Abstract
Background
Neuroprotection from therapeutic hypothermia (HT) is incomplete, therefore additional strategies are necessary to improve long-term outcomes. We assessed the neuroprotective efficacy of magnesium sulfate (MgSO4) bolus and infusion over 48 h plus HT in a piglet model of term neonatal encephalopathy (NE).
Methods
Fifteen newborn piglets were randomized following hypoxia–ischemia (HI) to: (i) MgSO4 180 mg/kg bolus and 8 mg/kg/h infusion with HT (Mg+HT) or (ii) HT and saline 0.5 ml/h (HT). Treatments were initiated 1 h post-HI; HT administered for 12 h (33.5 °C). HI was performed by transient carotid occlusion and inhalation of 6% O2 for 20–25 min. Primary outcomes included aEEG, magnetic resonance spectroscopy (MRS) at 24, and 48 h, and immunohistochemistry.
Results
MgSO4 bolus and infusion was well tolerated (no hypotension) and doubled serum magnesium (0.72 vs 1.52 mmol/L) with modest (16%) rise in CSF. In Mg+HT compared to HT, there was overall reduced cell death (p = 0.01) and increased oligodendrocytes (p = 0.002). No improvement was seen on aEEG recovery (p = 0.084) or MRS (Lac/NAA; PCr/Pi; NTP/epp) (p > 0.05) at 48 h.
Conclusion
Doubling serum magnesium with HT was safe; however, the small incremental benefit of Mg+HT compared to HT is unlikely to translate into substantive long-term improvement. Such an incremental effect might justify further study of MgSO4 in combination with multiple therapies.
Similar content being viewed by others
Introduction
Neonatal encephalopathy (NE) represents a significant global health burden and is the second leading cause of mortality in infants aged <28 days.1 Therapeutic hypothermia (HT) is currently the only routine neuroprotective strategy shown to be effective in intensive care settings; however, mortality and morbidity remain high at almost 50% despite treatment.2 Optimizing HT by cooling to lower temperatures (32 °C) and for longer duration (120 h) failed to improve neurological outcomes3 and attention is now directed toward adjunct pharmacological agents.
Magnesium sulfate (MgSO4) is a cheap and widely available drug with a well-documented side effect profile. It has recently been shown to reduce the incidence of cerebral palsy in preterm infants when administered antenatally4; MgSO4 may have potential as an adjunct treatment with HT in term NE. Experimental data suggest increasing serum magnesium to twice baseline values is neuroprotective5,6; however, studies demonstrating efficacy have been confounded by co-existing accidental hypothermia7 and those controlling core body temperature failed to demonstrate benefit.8 Clinical studies of MgSO4 in term infants with NE mostly predate the implementation of hypothermia and were limited by small numbers, variable dosing regimens, and inconsistent outcome measures.9 These trials implemented a daily bolus regimen of MgSO4, resulting in significant peaks and troughs in serum magnesium, limiting exposure to supra-systemic magnesium concentrations. Such peaks and troughs in the serum magnesium have been associated with hypotension in a large animal model of NE.8
MgSO4 provides neuroprotection through the blockade of the glutamatergic N-methyl-d-aspartate (NMDA) receptors at postsynaptic neuronal membranes, preventing excessive calcium entry that would otherwise activate several injurious cellular pathways, including catabolic enzyme induction and increased production of reactive oxygen species. Experimental data suggest that MgSO4 may also be anti-inflammatory; MgSO4 modified the inflammatory cytokine response in pregnant rodents following exposure to a bacterial endotoxin, lipopolysaccharide (LPS).10 Both MgSO4 and HT are thought to reduce excitotoxicity and modulate inflammation,11 and therefore it is plausible that these therapies may work synergistically. Indeed, combining MgSO4 with HT in rodent models of NE has been shown to reduce infarct size compared to cooling alone.12
We aimed to assess the neuroprotective benefit of a MgSO4 bolus and constant infusion in combination with HT (Mg+HT) compared to hypothermia (HT) in a clinically translational piglet model of NE. Primary outcome measures included amplitude-integrated electroencephalogram (aEEG) recovery after hypoxia–ischemia (HI), magnetic resonance spectroscopy (MRS) biomarkers, and immunohistochemistry.
Methods
Ethics
All experimental and surgical procedures were performed in accordance with UK Home Office Guidelines [Animals (Scientific Procedures) Act, 1986] and the study complies with the ARRIVE guidelines.
Anesthesia and surgical preparation
Large white male piglets aged <36 h and weighing 1.7–2.1 kg were anesthetized and surgically prepared as previously described.13 In brief, animals were sedated with intramuscular midazolam (0.2 mg/kg) and anesthetized with isoflurane mixed with air (3% v/v during surgery, 1.5–2.5% during experimentation), remaining insentient throughout experimentation. Animals were mechanically ventilated via tracheostomy (SLE 2000 infant ventilator, Surrey, UK) and settings guided by arterial blood gas analysis (PaO2 8–13 kPa, pCO2 4.5–6.5 kPa). The common carotid arteries were surgically isolated and carefully encircled by inflatable carotid occluders (OC2A, In Vivo Metric). An umbilical arterial line was inserted for mean arterial blood pressure (MABP) and heart rate (HR) monitoring and umbilical venous line for infusions. Infusions included maintenance 10% dextrose at 60 ml/kg/day (reduced to 40 ml/kg/day post-insult), fentanyl 3–6 mcg/kg/h, and antibiotics (benzylpenicillin 50 mg/kg/dose BD, gentamicin 5 mg/kg/dose OD). The arterial line was infused with heparinized saline (0.5 IU/ml in 0.9% sodium chloride) at 0.3 ml/h.
