Abstract
Background
We aimed at investigating whether early lung mechanics in non-intubated infants below 32 weeks of gestational age (GA) are associated with respiratory outcome.
Methods
Lung mechanics were assessed by the forced oscillation technique using a mechanical ventilator (Fabian HFOi, ACUTRONIC Medical Systems AG, Hirzel, Switzerland) that superimposed small-amplitude oscillations (10 Hz) on a continuous positive airway pressure. Measurements were performed during regular tidal breathing using a face mask on days 2, 4, and 7 of life. Respiratory system resistance (Rrs) and reactance (Xrs) were computed from flow and pressure.
Results
One hundred and seventy-seven measurements were successfully performed in 68 infants. Infants had a mean (range) GA of 29.3 (24.1–31.7) weeks and a birth weight of 1257 (670–2350)g. Xrs was associated with the duration of respiratory support (R2 = 0.39, p < 0.001). A multilevel regression model, including Xrs and GA, explained the duration of respiratory support better than GA alone (R2 = 0.51 vs. 0.45, p = 0.005, likelihood ratio test).
Conclusion
Assessment of Xrs in the first week of life is feasible and improves prognostication of respiratory outcome in very preterm infants on noninvasive respiratory support.
Similar content being viewed by others
Introduction
Very preterm infants, i.e., those born before 32 weeks of gestational age (GA), often develop respiratory distress. In some of these infants, the lung disease gradually improves toward the end of the first week of life, while others require prolonged respiratory support and oxygen supplementation, both of which are associated with protracted neonatal intensive care unit (NICU) stay and increased risk of developing bronchopulmonary dysplasia (BPD).1
Early identification of infants at high risk of requiring prolonged respiratory support may facilitate timely targeting of therapeutic interventions. Several authors reported that preterm infants who develop BPD have abnormal lung mechanics in the first week of life,2,3,4,5,6,7,8,9,10,11,12 but the results are conflicting as to which lung function parameter has the best prognostic value for medium-term pulmonary outcome. Some studies suggested that airway resistance is the best predictor,3,9,10,11 being higher in infants who later developed BPD, others reported that low compliance was a stronger predictor of poor respiratory outcome.2,4,5,6,7,8,12
The forced oscillation technique (FOT) measures respiratory system resistance (Rrs) and reactance (Xrs), the latter representing the elastic properties of the respiratory system.13 The advantages of FOT over other lung function techniques in preterm infants are that it (i) can be applied during invasive and noninvasive respiratory support modes, (ii) can be utilized during regular spontaneous breathing, and (iii) is readily available at the bedside. In intubated infants receiving mechanical ventilation, Rrs and Xrs discriminate infants with healthy lungs from those with respiratory distress or evolving BPD.14 Moreover, in intubated infants below 28 weeks of GA, the assessment of Xrs on the first day of life improved prognostication of respiratory outcomes compared with clinical parameters.4
The studies referenced above were all conducted in intubated and mechanically ventilated patients. However, most preterm infants nowadays receive noninvasive respiratory support, predominantly nasal continuous positive airway pressure (CPAP). The prognostic value of FOT in preterm infants under these conditions is unknown.
The aims of this study were in non-intubated preterm infants (e.g., on nasal CPAP, high-flow nasal cannula, or off respiratory support) below 32 weeks of GA, (1) to evaluate the feasibility of FOT measurements, (2) to prospectively and longitudinally describe lung mechanics by FOT during the first week of life, and (3) to investigate whether early lung mechanics are associated with respiratory outcome in the NICU.
Methods
Study design
This was a prospective, observational single-center study conducted in the NICU of the University Children’s Hospital Basel UKBB, Basel, Switzerland. The study was approved by the local ethics committee (EKNZ 233/13) and written informed consent was obtained from all parents prior to enrollment.
