset of viremia in the VAL group, compared with the group who did not receive VAL (p = 0.008). Compared with patients who received VAL, the mean TTV was 143 (±73) vs. 90 (±38) days for patients not receiving prophylaxis. Modeling the DOP with VAL identified a hazard ratio of 0.92 for each additional week of VAL prophylaxis (p = 0.003). Sequential testing of covariates in a bivariate analysis utilizing DOP as the base model of risk did not identify additional risk factors for TTV, and no other baseline predictors appeared to influence the treatment effect from DOP (Table 2). Among viremic patients, the median duration of viremia was similar in patients who did or did not receive VAL, 168 (IQR 47, 367) vs.135 (IQR 26, 282) days, respectively.
VAL is associated with reduced risk of viremia risk in the first-year posttransplantation
The DOP was modeled for its effect on TTV in the first-year posttransplantation, using the log-logistic AFT model. The peak hazard of viremia was 71 days in the no-VAL group, with a 2.6-fold increase in TTV relative to the VAL group (p < 0.001). Viremia-free survival at 12 months posttransplantation was also increased 2.6-fold (p = 0.03) in the VAL group. Modeling of DOP for TTV over the 12-month period of risk yielded a similar effect of VAL, with an additional 0.9% (95% confidence interval 0.4%, 1.4%, p < 0.001) increase in TTV for each additional day of VAL exposure.
EBV seroconversion
Rates were examined and 36 patients (63%) had serologic data available, obtained 12–18 months posttransplant. In patients who manifest viremia, the development of EBV IgG was associated with incident viremia in the first-year posttransplant (p = 0.005). Among those who did not manifest viremia, 5/17 (29.4%) are presumed to have had subclinical infection and became EBV IgG positive, including 4/12 (33%) from the VAL group and 1/5 (20%) from the no-VAL group (p = NS).
Patient and allograft survival
Five (11.4%) patients lost their allograft after a follow-up period ranging from 2 to 7 years posttransplant. The causes of graft loss in each patient were: EBV viremia complicated by acute T cell-mediated rejection grade IA; acute T cell-mediated rejection; combined acute T cell-mediated and antibody-mediated rejection; chronic allograft nephropathy; and one case was unknown. The patient with graft loss due to EBV and rejection had not received VAL prophylaxis. There were no cases of EBV-associated PTLD diagnosed in the first-year posttransplantation in either group. Late PTLD developed in one patient (EBV-positive Burkitt lymphoma) who did not receive VAL, after a prolonged period of low-grade EBV viremia.
Discussion
This is the largest pediatric cohort to investigate the association between antiviral prophylaxis with Val-ganciclovir and EBV viremia in high‐risk pediatric solid organ transplant recipients to date. The result of our retrospective study shows that VAL prophylaxis is effective in delaying the onset of EBV viremia in the first 6 months posttransplantation and incidence of EBV viremia in the first year. This effect was proportional to the DOP, with each additional week of VAL exposure prolonging the onset of viremia by 7.4%. We speculate that this might result from the inhibition of lytic replication during acute infection, thereby reducing the overall number of latently infected B cells with the potential to proliferate and escape immune control. These results challenge previously published reports refuting the effects of VAL prophylaxis in EBV viremic high‐risk pediatric solid organ transplant recipients.22,23,24,27 The incidence of EBV viremia was lower in patients who received prophylaxis, in spite of the fact that most received less than the recommended dose for CMV prophylaxis (7 mg × BSA × CrCl).28 Unlike Cameron et al.,21 we did not find an inverse correlation between VAL dose and incidence of EBV viremia, perhaps due to the lack of dosage variability. Of note, there were no cases of early PTLD observed in our study, which could be attributed to close monitoring and prompt reduction of immunosuppression.