Animals were nursed prone in a purpose-built MR-compatible transport incubator. Intensive care was provided throughout the 48 h experiment and complications (e.g., hypotension, seizures, hyperkalemia) were as per local neonatal guidelines. To maintain the MABP >40 mm Hg, 0.9% saline bolus (10 ml/kg), dopamine (5–20 μg/kg/min), dobutamine (5–20 μg/kg/min), noradrenaline (0.1–1.5 μg/kg/min), and adrenaline (0.1–1.5 μg/kg/min) were used as required by a neonatologist.
Blood tests
Blood tests included pH, pCO2, pO2, bicarbonate (HCO3), base excess (BE), lactate (Lac), glucose (Glu), urea, and electrolytes (Abbot Laboratories, UK). Serum and cerebrospinal fluid (CSF) magnesium levels were frozen (−20 °C) and processed on completion of the study (Royal Veterinary College, Hawkshead, UK). Serum magnesium was taken at baseline, end of insult (t = 0), and 2, 3, 6, 12, 18, 24, 30, 36, 42 and 48 h post-HI. CSF magnesium was measured at baseline and 48 h.
Study groups and protocol
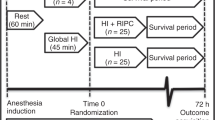
Block randomization of 15 animals was computer generated and sealed in opaque envelopes, opened on completion of HI. Animals were allocated to either MgSO4 plus hypothermia (Mg+HT; n = 8) or saline plus hypothermia (HT, n = 7). Interventions were initiated 1 h post-insult; whole-body HT to 33.5 °C was maintained for 12 h and MgSO4 therapy was administered as 180 mg/kg bolus plus 8 mg/kg/h infusion over 48 h (Fig. 1).
Study timeline. Following baseline data acquisition, piglets underwent cerebral hypoxia–ischemia (HI). At the end of HI (time = 0), piglets were randomized to (i) therapeutic hypothermia (HT; 33.5 °C)+Mg or (ii) HT. Treatment was started 1 h after HI. Piglets were maintained under intensive care for 48 h following HI, prior to euthanasia. Magnetic resonance spectroscopy (MRS) was acquired at 24 and 48 h. Amplitude-integrated electroencephalogram and near-infrared spectroscopy were acquired at baseline and in between MRS acquisitions
Magnetic resonance spectroscopy
MRS was performed at 24 and 48 h in a Philips clinical 3 T MRI scanner. A 7 × 5 cm elliptical transmit-receive MRS surface coil tuned to 31P frequency (51.6 MHz) was secured to the piglet’s head and spectra were acquired at 1 min intervals using a non-localized single pulse acquisition (repetition time 10 s, 6 summed acquisitions per spectrum). Spectra were analyzed using the Advanced Method for Accurate, Robust and Efficient Spectral (AMARES) fitting of MRS data14 as implemented in the jMRUI software. Nucleotide triphosphate (NTP) peaks were fitted as a doublet (α and γ) and triplet (β) structure with no assumption of the relative multiplet sizes. NTP is predominantly composed of adenosine triphosphate (ATP) and changes in this signal reflect changes in cerebral energetics. Measurements of inorganic phosphate (Pi), phosphocreatine (PCr), exchangeable phosphate pool (epp = Pi + PCr + 2γ-NTP + β-NTP) were acquired over the whole brain, and peak area ratios were calculated (Pi/epp, PCr/Pi, NTP/epp).
1H MRS spectra were acquired with a separate 6.5 × 5.5 cm elliptical receive surface coil tuned to the 1H resonance frequency. Metabolites were measured in a white matter voxel in the dorsal right subcortical region (8 × 8 × 15 mm) and deep gray matter voxel (15 × 15 × 10 mm) in the thalamus. The data was analyzed using the jMRUI software, and the lactate/N-acetylaspartate (Lac/NAA) peak area ratio was calculated.
Amplitude-integrated electroencephalogram
A multichannel EEG (Nicolet) was acquired at baseline and continued for 48 h post-insult. The aEEG score was classified as described by Hellstrom–Westas et al.15: isoelectric (0), continuous low voltage (1), burst suppression (2), discontinuous normal voltage (3), and continuous normal voltage (4). Scoring was performed hourly by two independent clinicians (I.L., K.M.) blinded to treatment allocation. aEEG scores were averaged in 6 h time epochs and mean differences were analyzed for significance.