Patients
Very preterm infants (<32.0 weeks of GA) not requiring endotracheal ventilation were eligible for inclusion into this study if they had no major congenital malformations. Respiratory support was managed at the discretion of the attending neonatal consultant and withdrawn according to standard operating procedures of our NICU (acceptable work of breathing, defined as no grunting and no severe recessions to achieve tolerable blood gases; fraction of inspired oxygen <0.3 to achieve preductal oxygen saturation of 87–95%; no bradycardia <100/min or manual stimulation for apnea >20 s for at least 24 h in non-intubated infants).
Lung function measurements
Lung mechanics were assessed by FOT using a mechanical ventilator (Fabian HFOi, ACUTRONIC Medical Systems AG, Hirzel, Switzerland) that already implements FOT during invasive mechanical ventilation. Sinusoidal pressure oscillations (5-cm H2O, 10 Hz) were superimposed on spontaneous breathing during CPAP by driving the expiratory valve with an oscillatory signal, using specifically designed software provided by the manufacturer. Pressure and flow were measured at the Y-piece, using the pressure sensor and the hot wire anemometer of the ventilator. Raw pressure and flow signals were sampled at 250 Hz and exported to a personal computer for offline analysis. Information on methodological details of data processing and the algorithms we used to compensate for leaks and for the compliance of the gas inside the face mask (required when applying FOT during noninvasive respiratory support) can be found in Supplementary Material (online).
Measurements were performed on days 2, 4, and 7 of life using a face mask during regular tidal breathing. Infants were either off respiratory support or on noninvasive respiratory support (CPAP, HFNC, and NIPPV) at the time of the measurement. For the FOT measurement, they were connected to the study ventilator, operating in a dual-limb CPAP mode that incorporated the expiratory valve in the flow circuit. The fraction of inspired oxygen on the study ventilator was set to pre-study levels and adjusted during the measurement if needed. Pulse-oximetric oxygen saturation (SpO2) limits were kept at pre-study levels (87–95% in infants on supplemental oxygen, 87–100% in those without supplemental oxygen). Lung mechanics were assessed sequentially at CPAP levels of 3, 5, and 7-cm H2O—a pressure range not exceeding clinical practice. Each pressure was applied for 1 min and followed by FOT lung function for 30 s according to European Respiratory Society (ERS)/American Thoracic Society (ATS) standards.15 Infants were studied in supine position during behaviorally defined quiet natural sleep or quiet awake. For each infant, the face mask providing the best seal was used (size 00, Dräger Medical, Lübeck, Germany; size 0 or 1, Homedica AG, Hünenberg, Switzerland). Heart rate and oxygen saturation were monitored continuously by SpO2 (Masimo SET, Irvine, CA).
Data analysis
We computed Rrs and Xrs from pressure and flow data using a least-squares algorithm. Data quality control prior to calculation of Rrs and Xrs included manually selecting the maximum number of breaths (minimum three breaths) with a stable breathing pattern and without obvious artifacts. Within-breath analysis of Rrs and Xrs allowed for separation of inspiratory and expiratory values. Only inspiratory values were used for the purpose of this study, as they are less sensitive to artifacts introduced by expiratory flow limitation or vocal cord adduction. For each lung function measurement, the Rrs and Xrs values at the CPAP level that maximized Xrs were included in the regression models.
