Abstract
Background
Propranolol is the preferred treatment for problematic proliferating infantile hemangioma (IH) by targeting the renin–angiotensin system (RAS) expressed by IH endothelium. (Pro)renin receptor (PRR) is a major component of the RAS associated with the canonical wnt signaling pathway. We proposed that activation of PRR by renin causes proliferation of IH.
Methods
The expression of PRR in IH tissue samples was investigated using immunohistochemical (IHC) staining and NanoString analysis. NanoString analysis was also used to confirm transcriptional expression of PRR in CD34-sorted proliferating IH-derived primary cell lines. MTT assay was utilized to determine the effect of exogenous renin on the number of viable IH cells. RT-qPCR was used to determine the effect of renin on the stem cell gene expression.
Results
NanoString analysis and IHC staining confirmed transcriptional and translational expression of PRR, which was localized to the non-endothelial and the endothelial IH cell populations. MTT assay demonstrated an increased number of viable IH cells by administration of renin and the effect was negated by the wnt receptor blocker dickkopf-1.
Conclusion
Our results present a model for renin-induced increased proliferation of IH cells through PRR acting via the wnt signaling pathway, which may account for accumulation of cells in IH during the proliferative phase of the tumor.
Similar content being viewed by others
Introduction
Infantile hemangioma (IH) is the most common tumor of infancy, affecting 4–10% of children.1,2,3 It is characterized by an initial rapid proliferation during infancy (proliferative phase), followed by gradual spontaneous regression over 1–5 years (involuting phase), with continual improvement until 6–12 years of age (involuted phase).4 There is a body of evidence supporting the stem cell basis of this hitherto enigmatic condition.5,6,7,8 Proliferating IH is composed of two broad subgroups of cellular phenotypes, with a relatively homogeneous CD34+ endothelial population and a more heterogeneous CD34− fraction, composed of pericytes and hematopoietic cells, with both fractions containing stem cells.9,10 We have also demonstrated the expression of embryonic stem cell (ESC) markers OCT4, SSEA-4, and pSTAT3 by the endothelium, and NANOG by cells within the interstitium, of proliferating IH.5
The renin–angiotensin system (RAS) is an endocrine system physiologically crucial to the maintenance of blood pressure and fluid homeostasis, and its dysregulation has been implicated in disease development, including IH.1,11 It has been previously proposed that angiotensin II, the downstream vasoactive peptide of RAS, is responsible for the proliferation of the stem cell-like blast colonies, predominantly via angiotensin II receptor 2,1 although angiotensin II receptor 1 has also been reported to be present.11
A significant component of the RAS is (pro)renin receptor (PRR), a transmembrane protein, with the ability to bind both the precursor, prorenin, and the active renin.12 Renin is a glycoproteolytic enzyme which catalyzes the conversion of angiotensinogen into angiotensin I.13 PRR has also been implicated in neovascularization of the choroidal plexus, independent of its role in RAS signaling.14 Interestingly, PRR has also been shown to form part of the wnt/frizzled receptor complex in association with organ development, cell–cell communication, and cellular proliferation, and its aberrant activation has also been implicated in diseases such as osteoporosis and cancer.15 Canonical wnt signaling (wnt/β-catenin) is initiated when the wnt ligand binds to the frizzled receptor and its co-receptor low-density lipoprotein receptor-related protein 5 and 6 (LRP5/6), subsequently stabilizing and translocating cytoplasmic β-catenin to the nucleus, where it activates a downstream transcriptional cascade.15,16 The secreted protein Dickkopf-1 (DKK1) functions as an inhibitor of the wnt/β-catenin signaling pathway by forming a complex with LRP5/6 and inducing endocytosis and its internalization from the cell surface.17 In 2010, Cruciat et al.18 reported that PRR is a component of the wnt receptor complex, functioning as an interface between LRP5/6 and the vacuolar H+-adenosine triphosphatase complex.
The role of PRR has been previously reported as being cell specific, with the resultant proliferation or neovascularization in vascular smooth muscle cells19,20 and endothelial cells,21 respectively.