Broadband near-infrared spectroscopy (NIRS)
An in-house developed broadband NIRS optical measuring device (Mini-CYRIL, Cytochrome Research Instrument and Application System) provided real-time in vivo measurements of changes in cerebral concentration of oxyhemoglobin (HbO2), deoxyhemoglobin, and oxidized cytochrome-c-oxidase (oxCCO). Changes in oxCCO during HI provided insight into the level of brain tissue metabolic suppression that is related to the degree of severity of the cerebral HI.16 The real-time and total fall in oxCCO during HI measured using the area under the curve below baseline (AUC oxCCO) was used as an indicator of insult severity.17
Transient HI
Animals were monitored for at least 1 h to ensure hemodynamic stability, normal EEG, and baseline broadband NIRS prior to HI. Carotid occluders were inflated and fraction of inspired oxygen (FiO2) reduced simultaneously at the start of insult. FiO2 was decreased to 6% and titrated to MABP, broadband NIRS, and EEG. Oxygen delivery was liberalized in the event of MABP <27 mm Hg or 3-fold decrease in brain tissue oxCCO as measured by broadband NIRS. Blood gas analysis was performed at 5 min intervals during insult. Total duration of HI was determined by the duration of isoelectric EEG, hypotension (BP < 30 mm Hg) in addition to total fall in oxCCO (AUC oxCCO), and reduction in FiO2 (AUC FiO2). Two experienced team members decided on the duration of insult using available information. At the end of insult, the animal was resuscitated, occluders deflated, and FiO2 increased to 21%. The duration of HI was typically 18–22 min.
Histology
Piglets were euthanized at 48 h post-HI using a pentobarbital injection and brain fixed with an intra-cardiac injection with cold 4% paraformaldehyde (PFA) in phosphate-buffered saline. The brain was dissected out and fixation continued in 4% PFA for 7 days. The right cerebral hemisphere was dissected in 5 mm coronal slices starting from the anterior optic chiasm and embedded in paraffin wax before being sectioned into 5 µm slides. Two sections per piglet were stained (bregma 00 and −2.0) and 8 brain regions were examined: cingulate cortex, sensorimotor cortex, hippocampus, internal capsule, periventricular white matter, caudate, putamen, and thalamus (Supplemental Fig. S1). All histological analysis was performed by an investigator blinded to treatment allocation.
To assess cell death and glial activation, sections were stained for nuclear DNA fragmentation using histochemistry with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), the appearance of activated caspase 3 (CC3), glial fibrillary acidic protein (GFAP), and microglial ionized calcium-binding adaptor molecule (Iba1) immunoreactivity. Oligodendrocytes were stained with oligodendrocyte transcription factor (OLIG2) as a marker of myelination. For each animal, 2 sections placed 5 mm apart were assessed for each stain.
For all histochemical and immunohistochemical stains, brain sections were dehydrated in xylene (3 × 10 min) and rehydrated in graded ethanol solutions (100–70%), followed by double-distilled water. For TUNEL, endogenous peroxidases were removed by pretreating sections in 3% H2O2 in methanol, followed by a 15-min peptidase pre-digestion with 20 µg/ml proteinase K (Promega) at 65 °C. Sections were incubated at 37 °C for 2 h with TUNEL solution (Roche) containing biotinylated dUTP. TUNEL-positive cells were counted in 3 fields (×40 magnification; area 0.066 mm2) and averaged per region (cells/mm2).
For activated caspase 3, Iba1, and OLIG2, pretreatment with Ventana CC1 (950–124), equivalent to EDTA buffer was used. For GFAP, Protease 1 (0.38 mg/mL alkaline protease enzyme activity) was used. Primary antibody incubation was performed with primary rabbit antibody against activated Caspase 3 (1:100, Cell Signalling 9661L) for 32 min, Iba1 (1:250, WAKO 019–19741) for 4 h, GFAP (1:1000, DAKO Z0334) for 32 min, and OLIG2 (1:100, Millipore AB9610) for 4 h. Incubation with a secondary swine anti-rabbit immunoglobulin (DAKO E0343) was performed for a duration of 44 min in activated caspase 3, 1 h for Iba1 and OLIG2, and for 32 min in GFAP staining.
The biotin residues were detected with avidin-biotinylated horseradish peroxidase complex (ABC, Vector Laboratories) and visualized with diaminobenzidine/H2O2 (Sigma), with CoCl2 and NiCl2 included to intensify TUNEL histochemistry. The sections were dehydrated in graded alcohol and xylene and mounted with Depex (VWR) or, alternatively, mounted with Vectashield+4′,6-diamidino-2-phenylindole aqueous mounting media (Vector Labs), to facilitate total cell number counts during analysis of Iba1 and activated caspase 3.
Iba1-positive microglial cells were assigned a ramification index based on body and branch density using a 0.049 mm × 0.049 mm square grid (×40 magnification) placed in three fields for each brain region. The microglial ramification index was calculated as B2/C. B represents the average number of branches crossing the 3 horizontal and 3 vertical 0.049 mm gridlines and C the number of cell bodies within the grid. Activated caspase 3 immunoreactive cells were counted in 3 fields (×20 magnification; area 0.164 mm2) and averaged per region (counts/mm2). Astroglial activation was quantified by measuring GFAP immunoreactivity optical luminosity values. The mean brightness values (two fields per region, ×20 magnification) were deducted from the mean brightness of the blank slide.18 OLIG2-positive cells were counted in 3 fields (×40 magnification; area 0.066 mm2) and averaged per brain region (cells/mm2).
Statistical analysis
Statistical analysis was performed using Prism v6.0 for Mac, GraphPad Software, CA, USA. Parametric data were analyzed using Student’s t tests and non-parametric data with Mann–Whitney U tests. MRS, aEEG, and immunohistochemistry were analyzed using analysis of variance of the least mean squared difference. Histology was log10 transformed to normalize distribution for parametric statistical analysis.