Sample size estimates and statistical analysis
Aiming for 80% power at the 5% significance level, we calculated a minimum required sample size of n = 67 for a multiple linear regression model with two independent continuous predictor variables of medium-effect size (f2 = 0.15).16 Baseline demographics of study participants were extracted from medical records. Weight z-scores were computed using revised Fenton growth charts.17 One-way ANOVA for repeated measurements with post hoc Tukey tests (or an equivalent nonparametric test) was used to compare lung mechanics at different CPAP levels. Rrs and Xrs were compared in infants who received respiratory support for <7 days (group 1) vs. 7–28 days (group 2) vs. ≥28 days (group 3), using two-way ANOVA for repeated measurements with group and study day as factors. Predefined outcomes included duration of respiratory support (endotracheal ventilation+CPAP+high-flow nasal cannula) and duration of supplemental oxygen therapy. We performed linear regression analyses to assess associations between outcomes and potential factors. Considered factors included lung mechanics over the first week of life, GA, birth weight, birth weight z-score, sex, surfactant treatment, and treatment with antenatal corticosteroids. To account for clustering on an individual level, we used multilevel, multivariable modeling, given that repeated measurements were analyzed. We first performed univariable regression analysis to determine potential associations of factors with outcomes. Then, we built multivariable, multilevel linear regression models and did stepwise backward elimination of factors that were not significantly associated with outcomes. Because of collinearity between GA and birth weight, birth weight z-score was used in the multilevel models. Nested models were compared using the likelihood ratio test. To evaluate whether the presence of missing data (infant deemed clinically unstable by the attending physician, intermittent endotracheal intubation, technical problems during data acquisition, and unavailability of the study team) influenced the results of our regression models, we compared the factors identified using all patients with those identified using only patients who had all three measurements. Statistical analysis was performed using Stata software (StataCorp. 2009. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP).
Results
Between October 2016 and June 2018, a total of 181 FOT measurements were performed in 70 infants. One hundred and seventy-seven (98%) measurements in 68 (97%) infants were of satisfactory technical quality. Measurements were highly feasible, well tolerated, and no adverse events (including hypoxia, bradycardia, and pneumothorax) were reported during the study.
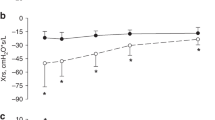
Study participants had a mean (range) GA of 29.3 (24.1–31.7) weeks and a mean (range) birth weight of 1257 (670–2350) g. Baseline characteristics of participants are summarized in Table 1. All measurements were done while the infants did not require tracheal ventilation. However, 31 patients (45%) were intubated and mechanically ventilated at some stage of the clinical course. In total, 29 infants were ventilated before the study (median (IQR) duration = 19 (9–27) h), 3 infants were ventilated between day 2 and day 4 measurements (median (IQR) duration = 47 (44–47)h), and 2 infants were re-intubated after the study (duration 8 and 21 days). After excluding infants with missing data, we used data from 49 infants (mean (range) GA of 29.6 (27.1–31.5) weeks, mean (range) birth weight of 1292 (670–2350) g) to evaluate the trend of lung mechanics over the first week of life. The CPAP level maximizing Xrs was 5-cm H2O in 56, 46, and 54% of the infants on days 2, 4, and 7, respectively, and 7- cm H2O in the remaining cases. At 3-cm H2O of CPAP, Xrs was always lower than at the higher CPAP levels. At the CPAP level that maximized Xrs, the mean (SD) Xrs was –26.13 (12.43) cm H2O*s/L on day 2, –22.47 (10.09) cm H2O*s/L on day 4, and –21.36 (7.60) cm H2O*s/L on day 7. The mean (SD) Rrs was 32.44 (17.05) cm H2O*s/L on day 2, 30.59 (13.56) cm H2O*s/L on day 4, and 30.59 (9.39) cm H2O*s/L on day 7. Figure 1 shows the trend of Rrs and Xrs, at the CPAP level that maximized Xrs, over the first week of life stratified by duration of respiratory support (<7 days (group 1, n = 18) vs. 7–28 days (group 2, n = 14) vs. ≥28 days (group 3, n = 17)). Infants who received respiratory support for greater than or equal to 28 days had significantly lower Xrs than infants with shorter respiratory support (p < 0.001 vs. groups 1 and 2, two-way ANOVA).