In this study, we investigated the expression and localization of PRR in IH and its functional effect on proliferation, and the effect of blocking the wnt/β-catenin signaling pathway, following activation with exogenous renin. Furthermore, we investigated the effect of exogenous renin on the transcript expression of stem cell-associated genes22 in both the CD34+ and the CD34− cellular populations of proliferating IH-derived primary cell lines.
Materials and methods
Proliferating IH tissue samples and proliferating IH-derived primary cell lines
Proliferating IH tissue samples from 10 patients, with a mean age of 6.6 (range, 3–10) months (Suppl. Table S1), were obtained from the Gillies McIndoe Research Institute Tissue Bank for this study, which was approved by the Central Health and Disability Ethics Committee (Ref. 13CEN130).
Histochemical and immunohistochemical staining
Four-micrometer-thick formalin-fixed and paraffin-embedded sections of 10 IH tissue samples underwent hematoxylin and eosin (H&E) and 3,3-diaminobenzidine (DAB) immunohistochemical (IHC) staining, using the primary antibody for IH marker GLUT-13 (1:200; cat# 355A-16, Cell Marque, Rocklin, CA, USA) and PRR (1:100; cat# HPA003156, Sigma, St. Louis, MO, USA), as previously described.23 All DAB IHC-stained slides were mounted in Surgipath Micromount (Leica, Nussloch, Germany). To confirm co-expression of PRR and CD34 (ready-to-use; cat# PA0212, Leica, Newcastle, United Kingdom), 4 samples from the original cohort included in DAB IHC staining underwent immunofluorescence (IF) IHC staining. Vectafluor Excel anti-mouse 488 (ready-to-use; cat# VEDK2488, Vector Laboratories, Burlingames, CA, USA) and Alexa Fluor anti-rabbit 594 (1:500; cat# A21207, Life Technologies, Carlsbad, CA, USA) were used to detect each protein.
All IF IHC-stained slides were mounted in Vecta Shield Hardset mounting medium with 4′,6′-diamino-2-phenylindone (Vector Laboratories). All antibodies were diluted in Bond primary diluent (Leica). All DAB and IF IHC staining was performed using the Leica Bond Rx auto-stainer (Leica), as previously described.24
Human tissues used for positive controls for the primary antibodies were placenta for PRR.25 Negative controls for DAB IHC staining were performed on sections of IH tissue samples, using a matched isotype control for both mouse (ready-to-use; cat# IR750, Dako, Copenhagen, Denmark) and rabbit (ready-to-use; cat# IR600, Dako) primary antibodies, to determine the specificity of the amplification cascade. Negative controls for IF IHC staining were performed using sections of IH tissue samples, with the combined use of primary isotype mouse (ready-to-use; cat# IR750, Dako) and rabbit (ready-to-use; cat# IR600, Dako) antibodies.
Image analysis
DAB IHC-stained slides were viewed and imaged using an Olympus BX53 microscope fitted with an Olympus DP21 digital camera (Olympus, Tokyo, Japan). IF IHC-stained slides were viewed and imaged using an Olympus FV1200 biological confocal laser-scanning microscope and processed with CellSens Dimension 1.11 software using 2D deconvolution algorithm (Olympus).
Proliferating IH-derived primary cell lines
Primary cell lines derived from proliferating IH samples from 6 patients from the original cohort of 10 patients included in DAB IHC staining, were sorted into endothelial (CD34+) and non-endothelial (CD34−) populations, using the Dynabeads™ CD34 positive isolation kit (cat# 11301D, ThermoFisher Scientific) protocol. All cultures used in this study were within 10 passages.