Sample size calculation based on previous piglet studies13 indicated that MRS biomarkers (Lac/NAA) over 48 h varied by 1.0 U between study groups (standard deviation 0.75) on a log scale. Using a significance threshold of 5 and 80% power, we determined that 7 subjects were needed in each group.
Results
Physiological measures
There were no significant differences in animal weight, baseline cardiovascular status (HR, MABP), and biochemistry (Na+, K+, urea, creatinine, glucose). Baseline blood gas was similar between groups with the exception of a slightly higher HCO3 and BE in the Mg+HT group, although these values were within normal limits (Table 1).
The duration of HI, hypotension, isoelectric EEG, AUC oxCCO, and AUC FiO2 were similar between magnesium-treated and control animals. In addition, there was no significant difference in the end of insult blood gas, indicating that the severity of HI was similar between groups (Table 2). One animal in each group had mild NE, defined a priori as aEEG recovery to at least discontinuous normal voltage within 1 h post-insult.
Piglets were nursed in a thermoregulated mattress and normothermia was maintained throughout HI. Mean HR, MABP, and temperature were similar between groups post-insult (0–1 h), during induction of hypothermia (1–3 h), hypothermia (3–13 h), rewarming (13–23 h), and normothermia (23–48 h). Animals in the HT group had a slightly raised HR compared to the Mg+HT group, although this value was still within normal limits. Magnesium infusion was not associated with a significant increase in inotrope usage during HT or rewarming/normothermia (Table 3).
One piglet in the control group was euthanized early at 21 h due to a rising lactate and intractable hypotension (diagnosed with bowel perforation on post-mortem). Overall, there was no difference in survival between animals receiving Mg+HT and HT (8/8 vs 6/7, p = 0.47).
Pharmacokinetics
Serum magnesium increased significantly by 1 h following MgSO4 bolus in the Mg+HT group (1.62 vs 0.72 mmol/L, p = 0.008); levels remained stable throughout infusion and were unaffected by HT (Fig. 2). Interim analysis demonstrated a gradual fall in serum magnesium after 30 h toward the lower end of the target range (1.40–2.00 mmol/L). The infusion was increased from 8 mg/kg/h (n = 5) to 10 mg/kg/h (n = 3); however, overall serum magnesium levels did not change (1.51 vs 1.52 mmol/L, p = 0.5). CSF magnesium increased significantly from baseline to 48 h post-infusion (1.04 vs 1.21 mmol/L, p = 0.008). Changes in CSF magnesium were relatively modest compared to serum; doubling serum magnesium resulted in a 16% rise in the CSF.
Pharmacokinetics of MgSO4 bolus and infusion. Serum magnesium concentrations a increased significantly from baseline and remained stable in the target range (1.4–2.0 mmol/L). Cerebrospinal fluid magnesium b in Mg+therapeutic hypothermia (HT) animals was significantly higher at 48 h than at baseline and in HT piglets (error bars represent SEM, *p < 0.05)
1H and 31P MRS
1H and 31P MRS were acquired in 14 out of 15 piglets at 24 and 48 h. White matter and thalamic Lac/NAA were plotted on a logarithm scale; there was no significant difference in white matter Lac/NAA, thalamic Lac/NAA, NTP/epp, and PCr/Pi observed between the Mg+HT and HT groups at 24 and 48 h (Fig. 3). Exclusion of the two animals with mild NE did not alter these findings.
Magnetic resonance spectroscopy at 24 and 48 h post-hypoxia–ischemia. All p values relate to the 48 h acquisition. Least square mean difference with 95% confidence intervals for: a lactate/N-acetylaspartate (Lac/NAA; white matter), p = 0.142; b Lac/NAA (thalamus), p = 0.131; c nucleotide triphosphate/exchangeable phosphate pool (NTP/epp), p = 0.317; d phosphocreatine/inorganic phosphate (PCr/Pi), p = 0.152. Overlapping bars show evidence of no difference. Thal thalamic, WM white matter
Amplitude-integrated electroencephalogram
All animals had normal aEEG activity pre-insult with peak and baseline voltage >10 μV. Typically, aEEG became isoelectric (<5 μV) within 3 min of HI and did not recover when oxygen was liberalized during periods of excessive hypotension. Cerebral electrical activity remained severely suppressed, except that two piglets demonstrated early aEEG recovery within 1 h of HI (one in each group). There was a trend toward aEEG improvement after 36 h in Mg+HT animals (p = 0.094), which became significant when excluding two animals with mild injury (aEEG recovery within 1 h post-HI) (Fig. 4). Two animals in each group had seizures requiring treatment with 20 mg/kg phenobarbitone (seizures were prolonged in one animal in each group).
Effects of Mg+therapeutic hypothermia (HT) compared to HT on amplitude-integrated electroencephalogram (aEEG) scores over 48 h post-hypoxia–ischemia (post-HI) in: a all piglets and b piglets with moderate-to-severe HI (after exclusion of two piglets with mild injury defined as aEEG recovery within 1 h after insult). Error bars represent 95% confidence interval (*p < 0.05). aEEG scored according to pattern classification (c)
Histology
Immunohistochemical staining with TUNEL, GFAP, Iba1, CC3, and OLIG2 was undertaken in all 15 piglets, and quantitative analysis was performed between treatment groups overall and by individual brain regions.