Our results support the prospective study by Hocker et al.,16 which showed a significant decrease in primary EBV infection among pediatric kidney transplant recipients who received prophylaxis compared with controls. Cameron et al.21 also reached a similar conclusion, their results showed that only three episodes of EBV viremia occurred during prophylaxis and all EBV viremia that occurred during prophylaxis was in under-dosed patients. Darenkov et al.17 found that preemptive antiviral therapy using ganciclovir or acyclovir was effective in reducing the incidence of PTLD, but it was limited to the duration of anti-lymphocyte antibody therapy. Findings by Malouf et al.18 supported earlier studies as none of the EBV-seronegative recipients receiving continuous antiviral prophylaxis were diagnosed with PTLD; however, 1 of the 3 (33%) of the EBV-seronegative recipients who did not receive antiviral prophylaxis was diagnosed with PTLD. Levine et al.19 reported significantly lower PTLD incidence and mortality (1.8% and 1%, respectively) in a series of 109 lung transplant recipients compared with published PTLD incidence in lung transplant and speculated that this was due to the initial use of ganciclovir followed by prolonged acyclovir use. Funch et al.20 concluded that antiviral treatment with ganciclovir for CMV prophylaxis reduced the risk of early PTLD by 83% in kidney transplant recipients. In this series, ganciclovir or its prodrug VAL may be the preferred drugs for EBV prophylaxis compared with valacyclovir or acyclovir because of their higher in vitro antiviral activity.20 These data are highly consistent with VAL inhibition of viral replication and lower rates of PTLD.
On the other hand, Aris et al.22 found that development of PTLD was not associated with the use of ganciclovir/acyclovir in a cohort of 80 EBV-naive lung transplant recipients. Humar et al.23 could not demonstrate an additive benefit of intravenous immunoglobulin to VAL prophylaxis in a small randomized nonblinded study of 34 patients but did not compare patients who did and did not receive the antiviral prophylaxis. Opelz et al.24 also showed in a registry analysis that lymphoma incidence in 12,470 patients who received antiviral prophylaxis for CMV was nearly identical (standardized incidence ratio 24.2, p = 0.62) to that in patients who did not receive CMV prophylaxis. This study, however, did not stratify risk according to EBV serostatus. Ville et al.29 as well reported in single retrospective cohort lower incidence of late PTLD (beyond 1-year posttransplant) in high-risk EBV (D+/R−) adult patients receiving antiviral prophylaxis with either (val)acyclovir or (val)ganciclovir post kidney/kidney–pancreas transplant comparing to early PTLD (within 1-year posttransplant). Lastly, Paulsen et al.27 did not demonstrate a significant impact of VAL prophylaxis on EBV infection but was unable to stratify according to EBV risk status and did not examine timing of viremia onset in relation to VAL treatment.
Several limitations of our study are noteworthy, in particular, the retrospective nature and small size of the cohort, and given this reason, we were not able to asses for PTLD outcomes. Also, the current assessment of plasma viral load only measures circulating free viruses from EBV lytic phase, thus cell-associated latent EBV is not measured. The link between lytic-phase virus replication, the load of latent infection and subsequent PTLD is speculated on but not evaluated by this study. However, we analyzed two comparable high-risk groups for EBV viremia, one that received prophylaxis and a control group that did not. The groups were similar in terms of their baseline characteristics, immunosuppressive regimen, and graft function during the first-year posttransplant. Dosing and duration of VAL among the patients was variable. There were also differences in the frequency of laboratory testing and variations in immunosuppressive therapy protocols, due to differences in the medical practice among physicians. Nevertheless, all the patients included had at least five viral PCR in the first 6-month posttransplantation and a minimum of nine viral PCR tests during the first-year posttransplantation. Lastly, different techniques of EBV viral titers were used over the period of the study as they were semi-quantitatively measured from 2003 to 2011 and transitioned to quantitative methods from 2012 onwards.