Cell cultures were grown in 96-well (cat# TCP010096, Jet BioFil, Guangzhou, China) and 24-well (cat# TCO010024, Jet BioFil) plates and cultured in DMEM media with high glucose and containing pyruvate (cat# 10569010, ThermoFisher Scientific), supplemented with 10% fetal bovine serum (cat# 10091148, ThermoFisher Scientific), 5% mTeSR™1 (cat# 85850, Stemcell Technologies, Vancouver, BC, Canada), 1% penicillin–streptomycin (cat# 15140122, ThermoFisher Scientific), and 0.2% gentamicin/amphotericin B (cat# R01510, ThermoFisher Scientific). Cultures were maintained in a Heracell VIOS 160i incubator (cat# 51030285, ThermoFisher Scientific) with 5% CO2 at 37 °C.
Cell suspensions were plated to final counts of 5000 cells/well in 96-well plates and 20,000 cells/well in 24-well plates, in parallel and left to adhere for 24 h. The cells were starved for 4 h prior to treatment, by replacing the media with non-supplemented DMEM. Treatment involved recombinant human renin (cat# 4090-AS, R&D Systems, MN, USA) at concentrations of 0 (media only), 5, 10, or 15 nM alone, or in combination with recombinant human DKK1 (cat# 120-30, Peprotech, Rehovot, Israel), at a concentration of 0.5 µg/mL, according to the manufacturers' guidelines. Treatment duration was 20 h, and where DKK1 was used, this was added 30 min before the addition of renin. Following treatment, the media was removed and cells from the 24-well plates were immediately frozen at −80 °C, whereas the cells from the 96-well plates were analyzed for the number of viable cells using the MTT assay (cat# 211091, Abcam, Cambridge, United Kingdom).
Transcriptional analysis
Snap-frozen proliferating IH tissue samples from 6 patients and the matching CD34+ and CD34− sorted cells from the 6 proliferating IH-derived primary cell lines from the cohort of 10 patients used for DAB IHC staining were used to isolate total RNA for NanoString nCounter Gene Expression Assay (NanoString Technologies, Seattle, WA, USA), to investigate transcript expression of PRR and ESC markers OCT4 and NANOG, prior to treatment with renin and DKK1. RNA was extracted from snap-frozen tissues using RNeasy Mini Kit (Qiagen) and cells using RNeasy Micro Kit (Qiagen) and subjected to the NanoString nCounter gene expression assay by New Zealand Genomics Ltd. (Dunedin, New Zealand). To detect the expression of PRR and ESC markers OCT4 and NANOG that had been shown to be expressed by the endothelium and interstitial cells, respectively, in proliferating IH,5 probes (designed and manufactured by NanoString Technologies) for the genes encoding PRR (NM_005765.2), OCT4 (NM_002701.4), and NANOG (NM_024865.2) were used, along with the probe for the housekeeping gene GUSB (NM_00181.3) Raw data were analyzed by nSolver software (NanoString Technologies) using standard settings and were normalized against the housekeeping gene GUSB.
MTT assays
The MTT assays (cat# 211091, Abcam) were used to analyze viable CD34+ and CD34− sorted cells from the 6 proliferating IH-derived primary cell lines from the same cohort used for DAB IHC staining, which had been treated with renin and DKK1. All steps of the experiment were performed according to the manufacturer’s protocol. The MTT substrate was incubated at room temperature for 15 min, and absorbance at OD = 590 nm was immediately detected using the Varioskan plate reader (ThermoFisher Scientific).
RT-qPCR
Total RNA was extracted from the same CD34+ and CD34− sorted cells from the 6 proliferating IH-derived primary cell lines used for MTT analysis. Cells were lysed in 350 µL of lysis buffer RLT (cat# 79216, Qiagen, Hilden, Germany) and RNA extraction was performed using the RNeasy Micro Kit (Qiagen) and QIAcube (Qiagen). The quantity and quality of the RNA extracted were assessed using the NanoDrop 2000 Spectrophotometer (ThermoFisher Scientific). RT-qPCR was performed using the Rotor-Gene Q (Qiagen) and the Rotor-Gene Multiplex RT-PCR kit (Qiagen). The primer probes used were OCT4 (HS009993621_G1), NANOG (HS04399610_G1), and c-MYC (HS00153408_M1), all obtained from ThermoFisher Scientific (cat# 4331182). Threshold cycle values were compared against the housekeeping gene GAPDH (HS99999905_M1) (cat# 4351370, ThermoFisher Scientific) to obtain a ΔCT value. PC3 cell RNA extractions were used as a positive control. The specificity of the probes was confirmed using a no-template control.