Resistance (Rrs) and reactance (Xrs) over the first week of life in infants who received respiratory support for (1) < 7 days (closed circles, solid line, n = 18, mean (SD) GA = 30.5 (1.1) weeks), (2) 7–28 days (open circles, dashed line, n = 14, mean (SD) gestational age (GA) = 29.9 (0.8) weeks), and (3) ≥ 28 days (triangles, dotted line, n = 17, mean (SD) GA = 28.4 (1.2) weeks). For each lung function test, the Rrs and Xrs values at the CPAP level that maximized Xrs were considered. Data are expressed as means and SD. *p < 0.05 vs. respiratory support ≥ 28 days within the same day. **p < 0.05 vs. day 2 within the same group
The results of univariable and multivariable regression analyses (n = 68) are summarized in Tables 2 and 3, respectively. In univariable analysis, duration of respiratory support was significantly associated with Xrs (R2 = 0.39, p < 0.001), GA (R2 = 0.45, p < 0.001), birth weight (R2 = 0.25, p < 0.001), and surfactant administration (R2 = 0.17, p < 0.001). Duration of oxygen therapy was significantly associated with Xrs (R2 = 0.19, p < 0.001), GA (R2 = 0.32, p < 0.001), birth weight (R2 = 0.12, p = 0.004), and surfactant administration (R2 = 0.12, p = 0.003). Rrs was significantly but weakly associated with the duration of respiratory support (R2 = 0.09, p = 0.018) and not associated with the duration of oxygen therapy (R2 = 0.04, p = 0.119). Outcomes were not associated with any of the other considered factors.
The best multilevel, multivariable regression model (R2 = 0.51) estimating the duration of respiratory support included GA (beta (95% CI) = –4.94 (–7.50, –2.38), p < 0.001) and Xrs (beta (95% CI) = –0.62 (–1.07, –0.17), p = 0.008), while the best model (R2 = 0.32) estimating duration of oxygen therapy included only GA (beta (95% CI) = –2.92 (–3.96, –1.87), p < 0.001). A regression model including Xrs and GA explained the duration of respiratory support better than GA alone (p = 0.005, likelihood ratio test). Figure 2 shows the association of the duration of respiratory support with GA and Xrs.
Discussion
The main findings of this study are: (1) Measurements of Rrs and Xrs by FOT during noninvasive respiratory support were feasible and well tolerated in preterm infants during the first week of life; (2) low Xrs and high Rrs values obtained from these measurements were associated with longer duration of respiratory support; (3) low Xrs values were associated with longer supplemental oxygen requirement; (4) Xrs added significantly to clinical parameters (GA) in estimating the duration of respiratory support.
Comparison with previous literature
Hantos et al. have previously described respiratory mechanics by FOT through a noninvasive interface in the first few days of life and during natural sleep.18 However, in this study, lung mechanics were not associated with any respiratory outcome, as measurements were conducted in healthy-term infants not requiring respiratory support. Other studies investigated the prognostic value of lung mechanics in the first week of life in infants with respiratory distress.2,3,4,5,6,7,8,9,10,11,12,19,20,21 Some12,19,20,21 found that lung mechanics do not add significantly to clinical variables in the estimation of poor respiratory outcome. Other authors demonstrated that early lung mechanics are associated with the duration of respiratory support and the development of a chronic lung disease,2,3,4,5,6,7,8,9,10,11 but there are conflicting results about which parameter has the strongest prognostic power. Comparison of our results with published data is difficult due to differences in terms of population characteristics (level of respiratory support, use of exogenous surfactant) and methods (technique used to measure lung mechanics, definition of respiratory outcomes, and confounders included in the regression model). While in most studies lung mechanics were assessed using either the single-occlusion technique,2,7,9,10,11,19,21 or the the interrupter technique,7 or by fitting the equation of motion of the respiratory system,3,5,6,7,12,20 we measured lung mechanics using FOT. The latter has several advantages over other techniques. First, it can be applied without disconnecting the patient from the ventilator circuit and does not interfere with the ventilator. Second, it does not require measuring transpulmonary pressure with an esophageal catheter to accurately estimate lung mechanics when the subject is breathing spontaneously. Third, there is no need to invoke the Hering–Breuer inflation reflex to obtain respiratory muscle relaxation.