In summary, donor-derived primary EBV infection poses serious risks to children post-solid organ transplantation, including the development of EBV-related PTLD. Management of EBV viremia early after transplantation remains a challenge. Reduction or withdrawal of immunosuppression is the standard first-line response to EBV viremia, with the goal of preventing PTLD.4,13,30 Although this approach improves outcomes related to PTLD, prevention of EBV infection or proliferation of infected B cells would be ideal, given the risk of rejection associated with reducing immunosuppression.31 Despite the standard use of VAL for EBV prophylaxis in many centers, data to support its efficacy to prevent EBV infections has been limited. In this cohort of high-risk patients, we have demonstrated benefit with this approach with delayed onset of viremia that is associated with the duration of prophylaxis. Because immunosuppression reduction upon onset of EBV viremia may increase risk of rejection, prevention or delay of EBV viremia onset may be beneficial in and of itself, until a time when patients have been weaned to more reduced levels of immunosuppression, have lower risk of rejection, and may more effectively respond to the infection. Thus we suggest that VAL be considered for EBV D+/R− pediatric organ transplant recipients to delay the onset of EBV viremia.
References
Hong, G. K. et al. Epstein-Barr virus lytic infection is required for efficient production of the angiogenesis factor vascular endothelial growth factor in lymphoblastoid cell lines. J. Virol. 79, 13984–13992 (2005).
Yang, J. et al. Characterization of Epstein-Barr virus–infected B cells in patients with posttransplantation lymphoproliferative disease: disappearance after rituximab therapy does not predict clinical response. Blood 96, 4055–4063 (2000).
Holmes, R. D. et al. Response of elevated Epstein-Barr virus DNA levels to therapeutic changes in pediatric liver transplant patients: 56-month follow up and outcome. Transplantation 74, 367–372 (2002).
Allen, U. D., Preiksaitis, J. K. & AST Infectious Diseases Community of Practice. Epstein-Barr virus and posttransplant lymphoproliferative disorder in solid organ transplantation. Am. J. Transplant. 13 (Suppl 4), 107–120 (2013).
Allen, U. et al. Epstein-Barr virus infection in transplant recipients: Summary of a workshop on surveillance, prevention and treatment. Can. J. Infect. Dis. 13, 89–99 (2002).
Ishihara, M. et al. Epstein-Barr virus load for early detection of lymphoproliferative disorder in pediatric renal transplant recipients. Clin. Nephrol. 76, 40–48 (2011).
Green, M. & Webber, S. Posttransplantation lymphoproliferative disorders. Pediatr. Clin. North Am. 50, 1471–1491 (2003).
Gulley, M. L. & Tang, W. Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder. Clin. Microbiol. Rev. 23, 350–366 (2010).
Gärtner, B. & Preiksaitis, J. K. EBV viral load detection in clinical virology. J. Clin. Virol. 48, 82–90 (2010).
Holman, C. J. et al. Quantitative Epstein-Barr virus shedding and its correlation with the risk of post-transplant lymphoproliferative disorder. Clin. Transpl. 26, 741–747 (2012).
Hierro, L. et al. Efficacy and safety of valganciclovir in liver-transplanted children infected with Epstein-Barr virus. Liver Transpl. 14, 1185–1193 (2008).
Gershburg, E. & Pagano, J. S. Epstein-Barr virus infections: prospects for treatment. J. Antimicrob. Chemother. 56, 277–281 (2005).
Reshef, R. et al. Reduction of immunosuppression as initial therapy for post-transplantation lymphoproliferative disorder. Am. J. Transplant. 11, 336–347 (2011).
Franke, A. J. et al. Association of allograft rejection with reduction of immunosuppression for post-transplant lymphoproliferative disorder: analysis of a 20-year single-institutional experience. J. Clin. Oncol. 35, e19047 (2017).
Smith, J. M. et al. Subclinical viremia increases risk for chronic allograft injury in pediatric renal transplantation. J. Am. Soc. Nephrol. 21, 1579–1586 (2010).
Hocker, B. et al. (Val-)Ganciclovir prophylaxis reduces Epstein-Barr virus primary infection in pediatric renal transplantation. Transpl. Int. 25, 723–731 (2012).
Darenkov, I. A. M. et al. Reduced incidence of epstein-barr virus-associated posttransplant lymphoproliferative disorder using preemptive antiviral therapy. Transplantation 64, 848–852 (1997).