Statistical analysis
Data obtained from MTT assays (n = 6) and RT-qPCR (n = 6) were subjected to paired t tests, using SPSS version 24 software, comparing the means of all replicates to a significance of p = 0.05.
Results
Histochemical and immunohistochemical staining
H&E of all 10 proliferating IH tissue samples showed the typical microvessels lined by plump endothelial cells with tiny lumens organized in lobules (Fig. 1a). DAB IHC staining demonstrated the expression of the IH marker GLUT-1 (Fig. 1b, brown) on the endothelium of the microvessels and PRR (Fig. 1c, brown) by the endothelium of the microvessels, and cells away from the microvessels, in all 10 proliferating IH tissue samples were examined. Human placenta used for positive control demonstrated the expected staining patterns for GLUT-1 (Fig. 1d, brown) and PRR (Fig. 1e, brown). Negative controls performed on proliferating IH samples using an IgG isotype (Fig. 1f) showed no staining.
Representative images of hematoxylin and eosin staining showing microvessels lined by plump endothelial cells with tiny lumens organized in lobules a, and 3,3-diaminobenzidine immunohistochemical staining of proliferating infantile hemangioma sections, showing the expression of GLUT-1 (b, brown) on the endothelium of the microvessels, and PRR (c, brown) on the endothelium of the microvessels and cells away from the microvessels. Positive staining for GLUT-1 (d, brown) and PRR (e, brown) in human placenta. A negative control by staining with an IgG isotype antibody f. Nuclei were counterstained with hematoxylin (a–f, blue). Original magnification: ×400
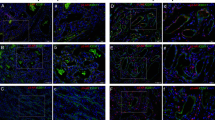
IF IHC staining was performed on 4 of the 10 proliferating IH tissue samples used for DAB IHC staining, in order to further determine the expression of PRR in relation to the CD34+ population. PRR (Fig. 2a, b, red) was expressed by both the CD34+ population (Fig. 2a, c, green, arrows) as well as the CD34− population (Fig. 2a, arrowheads) within the proliferating IH tissue samples. Minimal staining was present on the negative controls for PRR (Fig. 2d, red) and CD34 (Fig. 2e, green), confirming the specificity of the antibodies.
Representative immunofluorescence immunohistochemical-stained sections of proliferating infantile hemangioma tissue samples, demonstrating the expression of PRR (a, red) by the CD34+ cells (a, green, arrows), as well as the CD34− cells (arrowheads) with split images of PRR (b, red) and CD34 (c, green). A negative control of PRR (d, red) and CD34 (e, green) showing minimal staining, confirming the specificity of the antibodies. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (a–e, blue). Original magnification: ×400
NanoString mRNA analysis
NanoString mRNA analysis confirmed the presence of mRNA transcripts for PRR, OCT4, and NANOG, relative to the housekeeping gene GUSB, in all 6 snap-frozen proliferating IH tissue samples (Fig. 3a) and the 6 matched proliferating IH-derived primary cell lines (Fig. 3b). In both CD34+ and CD34− cell populations and in the IH tissues, OCT4 mRNA was found at a greater abundance than NANOG. PRR was expressed at a similar level in both cell populations, but at a far greater level in the tissue compared with the cells.
NanoString mRNA analysis of 6 snap-frozen proliferating infantile hemangioma tissue samples a and the matched CD34+ and CD34− sorted cells from 6 proliferating infantile hemangioma-derived primary cell lines b, demonstrating transcriptional expression of PRR, OCT4, and NANOG, normalized against the housekeeper gene GUSB. Error bars represent standard error of the mean
MTT assays
The MTT assays demonstrated a statistically significant increase in the number of viable CD34+ cells (Fig. 4a) treated with renin at 5 (p < 0.05), 10 (p < 0.05), and 15 nM (p < 0.01) concentrations relative to the controls. Furthermore, there was a statistically significant decrease in the number of viable CD34+ cells, when 10 nM renin was added in the presence of 0.5 µg/mL DKK1 compared with 10 nM renin alone (p < 0.05). No significant difference was found in any of the renin and DKK1 concentrations in the CD34− cells (Fig. 4b).