We demonstrated that the association of respiratory support duration with Xrs was stronger than that with Rrs. Our results are in agreement with Veneroni et al., who, in mechanically ventilated preterm infants, found that Xrs but not Rrs was associated with the duration of mechanical ventilation and the development of BPD.4 Since Rrs mainly accounts for the resistance of the upper and central airways and Xrs accounts for the elastic and inertial properties of the respiratory system, our results are also in agreement with those studies that found that early measurement of dynamic lung compliance is strongly associated with poor respiratory outcome in preterm infants with respiratory distress syndrome requiring mechanical ventilation.5,6,7 Further, Xrs better reflects the underlying, prematurity-related lung disease, which is primarily characterized by poor alveolarization, reduced lung volume, and low compliance of the lung, due to preterm birth and surfactant deficiency. Nevertheless, caution is required in the interpretation of these results, as the vast majority of infants in our cohort had mild-to-moderate lung disease (median (range) duration of endotracheal ventilation, 0 (0–23) days; median (range) duration of supplemental oxygen requirement, 0 (0, 37) days; no infants diagnosed with BPD at 36 weeks of postmenstrual age; no infants discharged home on supplemental oxygen). Rrs may well be elevated in preterm infants with severe lung disease who require prolonged endotracheal ventilation, as subsequent airway inflammation and/or ventilator-induced lung injury may lead to decreased airway diameter.9 Another possible reason for the low prognostic power of Rrs is that during CPAP, Rrs may be affected by several factors other than the underlying lung disease. Specifically, Rrs is more sensitive than Xrs to the breathing pattern, the presence of a nasogastric tube, and shunting effect of the interface. On the other hand, the pressure applied at the airway opening may significantly affect Xrs and compliance. Thus, we standardized lung volume recruitment by performing measurements at three different CPAP levels and considering, for each measurement, the pressure level that maximized Xrs.
We found a stronger association of Xrs with the duration of respiratory support vs. the duration of oxygen requirement. This is plausible, as respiratory distress syndrome in preterm infants reduces lung volume and results in poor lung mechanics requiring positive pressure support. Thus, prolonged oxygen requirement may not be necessary, if lung volumes are optimized using CPAP or a high-flow nasal cannula, at least in patients with mild-to-moderate lung disease.
Typically, lung mechanics measurements in infants and children are normalized to body size.6,9 Reference ranges for Rrs and Xrs in older children and adults are a nonlinear function of sex, length, and body weight. Since such reference equations for infants are not available, we used absolute values of Rrs and Xrs and adjusted models for GA, the latter being highly colinear with body weight in the first week of life. Although the results were not significantly associated with body weight after adjusting for GA, FOT measurements in infants are very likely related to body size; thus, it is important to establish those reference equations for infants tested at various ages in future studies.
Strengths and limitations of the study
The strengths of this study include the recruitment of preterm infants from 24 weeks of GA who received contemporary perinatal care, including prenatal corticosteroids, postnatal surfactant, and lung-protective (volume-targeted) ventilation followed by CPAP; the noninvasive nature of repeated FOT measurements; and close adherence to ERS/ATS guidelines. The limitations include a single-center study design that precludes the generalizability of the results, due to potential differences between NICUs in patient populations and respiratory management. Further, we restricted the inclusion criteria to non-intubated infants; thus, spectrum bias cannot be excluded. Although it is likely that Xrs in intubated infants is associated with the duration of respiratory support in a similar fashion to that observed in non-intubated infants, we had designed the study specifically for infants on noninvasive respiratory support, because in this setting, it is extremely difficult for clinicians to monitor lung function; therefore, new bedside tools are particularly required for those conditions. Besides, combining measurements of intubated and non-intubated infants in a single regression model would have required validated equations to account for different interfaces and a big sample size to correct for the different respiratory support modes.