TUNEL
We observed a significant reduction in total brain TUNEL-positive cells in Mg+HT piglets compared to HT (21.1 vs 47.1 log10 count/mm2, p = 0.014) and a trend toward reduced cell death in the periventricular white matter (4.8 vs 22.3 log10 count/mm2, p = 0.094). There was no significant difference in cell death in the cingulate cortex, sensorimotor cortex, hippocampus, periventricular white matter, caudate, and putamen (Fig. 5, Table 4).
Scatter plots with median and interquartile range for overall and regional brain terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cell counts. Comparing Mg+therapeutic hypothermia (HT) with HT, there was significant reduction in overall cell death (p = 0.014). There was a non-significant trend toward reduced TUNEL-positive cells in the Mg+HT group in the periventricular white matter (p = 0.094)
Cleaved caspase 3
There was no difference in CC3-positive cells in Mg+HT piglets compared to HT (0.7 vs 0.5 log10 count/mm2, p = 0.292). There was, however, a significant increase in CC3-positive cells in the periventricular white matter in Mg+HT piglets compared to HT (1.4 vs 0.6 log10 count/mm2) (Fig. 6a–d).
Scatter plots with median and interquartile range for overall and regional immunohistochemistry. Data are shown for CC3 (a–d), IBA1 ramification index (e–h), glial fibrillary acidic protein (GFAP) (i–l), and OLIG2 (m–p). For CC3, there was no difference between study groups overall (p = 0.292); however, in the periventricular white matter we observed increased CC3 in Mg+therapeutic hypothermia (HT) animals (p = 0.007). For IBA1 ramification index, there was a trend toward an overall increased ramification index (less activation of microglia) (p = 0.086) in Mg+HT animals with a similar trend in the hippocampus (p = 0.071). For GFAP, there was no significant difference in overall astrogliosis but localized increases in the cingulate cortex (p = 0.008) and caudate (p = 0.022) in Mg+HT animals. For OLIG2, there was a significant overall increase in oligodendrocytes in Mg+HT animals (p = 0.002) with regional differences in the hippocampus (p = 0.024) and thalamus (p = 0.004)
Iba1
There was a trend toward increased ramification index (less microglial activation) for Iba1-positive cells in Mg+HT piglets compared to HT (1.6 vs 1.1, p = 0.086). No significant differences were identified on analysis of individual brain regions (Fig. 6e–h).
GFAP
There was no significant difference in overall GFAP optical luminosity in piglets receiving Mg+HT compared to HT (64.7 vs 57.1, p = 0.110). On regional analysis, there was significantly higher luminosity in the cingulate cortex (59.2 vs 42.5, p = 0.008) and caudate (49.7 vs 35.4, p = 0.022) in piglets treated with Mg+HT compared to HT (Fig. 6i–l).
OLIG2
We observed an increase in the overall number of OLIG2-positive cells in Mg+HT piglets compared to HT (2.44 vs 2.05 log10 count/mm2, p = 0.002). Analysis of individual brain regions demonstrated a significant increase in OLIG2-positive cells in the hippocampus (2.49 vs 1.67 log10 count/mm2, p = 0.024) and thalamus (2.56 vs 1.6 log10 count/mm2, p = 0.004) in piglets treated with Mg+HT compared to HT (Fig. 6m–p).
Discussion
This is the first large animal study comparing the neuroprotective efficacy of MgSO4 with HT against HT alone. MgSO4 administered as a bolus and infusion was well tolerated and provided a stable, raised serum magnesium concentration with significant rise in CSF 48 h post-infusion. We observed an overall reduction in TUNEL cell death in animals treated with Mg+HT compared to HT, but on regional analysis there was no significant difference between groups. We observed an increase in surviving oligodendroglia in the hippocampus and thalamus. We saw more rapid aEEG recovery after 30 h of treatment on post hoc analysis in the Mg+HT group (excluding the mild NE animals) but no improvement on MRS biomarkers at 24 or 48 h post-HI.
Hypotension is a common side effect of MgSO4 and previously observed following repeated boluses of MgSO4 in normothermic piglets.8 Given the physiological effects of HT and cardiovascular depression following HI, hypotension is a significant concern with combination therapy. Rahman and colleagues reported a favorable safety profile in a clinical trial of MgSO4 boluses with HT but did not report cardiovascular parameters immediately following drug administration.19 Reassuringly, we did not observe significant hypotension or increased inotrope usage during HT, rewarming, or normothermia. Serum magnesium pharmacokinetics remained stable during hypothermia and we did not observe toxic accumulation. Of note, a two-fold rise in serum magnesium resulted in a modest 16% increase in CSF concentration, consistent with data from adult neurosurgical studies.20 Magnesium is actively transported across the blood–brain barrier through ATP-dependent ion exchangers and cation channels.21 Saturation of these ion channels may explain the limited rise in CSF concentration compared to serum.