MTT assay analysis of CD34+ a and CD34− b sorted cells from 6 proliferating infantile hemangioma-derived primary cell lines, demonstrating proliferation under different renin concentrations and in the presence of the wnt signaling inhibitor DKK1. A statistically significant difference was observed in the CD34+ populations when comparing 0 nM with 5, 10, and 15 nM, and at 10 nM renin compared with when DKK1 was added. Error bars represent standard error of the mean. *p < 0.05; **p < 0.01
RT-qPCR
To determine whether the renin-induced proliferation in the CD34+ sorted cells from the proliferating IH-derived primary cell lines was due to an increase in stem cell-like properties, RT-qPCR was performed on cells grown in the same conditions as those analyzed by the MTT assay. In the CD34+ population (Fig. 5a), the stem cell markers OCT4, NANOG, and c-MYC were expressed in all cell lines, with no statistically significant difference in the expression observed across the different renin concentrations or between the renin and DKK1-treated cells, suggesting that the renin-induced proliferation was not directly associated with the stem cell nature of IH. A similar trend is shown in the CD34− population (Fig. 5b), with most genes having a higher average expression, potentially due to the more heterogeneous nature of the non-endothelial population.
Graphs showing average ΔCT values of duplicate RT-qPCR analyses performed on CD34+ a and CD34− b sorted cells from 6 proliferating infantile hemangioma-derived primary cell lines treated with renin and DKK1. ΔCT values were calculated by comparing CT values of the experimental genes with those of the housekeeping gene GAPDH. Error bars represent standard error of the mean
Discussion
A critical role for the RAS in the pathoetiology of IH has been proposed by us1,26 and others,11 predominantly relating the downstream signaling receptors.1,26 The efficacy of propranolol,27 a β-blocker, and captopril,28 an angiotensin-converting enzyme inhibitor, in the treatment of problematic proliferating IH, underscores the targeting of the RAS in the treatment of this tumor. The involvement of the RAS in IH is supported by the observation that serum levels of renin in the first 3 months of life are approximately 10 folds greater than those of adults, and decrease to approximately 3 folds at 3–12 months, and gradually decline to normal adult levels from 8 years of age29—a pattern that reflects the biologic behavior of IH.1
In this study, we investigated the role for the upstream receptor, PRR, and to elucidate the effect of its ligand, renin, on the proliferation of IH-derived cells. We present the novel finding of PRR being transcriptionally and translationally expressed in both the endothelial and non-endothelial populations of proliferating IH tissues and proliferating IH-derived primary cell lines, suggesting a role for PRR in the pathogenesis of IH.
We have also demonstrated an association between renin and the proliferation of IH endothelial cells, suggesting a possible mode of action for the PRR in IH. Our results show that when 5, 10, or 15 nM renin is added to the CD34+ population, there is a statistically significant increase in the number of viable cells compared with the untreated cells. Furthermore, this increase in the number of viable cells is reversed at 10 nM, with the addition of the wnt signaling inhibitor DKK1. This would infer that exogenous renin stimulates proliferation in IH endothelial cells, presumably via the canonical wnt signaling pathway. It is interesting to note that DKK1 does not have a significant effect on reducing proliferation at both 5 and 15 nM, the latter dose maybe simply accounted for by the overwhelming effect of the relatively high concentration of renin. It is intriguing that at 5 nM of renin, DKK1 does not cause a significant decrease in the number of viable cells. This may be attributed to possible interaction with its other receptor(s) Kremen1 or 2,30 although the presence of these latter receptors in proliferating IH remains the topic of further investigation.