Clinical implications
The results of this study are encouraging, as FOT was significantly associated with duration of respiratory support after adjusting for GA, suggesting that it provides additional useful information on the severity of lung disease. Arguably, the effect size of FOT is modest, as the additional amount of variance, that could be explained upon adding FOT to the model, was only 6% (increase in R2 from 0.45 to 0.51). Its utility is also confirmed by the fact that Xrs was significantly associated with the maximal level of respiratory support required during hospitalization (invasive mechanical ventilation, CPAP, high-flow nasal cannula, or none). Furthermore, the single Xrs measurement that was most strongly associated with the duration of respiratory support was the one performed on day 2 of life, i.e., FOT may contribute to estimating the time course of respiratory disease in the NICU at a very early postnatal age. However, considerable challenges remain until FOT can be applied in non-intubated infants during routine clinical care, as leaks, irregular tidal breathing, and different interfaces (e.g., different types and sizes of face or nasal masks) have a strong influence on FOT outcomes. In this study, two investigators were present at the bedside and performed measurements and manual quality control. Also, raw data were analyzed and processed offline. Thus, methodology of measurement, quality control, and ventilator software need to be improved and simplified, to allow for reliable clinical use of FOT in non-intubated infants in the future.
Implications for future research
Based on this study, further research of FOT in infants is required, including the development of reference equations related to body size during a period of very rapid growth, cross-sectional comparison of FOT values in intubated vs. non-intubated infants, intra-individual changes of FOT values pre- and post extubation, the use of FOT under conditions of severe neonatal lung disease, and the effect of different mask interfaces when using FOT during noninvasive respiratory support. Studies addressing the clinical utility of FOT in guiding specific respiratory treatments (e.g., surfactant administration) are also warranted.
Conclusion
FOT measurements in the first week of life are feasible in very preterm infants managed with noninvasive respiratory support. Xrs adds to clinical parameters in estimating the duration of respiratory support. Further research is needed to generalize our results and to allow for clinical use of FOT in non-intubated infants.
References
Laughon, M. et al. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics 123, 1124–1131 (2009).
Simbruner, G., Coradello, H., Lubec, G., Pollak, A. & Salzer, H. Respiratory compliance of newborns after birth and its prognostic value for the course and outcome of respiratory disease. Respiration 43, 414–423 (1982).
Goldman, S. L. et al. Early prediction of chronic lung disease by pulmonary function testing. J. Pediatr. 102, 613–617 (1983).
Veneroni, C., Wallström, L., Sindelar, R. & Dellacaʼ, R. L. Oscillatory respiratory mechanics on the first day of life improves prediction of respiratory outcomes in extremely preterm newborns. Pediatr. Res. 85, 312–317 (2018).
Graff, M. A. et al. Compliance measurement in respiratory distress syndrome: The prediction of outcome. Pediatr. Pulmonol. 2, 332–336 (1986).
Bhutani, V. K. & Abbasi, S. Relative likelihood of bronchopulmonary dysplasia based on pulmonary mechanics measured in preterm neonates during the first week of life. J. Pediatr. 120, 605–613 (1992).
Freezer, N. J. & Sly, P. D. Predictive value of measurements of respiratory mechanics in preterm infants with HMD. Pediatr. Pulmonol. 16, 116–123 (1993).
Kavvadia, V., Greenough, A. & Dimitriou, G. Early prediction of chronic oxygen dependency by lung function test results. Pediatr. Pulmonol. 29, 19–26 (2000).
Lui, K., Lloyd, J., Ang, E., Rynn, M. & Gupta, J. M. Early changes in respiratory compliance and resistance during the development of bronchopulmonary dysplasia in the era of surfactant therapy. Pediatr. Pulmonol. 30, 282–290 (2000).