Magnesium ions bind in a voltage-dependent manner to the glutamatergic NMDA receptor channel and competitively antagonize calcium ion entry. The excessive release of excitatory neurotransmitters such as glutamate is a key mechanism of injury in the hours following HI and is a target for neuroprotective intervention. We observed a significant reduction in overall, but not regional, cell death in animals treated with Mg+HT, compared to HT. A trend to lower TUNEL-positive cells was seen in the periventricular white matter (p = 0.094). Our data are consistent with those from an adult rodent study in which Mg+HT started 2 h after global ischemia showed a small incremental improvement in neuroprotection with both mild and moderate HT, but the improvement was not significant.22 Such incremental improvement in cell survival would be difficult to detect in a reasonably sized clinical trial but may be important in future preclinical studies aimed at optimizing outcomes with a cocktail of therapies.
Although excitotoxic-mediated injury may affect all neuronal cells, the myelin-producing oligodendrocytes are particularly vulnerable.23 This may explain the increase in overall oligodendrocyte count (OLIG2-positive cells) and trend toward reduced cell death on TUNEL-positive cells in the white matter in animals receiving Mg+HT. Prolonged infusions of MgSO4 have been associated with a reduction in the number of immature and mature oligodendrocytes in the intragyral and periventricular white matter in a preterm fetal sheep model.24 In our study, we did not measure changes in immature or mature oligodendrocytes or myelination. In future studies, it will be important to assess whether add on treatment with MgSO4 has differential effects on survival within the oligodendrocyte lineage and whether or not it can improve myelination.
Surprisingly, there was a non-significant increase in the CC3 cell count, particularly in the periventricular white matter in the Mg+HT compared to the HT group. Apoptotic pathways are sexually dimorphic,25 and in the male piglet (all piglets in our study were male), apoptosis mainly occurs via caspase-independent pathways,26 making CC3 a poor apoptotic marker in this model. CC3 alterations in our model appear to reflect caspase’s non-apoptotic functions, including promoting microglial and lymphocyte function, cell differentiation, and autophagy.27,28,29 We have previously observed a poor correlation between TUNEL-positive cell counts and CC3. MRS Lac/NAA used in both babies with NE and in our piglet model shows a strong correlation with TUNEL-positive cells and weak correlation with CC3, suggesting that TUNEL-positive cells are the most relevant marker for outcome in this model.30 Lastly, termination of the studies at 48 h may be insufficient time for apoptotic cell death to fully evolve and may partially explain the discrepant CC3 data.
aEEG is a useful clinical tool to stratify the severity of NE and recovery of electrical activity is associated with good clinical outcomes,31 although this may be delayed in infants undergoing HT.32 The trend toward aEEG recovery in the Mg+HT group is an indication of the potential incremental efficacy of MgSO4 in animals, particularly in moderate-to-severe injuries. A similar number of piglets in each group had seizures detected on aEEG (two in each group) and were treated with phenobarbitone. Unlike recent studies with MgSO4 in fetal sheep,33 we did not observe any reduction in seizure burden in piglets who received Mg+HT. The possible brain injury related to phenobarbitone use itself34 is likely to have been balanced in our study due to the equal incidence of seizures in each group.
Cerebral Lac/NAA is a robust prognostic marker in the first 2 weeks after birth in babies with NE35 and used as a surrogate measure of long-term outcomes in experimental trials of neuroprotection in NE.13,36 It was surprising that we did not observe significant improvement in MRS biomarkers in the Mg+HT treated piglets at 24 or 48 h post-insult. It is possible that the additional protective effect of MgSO4 was too small to be observed with MRS.
Magnesium has previously been reported to attenuate the inflammatory response after HI. MgSO4 exposure significantly decreased interleukin-6 and tumor necrosis factor-α production in LPS-stimulated cord blood monocytes.37 MgSO4 administered to pregnant rodents attenuated LPS-induced pro-inflammatory mediator expression38 and improved offspring learning at 3 months.10 In our study, there was a trend toward increased overall ramification index in the Mg+HT group (p = 0.086) compared to HT. Activation of brain-resident microglia comprises a set of highly conserved cellular responses to brain injury. Metabolic activation, migration, and loss of ramification associated with phagocytosis are rapid events that can occur within hours following injury. The partial preservation of the microglial ramification index is in keeping with the incremental neuroprotection seen with Mg+HT in this study. The ramification index provides a quantitative measure to rapidly assess total brain injury and has been seen previously to correlate negatively with TUNEL cell density and brain MRS measures.39 We observed an increase in astrogliosis in the cingulate cortex and caudate in the Mg+HT group, which was unexpected.
This study was the first to utilize a MgSO4 bolus and infusion to achieve a stable supra-systemic concentration of magnesium in a term-equivalent piglet model of NE. We have demonstrated that baseline serum magnesium levels can be doubled safely and maintained over 48 h. We felt that initiating therapy by 1 h is feasible in clinical practice and provides sufficient time to initiate resuscitation, gain intravenous access, and commence magnesium therapy.
The main limitation of this study was the short duration (48 h) after HI for injury to develop; the fate of microglia and apoptosis may therefore still be evolving. The main strengths of this study were its standardized HI insult, known timing of injury, and clinically relevant outcomes measures. Although the study was powered a priori to detect a difference based on previous piglet studies, the minimal numbers used, keeping the 3Rs in mind, may explain the absence of a statistically significant reduction in MRS biomarkers. Animals were cooled for 12 h, rather than for 24 h as in our previous studies13; this was justified as we are modeling a situation where cooling is partially effective and the original study demonstrating efficacy of cooling in piglets showed efficacy with 12 h HT.40 Indeed, the possible additional benefit of longer HT may abolish any incremental neuroprotection provided by Mg.