The limitations of this study include the relatively small sample size, a lack of normal tissue controls, and the need for further in vivo work to confirm our preliminary finding of the role of PRR in the pathogenesis of IH.
The absence of a significant change in the number of viable CD34− population maybe explained by the relatively low abundance of cells that expressed PRR within proliferating IH tissue, as demonstrated on IHC staining.
Furthermore, our transcriptional analyses show no significant change in the transcript expression of ESC markers, suggesting that this effect is not directly related to an increase in stem cell population/s. The precise mechanisms for the effect of renin on IH are a focus for future research.
Conclusion
We have demonstrated transcriptional and translational expression of PRR in both the endothelial and non-endothelial populations of IH, at both the tissue and the cellular level. We have also shown the effect of renin in promoting proliferation of the endothelial population, which is inhibited by blocking the canonical wnt signaling pathway, suggesting a pathway linking renin, PRR, and wnt signaling, which results in proliferation and differentiation of IH. These novel findings support the hypothesis that PRR promotes the proliferation of IH and suggests PRR as a potential novel therapeutic target in the treatment of IH.
References
Itinteang, T., Brasch, H. D., Tan, S. T. & Day, D. J. Expression of components of the renin-angiotensin system in proliferating infantile haemangioma may account for the propranolol-induced accelerated involution. J. Plast. Reconstr. Aesthet. Surg. 64, 759–765 (2011).
Tan, S. T. et al. A novel in vitro human model of hemangioma. Mod. Pathol. 13, 92–99 (2000).
North, P. E. et al. A unique microvascular phenotype shared by juvenile hemangiomas and human placenta. Arch. Dermatol 137, 559–570 (2001).
Takahashi, K. et al. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J. Clin. Invest 93, 2357–2364 (1994).
Itinteang, T. et al. Infantile haemangioma expresses embryonic stem cell markers. J. Clin. Pathol. 65, 394–398 (2012).
Harbi, S. et al. Infantile hemangioma originates from a dysregulated but not fully transformed multipotent Stem Cell. Sci. Rep. 6, 35811 (2016).
Itinteang, T., Tan, S. T., Brasch, H. & Day, D. J. Primitive mesodermal cells with a neural crest stem cell phenotype predominate proliferating infantile haemangioma. J. Clin. Pathol. 63, 771–776 (2010).
Khan, Z. A. et al. Multipotential stem cells recapitulate human infantile hemangioma in immunodeficient mice. J. Clin. Invest 118, 2592–2599 (2008).
Huang, L., Nakayama, H., Klagsbrun, M., Mulliken, J. B. & Bischoff, J. Glucose transporter 1-positive endothelial cells in infantile hemangioma exhibit features of facultative stem cells. Stem Cells 33, 133–145 (2015).
Ritter, M. R., Reinisch, J., Friedlander, S. F. & Friedlander, M. Myeloid cells in infantile hemangioma. Am. J. Pathol. 168, 621–628 (2006).
Dornhoffer, J. R., Wei, T., Zhang, H., Miller, E., MA, C. & Richter, G. T. The expression of renin-angiotensin-aldosterone axis components in infantile hemangioma tissue and the impact of propranolol treatment. Pediatr. Res 82, 155–163 (2017).
Nguyen, G. et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 109, 1417–1427 (2002).
Griendling, K. K., Murphy, T. J. & Alexander, R. W. Molecular biology of the renin-angiotensin system. Circulation 87, 1816–1828 (1993).
Satofuka, S. et al. Pro)renin receptor promotes choroidal neovascularization by activating its signal transduction and tissue renin-angiotensin system. Am. J. Pathol. 173, 1911–1918 (2008).
Balakumar, P. & Jagadeesh, G. Potential cross-talk between (pro)renin receptors and Wnt/frizzled receptors in cardiovascular and renal disorders. Hypertens. Res. 34, 1161–1170 (2011).
MacDonald, B. T., Tamai, K. & He, X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 (2009).