Malouf, M. A. et al. Anti-viral prophylaxis reduces the incidence of lymphoproliferative disease in lung transplant recipients. J. Heart Lung Transplant. 21, 547–554 (2002).
Levine, S. M. et al. A low incidence of posttransplant lymphoproliferative disorder in 109 lung transplant recipients. Chest 116, 1273–1277 (1999).
Funch, D. P., Walker, A. M., Schneider, G., Ziyadeh, N. J. & Pescovitz, M. D. Ganciclovir and acyclovir reduce the risk of post-transplant lymphoproliferative disorder in renal transplant recipients. Am. J. Transplant. 5, 2894–2900 (2005).
Cameron, B. M., Kennedy, S. E., Rawlinson, W. D. & Mackie, F. E. The efficacy of valganciclovir for prevention of infections with cytomegalovirus and Epstein-Barr virus after kidney transplant in children. Pediatr. Transplant. https://doi.org/10.1111/petr.12816 (2017).
Aris, R. M. et al. Post-transplantation lymphoproliferative disorder in the Epstein-Barr virus-naive lung transplant recipient. Am. J. Respir. Crit. Care Med. 154, 1712–1717 (1996).
Humar, A. et al. A randomized trial of ganciclovir versus ganciclovir plus immune globulin for prophylaxis against Epstein-Barr virus related posttransplant lymphoproliferative disorder. Transplantation 81, 856–861 (2006).
Opelz, G., Daniel, V., Naujokat, C., Fickenscher, H. & Dohler, B. Effect of cytomegalovirus prophylaxis with immunoglobulin or with antiviral drugs on post-transplant non-Hodgkin lymphoma: a multicentre retrospective analysis. Lancet Oncol. 8, 212–218 (2007).
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients (2009). Am. J. Transplant. 9 (Suppl 3), S1–S155 (2009).
R. C. Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, 2015).
Paulsen, G. et al. Cytomegalovirus and Epstein-Barr virus infections among pediatric kidney transplant recipients at a center using universal valganciclovir prophylaxis. Pediatr. Transplant. 23, e13382 (2019).
Valcyte® Prescribing Information. Genentech USA, Inc., South San Francisco, CA, (2017).
Ville, S. et al. Impact of antiviral prophylaxis in adults Epstein–Barr Virus-seronegative kidney recipients on early and late post-transplantation lymphoproliferative disorder onset: a retrospective cohort study. Transpl. Int. 31, 484–494 (2018).
Swinnen, L. J. et al. Prospective study of sequential reduction in immunosuppression, interferon alpha-2b, and chemotherapy for posttransplantation lymphoproliferative disorder. Transplantation 86, 215–222 (2008).
Rabot, N. et al. CNI withdrawal for post-transplant lymphoproliferative disorders in kidney transplant is an independent risk factor for graft failure and mortality. Transpl. Int. 27, 956–965 (2014).
Author information
Authors and Affiliations
Contributions
S.A.: Concept/design of the study, data collection, data analysis, manuscript preparation and draft; A.S.: Data analysis, critical revision of article; K.H., A.W., and S.G.: Concept/design of the study, critical revision of article; T.D.B.-H.: Concept/design of study, data interpretation, critical revision of article.
Corresponding author
Ethics declarations
Competing interests
S.G. receives research funding and consulting fees from Merck, GSK, VBI, and Meridian Biosciences. T.D.B.-H. receives research funding from Astellas. A.W. has received consulting fees or honoraria from Merck, AVIR Pharma, Pendopharm, and Astellas. A.S. has received payment for “conducting or overseeing statistical analyses.” The other authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Albatati, S., Sharma, A., Haubrich, K. et al. Valganciclovir prophylaxis delays onset of EBV viremia in high-risk pediatric solid organ transplant recipients. Pediatr Res 87, 892–896 (2020). https://doi.org/10.1038/s41390-019-0523-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0523-4