In conclusion, we demonstrated in a piglet model of NE treated with HT that a MgSO4 bolus and infusion is a safe regimen to raise and maintain supra-physiological magnesium serum levels. Combined Mg+HT therapy significantly reduced overall neuronal cell death and increased numbers of myelin-producing oligodendrocytes. We did not, however, demonstrate significant improvement in aEEG score or MRS biomarkers, indicating that the overall neuroprotective benefit of combined Mg+HT is incremental and therefore unlikely to translate into substantive improvement in clinical trials. However, such an incremental effect with a good safety profile justifies further preclinical studies of MgSO4 in combination with complementary therapies in the future.
References
Global Burden of Disease Pediatrics Collaboration et al.Global and national burden of diseases and injuries among children and adolescents between 1990 and 2013: findings from the Global Burden of Disease 2013 Study. JAMA Pediatr. 170, 267–287 (2016).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy (Review). Cochrane Database Syst. Rev. CD003311 (2013).
Shankaran, S. et al. Effect of depth and duration of cooling on deaths in the NICU among neonates with hypoxic ischemic encephalopathy: a randomized clinical trial. JAMA 312, 2629–2639 (2014).
Doyle, L. W., Anderson, P. J., Haslam, R., Lee, K. J. & Crowther, C. School-age outcomes of very preterm infants after antenatal treatment with magnesium sulfate vs placebo. JAMA 312, 1105–1113 (2014).
McKee, J. A. et al. Magnesium neuroprotection is limited in humans with acute brain injury. Neurocrit. Care 2, 342–351 (2005).
Westermaier, T., Zausinger, S., Baethmann, A. & Schmid-Elsaesser, R. Dose finding study of intravenous magnesium sulphate in transient focal cerebral ischemia in rats. Acta Neurochir. 147, 525–532 (2005).
Galinsky, R. et al. Magnesium is not consistently neuroprotective for perinatal hypoxia-ischemia in term-equivalent models in preclinical studies: a systematic review. Dev. Neurosci. 36, 73–82 (2014).
Penrice, J. et al. Magnesium sulfate after transient hypoxia-ischemia fails to prevent delayed cerebral energy failure in the newborn piglet. Pediatr. Res. 41, 443–447 (1997).
Tagin, M., Shah, P. S. & Lee, K.-S. Magnesium for newborns with hypoxic-ischemic encephalopathy: a systematic review and meta-analysis. J. Perinatol. 33, 663–669 (2013).
Lamhot, V. B. et al. Magnesium sulfate prevents maternal inflammation-induced impairment of learning ability and memory in rat offspring. Am. J. Obstet. Gynecol. 213, 851.e1–851.e8 (2015).
Jenkins, D. D. et al. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Cereb. Blood Flow Metab. 32, 1888–1896 (2012).
Zhu, H., Meloni, B. P., Bojarski, C., Knuckey, M. W. & Knuckey, N. W. Post-ischemic modest hypothermia (35C) combined with intravenous magnesium is more effective at reducing CA1 neuronal death than either treatment used alone following global cerebral ischemia in rats. Exp. Neurol. 193, 361–368 (2005).
Robertson, N. J. et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain 136, 90–105 (2013).
Vanhamme, L., van den Boogaart, A. & Van Huffel, S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J. Magn. Reson. 129, 35–43 (1997).
Hellstrom-Westas, L., Rosen, I., de Vries, L. S. & Greisen, G. Amplitude-integrated EEG classification and interpretation in preterm and term infants. Neoreviews 7, e76–87 (2006).
Bale, G., Elwell, C. E. & Tachtsidis, I. From Jöbsis to the present day: a review of clinical near-infrared spectroscopy measurements of cerebral cytochrome-c-oxidase. J. Biomed. Opt. 21, 091307 (2016).
Bainbridge, A. et al. Brain mitochondrial oxidative metabolism during and after cerebral hypoxia-ischemia studied by simultaneous phosphorus magnetic-resonance and broadband near-infrared spectroscopy. Neuroimage 102, 173–183 (2014).
Möller, J. C. et al. Regulation of thrombospondin in the regenerating mouse facial motor nucleus. Glia 17, 121–132 (1996).
Rahman, S. et al. Multicenter randomized controlled trial of therapeutic hypothermia plus magnesium sulfate versus therapeutic hypothermia plus placebo in the management of term and near-term infants with hypoxic ischemic encephalopathy (The Mag Cool study): a pilot study. J. Clin. Neonatol. 4, 158–163 (2015).
Wong, G. K. C., Lam, C. W. K., Chan, M. T. V., Gin, T. & Poon, W. S. The effect of hypermagnesemic treatment on cerebrospinal fluid magnesium level in patients with aneurysmal subarachnoid hemorrhage. Magnes. Res. 22, 60–65 (2009).
Morris, M. E. Brain and CSF magnesium concentrations during magnesium deficit in animals and humans: neurological symptoms. Magnes. Res. 5, 303–313 (1992).