Niida, A. et al. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene 23, 8520–8526 (2004).
Cruciat, C. M. et al. Requirement of prorenin receptor and vacuolar H + -ATPase-mediated acidification for Wnt signaling. Science 327, 459–463 (2010).
Liu, F. Y., Liu, X. Y., Zhang, L. J., Cheng, Y. P. & Jiang, Y. N. Binding of prorenin to (pro)renin receptor induces the proliferation of human umbilical artery smooth muscle cells via ROS generation and ERK1/2 activation. J. Renin Angiotensin Aldosterone Syst. 15, 99–108 (2014).
Yisireyili, M. et al. Indoxyl sulfate-induced activation of (pro)renin receptor promotes cell proliferation and tissue factor expression in vascular smooth muscle cells. PLoS ONE 9, e109268 (2014).
Uraoka, M. et al. Prorenin induces ERK activation in endothelial cells to enhance neovascularization independently of the renin-angiotensin system. Biochem. Biophys. Res. Commun. 390, 1202–1207 (2009).
van Schaijik, B., Davis, P. F., Wickremesekera, A. C., Tan, S. T. & Itinteang, T. Subcellular localisation of the stem cell markers OCT4, SOX2, NANOG, KLF4 and c-MYC in cancer: a review. J. Clin. Pathol. 71, 88–91 (2018).
Ram, R. S. et al. Cancer stem cells in moderately differentiated lip squamous cell carcinoma express components of the renin-angiotensin system. Front Surg. 4, 30 (2017).
Koh, S. P. et al. Embryonic stem cell-like population in Dupuytren’s disease. Plast. Reconstr. Surg. Glob. Open 4, e1064 (2016).
Narita, T. et al. Placental (pro)renin receptor expression and plasma soluble (pro)renin receptor levels in preeclampsia. Placenta 37, 72–78 (2016).
Itinteang, T., Marsh, R., Davis, P. F. & Tan, S. T. Angiotensin II causes cellular proliferation in infantile haemangioma via angiotensin II receptor 2 activation. J. Clin. Pathol. 68, 346–350 (2015).
Tan, C. E., Itinteang, T., Leadbitter, P., Marsh, R. & Tan, S. T. Low-dose propranolol regimen for infantile haemangioma. J. Paediatr. Child Health 51, 419–424 (2015).
Tan, S. T. et al. Treatment of infantile haemangioma with captopril. Br. J. Dermatol 167, 619–624 (2012).
Itinteang, T., Withers, A. H., Davis, P. F. & Tan, S. T. Biology of infantile hemangioma. Front Surg. 1, 38 (2014).
Christodoulides, C. et al. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J. Cell Sci. 119, 2613–2620 (2006).
Acknowledgements
We thank Ms. Liz Jones and Dr. Helen Brasch of the Gillies McIndoe Research Institute for their assistance in IHC staining and the diagnosis of IH and interpretation of the DAB IHC results. No external funding was secured for this project.
Author information
Authors and Affiliations
Contributions
T.I. formulated the hypothesis. T.I., S.T.T., and B.v.S. designed the study. T.I. and S.T.T. interpreted the IHC and NanoString data. B.v.S., S.T.T., and T.I. interpreted the cell culture and RT-PCR data. R.M. carried out the statistical analysis. B.v.S., T.I., and S.T.T. drafted the paper. All authors approved the paper.
Corresponding author
Ethics declarations
Competing interests
T.I. and S.T. are inventors of a provisional patent Treatment of Vascular Anomalies (PCT/NZ2017/050032). The remaining authors declare no competing interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
van Schaijik, B., Tan, S.T., Marsh, R.W. et al. Expression of (pro)renin receptor and its effect on endothelial cell proliferation in infantile hemangioma. Pediatr Res 86, 202–207 (2019). https://doi.org/10.1038/s41390-019-0430-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0430-8
This article is cited by
-
Targeting the (pro)renin receptor in cancers: from signaling to pathophysiological effects
Journal of Cancer Research and Clinical Oncology (2023)