Choukroun, M. L., Tayara, N., Fayon, M. & Demarquez, J. L. Early respiratory system mechanics and the prediction of chronic lung disease in ventilated preterm neonates requiring surfactant treatment. Biol. Neonate 83, 30–35 (2003).
Snepvangers, Y., de Winter, J. P., Burger, H., Brouwers, H. & van der Ent, C. K. Respiratory outcome in preterm ventilated infants: importance of early respiratory system resistance. Eur. J. Pediatr. 163, 378–384 (2004).
Williams, O., Dimitriou, G., Hannam, S., Rafferty, G. F. & Greenough, A. Lung function and exhaled nitric oxide levels in infants developing chronic lung disease. Pediatr. Pulmonol. 42, 107–113 (2007).
Dubois, A. B., Brody, A. W., Lewis, D. H. & Burgess, B. F. Oscillation mechanics of lungs and chest in man. J. Appl Physiol. 8, 587–594 (1956).
Dellacà, R. L. et al. Relationship between respiratory impedance and positive end-expiratory pressure in mechanically ventilated neonates. Intensive Care Med. 39, 511–519 (2013).
Bates, J. H., Schmalisch, G., Filbrun, D. & Stocks, J. Tidal breath analysis for infant pulmonary function testing. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. European Respiratory Society/American Thoracic Society. Eur. Respir. J. 16, 1180–1192 (2000).
Cohen Jacob, Cohen Patricia, West Stephen G. ALS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences - Jacob Cohen, Patricia Cohen, Stephen G. West, Leona S. Aiken - Google Libri. Third edit. 2002.
Fenton, T. R. & Kim, J. H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 13, 59 (2013).
Hantos, Z. et al. Assessment of respiratory mechanics with forced oscillations in healthy newborns. Pediatr. Pulmonol. 50, 344–352 (2015).
Kirpalani, H., Schmidt, B., Gaston, S., Santos, R. & Wilkie, R. Birthweight, early passive respiratory system mechanics, and ventilator requirements as predictors of outcome in premature infants with respiratory failure. Pediatr. Pulmonol. 10, 195–198 (1991).
van Lierde, S., Smith, J., Devlieger, H. & Eggermont, E. Pulmonary mechanics during respiratory distress syndrome in the prediction of outcome and differentiation of mild and severe bronchopulmonary dysplasia. Pediatr. Pulmonol. 17, 218–224 (1994).
May, C. et al. Prediction of bronchopulmonary dysplasia. Arch. Dis. Child Fetal Neonatal Ed. 96, F410–F416 (2011).
Acknowledgements
This work was supported by the Swiss Kommission für Technologie und Innovation (KTI, Grant-Nr. 25768.2 PFLS). ACUTRONIC Medical Systems AG provided the device and disposables to perform the measurements. Dr. Zannin was supported in this work by a European Respiratory Society Long-Term Fellowship (LTRF 2015-4459). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Author information
Authors and Affiliations
Contributions
All authors substantially contributed to the conception and design of the study and to the interpretation of data. R.P.N. and E.Z. acquired the data, E.Z. performed data analysis, E.Z. wrote the first draft of the paper, all authors revised it critically, and approved the final version of the paper to be published.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zannin, E., Neumann, R.P., Dellacà, R. et al. Forced oscillation measurements in the first week of life and pulmonary outcome in very preterm infants on noninvasive respiratory support. Pediatr Res 86, 382–388 (2019). https://doi.org/10.1038/s41390-019-0432-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0432-6
This article is cited by
-
Oscillatory mechanics trajectory in very preterm infants: a cohort study
Pediatric Research (2023)
-
Combining lung ultrasound and oscillatory mechanics for assessing lung disease in very preterm infants
Pediatric Research (2023)
-
Oscillatory mechanics at birth for identifying infants requiring surfactant: a prospective, observational trial
Respiratory Research (2021)
-
Non-invasive forced oscillometry to quantify respiratory mechanics in term neonates
Pediatric Research (2020)