Li, L.-X., Campbell, K., Zhao, S., Knuckey, N. W. & Meloni, B. P. Comparison of the efficacy of mild hypothermia (35 °C) and moderate hypothermia (33 °C), alone or combined with magnesium treatment, when commenced 2 or 4 h after global cerebral ischemia in rats. Ther. Hypothermia Temp. Manag. 1, 151–158 (2011).
Dewar, D., Underhill, S. M. & Goldberg, M. P. Oligodendrocytes and ischemic brain injury. J. Cereb. Blood Flow. Metab. 23, 263–274 (2003).
Galinsky, R. et al. Magnesium sulfate reduces EEG activity but is not neuroprotective after asphyxia in preterm fetal sheep. J. Cereb. Blood Flow Metab. 37, 1362–1373 (2017).
Charriaut-Marlangue, C., Besson, V. C. & Baud, O. Sexually dimorphic outcomes after neonatal stroke and hypoxia-ischemia. Int. J. Mol. Sci. 19, E61 (2018).
Cho, B. B. & Toledo-Pereyra, L. H. Caspase-independent programmed cell death following ischemic stroke. J. Investig. Surg. 21, 141–147 (2008).
Abraham, M. C. & Shaham, S. Death without caspases, caspases without death. Trends Cell Biol. 14, 184–193 (2004).
McComb, S., Mulligan, R. & Sad, S. Caspase-3 is transiently activated without cell death during early antigen driven expansion of CD8+ T cells in vivo. PLoS ONE 5, e15328 (2010).
Northington, F. J., Chavez-Valdez, R. & Martin, L. J. Neuronal cell death in neonatal hypoxia-ischemia. Ann. Neurol. 69, 743–758 (2011).
Pang, R. et al. 1H MRS Lactate/N-acetylaspartate is associated with whole brain cell death and inflammation in a piglet model of perinatal asphyxia. Paediatr. Acad. Soc. Abstr. E-PAS2019:4153.49 (2019).
Merchant, N. & Azzopardi, D. Early predictors of outcome in infants treated with hypothermia for hypoxic-ischaemic encephalopathy. Dev. Med. Child Neurol. 57, 8–16 (2015).
Thoresen, M., Hellström-Westas, L., Liu, X. & de Vries, L. S. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics 126, e131–e139 (2010).
Bennet, L. et al. Time and sex dependent effects of magnesium sulphate on post-asphyxial seizures in preterm fetal sheep. J. Physiol. 596, 6079–6092 (2018).
Torolira, D., Suchomelova, L., Wasterlain, C. G. & Niquet, J. Phenobarbital and midazolam increase neonatal seizure-associated neuronal injury. Ann. Neurol. 82, 115–120 (2017).
Thayyil, S. et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: a meta-analysis. Pediatrics 125, e382–e395 (2010).
Robertson, N. J., Thayyil, S., Cady, E. B. & Raivich, G. Magnetic resonance spectroscopy biomarkers in term perinatal asphyxial encephalopathy: from neuropathological correlates to future clinical applications. Curr. Pediatr. Rev. 10, 37–47 (2014).
Sugimoto, J. et al. Magnesium decreases inflammatory cytokine production: a novel innate immunomodulatory mechanism. J. Immunol. 188, 6338–6346 (2012).
Tam Tam, H. B. et al. Magnesium sulfate ameliorates maternal and fetal inflammation in a rat model of maternal infection. Am. J. Obstet. Gynecol. 204, 364.e1–8 (2011).
Faulkner, S. et al. Xenon augmented hypothermia reduces early lactate/N-acetylaspartate and cell death in perinatal asphyxia. Ann. Neurol. 70, 133–150 (2011).
Thoresen, M. et al. Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr. Res. 37, 667 (1995).
Acknowledgements
We would like to thank Debbie Kraus for her support in statistical analysis. This study was funded by Action Medical Research for Children (GN2295). This work was undertaken at University College London Hospital and University College London, which received a proportion of funding from the UK Department of Health’s National Institute for Health Research Biomedical Research Centers funding scheme.
Author information
Authors and Affiliations
Contributions
The manuscript was authored by I.L. and edited by N.J.R. Experiments were performed by I.L., C.M., A.A.-B. and K.M. Immunohistochemistry staining was undertaken by M.H.; counting was performed by I.L., C.M. and K.M. NIRS data was collected with support from P.K., C.B. and I.T. MRS data acquisition was led by X.G. All co-authors have reviewed this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lingam, I., Meehan, C., Avdic-Belltheus, A. et al. Short-term effects of early initiation of magnesium infusion combined with cooling after hypoxia–ischemia in term piglets. Pediatr Res 86, 699–708 (2019). https://doi.org/10.1038/s41390-019-0511-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0511-8
This article is cited by
-
Neuroprotective therapies in the NICU in preterm infants: present and future (Neonatal Neurocritical Care Series)
Pediatric Research (2023)
-
New possibilities for neuroprotection in neonatal hypoxic-ischemic encephalopathy
European Journal of Pediatrics (2022)
-
Hypothermia is not therapeutic in a neonatal piglet model of inflammation-sensitized hypoxia–ischemia
Pediatric Research (2022)
-
High-Dose Melatonin and Ethanol Excipient Combined with Therapeutic Hypothermia in a Newborn Piglet Asphyxia Model
Scientific Reports (2020)
-
Magnesium sulfate: a last roll of the dice for anti-excitotoxicity?
Pediatric Research (2019)