Abstract
Background
Congenital diaphragmatic hernia (CDH) is a complex birth anomaly with significant mortality and morbidity. Lung hypoplasia and persistent pulmonary hypertension (PPHN) limit survival in CDH. Macrophage migration inhibitory factor (MIF), a key regulator of innate immunity, is involved in hypoxia-induced vascular remodeling and PPHN. We hypothesized that antenatal inhibition of MIF in CDH fetuses, would reduce vascular remodeling, and improve angiogenesis and lung development.
Methods
Pregnant rats were randomized into three groups: Control, nitrofen, and nitrofen + ISO-92. Lung volumes of pups were measured by CT scanning. Right ventricular systolic pressure (RVSP) and vascular wall thickness (VWT) were measured together with MIF concentration, angiogenesis markers, lung morphometry, and histology.
Results
Prenatal treatment with ISO-92, an MIF inhibitor, improved normalization of static lung volume, lung volume-to-body weight ratio, decreased alveolar septal thickness, RVSP and VWT and improved radial alveolar count as compared to the non-treated group. Expression of MIF was unaffected by ISO-92; however, ISO-92 increased p-eNOS and VEGF activities and reduced arginase 1, 2 and Sflt-1.
Conclusion
Prenatal inhibition of MIF activity in CDH rat model improves angiogenesis and lung development. This selective intervention may be a future therapeutic strategy to reduce the morbidity and mortality of this devastating condition.
Similar content being viewed by others
Introduction
Congenital diaphragmatic hernia (CDH) is a complex birth anomaly and a major cause of respiratory failure at birth. One in 2500–3500 babies is diagnosed with CDH every year in the USA.1 CDH is a defect in the diaphragm leading to herniation of the abdominal content into the thoracic cavity causing poor lung growth at a critical stage of fetal development. Further, CDH-induced lung hypoplasia and pulmonary vascular malformation that occur secondary to defective angiogenesis lead to pulmonary hypertension.2 Surgical repair of the diaphragm at birth is not always associated with improved outcome and survival.3 The two main factors which limit survival in CDH (even with surgical repair) are lung hypoplasia and persistent pulmonary hypertension of the newborn (PPHN).4 At birth, infants with CDH usually present with refractory pulmonary hypertension and show limited response to routine vasodilator therapy, due to changes in the pulmonary circulation and vascular remodeling.5 Pulmonary hypertension (PH) can persist beyond the neonatal period and is associated with a devastating prognosis.
Despite advances in neonatal care and new treatment modalities, CDH remains associated with up to 50% mortality.6 Antenatal therapy, focused on improving lung and pulmonary vascular growth in utero, remains an ultimate treatment goal towards achieving a better postnatal outcome. However, so far, there is no proven antenatal therapeutic approach available to limit CDH mortality and morbidity. A few studies using experimental animal models of CDH, have attempted to implement and test antenatal interventional therapy to improve postnatal outcome.2,7
Macrophage migration inhibitory factor (MIF) is a pro-inflammatory cytokine capable of inhibiting random migration of macrophages.8 MIF is also recognized as a critical regulator of innate immunity and has been shown to play an important role in the development of hypoxia-induced pulmonary hypertension.9 MIF is a protein assembled as a trimer and is expressed in multiple cell types including vascular endothelial cells, vascular smooth muscle cells, fibroblasts and macrophages. Newborns express 10-fold higher levels of MIF compared to children and adults.10 MIF enhances cell proliferation as well as cell survival and has potent antiapoptotic activity.11 Enhanced secretion of MIF leads to increased growth of pulmonary arterial smooth muscles cells via activation of ERK1/2 and c-Jun N-terminal kinase (JNK) pathways. MIF inhibition suppresses the ERK1/2 and JNK phosphorylation induced by MIF.
Angiogenesis is a complex phenomenon regulated by proangiogenic factors such as vascular endothelial growth factor (VEGF) and anti-angiogenesis proteins including soluble fms-like tyrosine kinase-1 (sFlt-1). MIF inhibition decreases sFlt-1, and Angiopoietin 1 thereby increasing VEGF and subsequently angiogenesis. Simultaneous increases in Phospho-endothelial nitric oxide synthase (P-eNOS) expression with MIF inhibition occurs secondary to an inhibitory effect on arginase 1 and 2 in the urea cycle. Increased production of nitric oxide (NO) leads to enhanced angiogenesis with endothelial cell migration and endothelial cell growth.12
We hypothesized that prenatal inhibition of maternal circulating MIF activity would improve fetal lung development despite CDH and the presence of gut in the thoracic cavity. Specifically, we hypothesized that prenatal MIF activity inhibition would attenuate the severity of lung hypoplasia and the severity of pulmonary hypertension after birth.
Methods
All procedures and protocols were approved by the Institutional Animal Care and Use Committees at The Feinstein institute for Medical Research at Manhasset and at Stony Brook University Hospital.
Animal model
Nitrofen is an herbicide that is not toxic to adult rats, but is known to produce CDH in rodent offspring with variable incidence, depending on dose and time of exposure.13 Experimental nitrofen-induced CDH in rats is a well-established model that mimics the pulmonary abnormalities described in human CDH, including lung hypoplasia and pulmonary vascular remodeling.14
Our CDH animal model was established by gavage feeding pregnant female rats at gestational age (GA) day 8–9,15 with nitrofen (200 mg dissolved in 1 ml olive oil). In this particular model, 60–70% of pups are expected to develop CDH and lung hypoplasia.13 Timed pregnant rats were randomized to one of the three following groups: (i) Controls that received olive oil alone at GA day 8–9; (ii) nitrofen group that received nitrofen on GA day 8–9; and (iii) MIF-inhibitor-treated group that received nitrofen (200 mg) on GA day 8–9 and ISO-92 (MIF inhibitor) at a dose of 1.8 mg/kg/day, started at GA day 10–11 and administered continuously via a subcutaneous osmotic device (Alzet, CA). The osmotic delivery device was surgically implanted on the dorsum of pregnant rats under general anesthesia, as previously described.9 At term (E21.5), fetuses from each group were harvested by cesarean section and assessed for various parameters including: static lung volumes by CT analysis (n = 6), lung histopathology (n = 6); hemodynamic measurements (n = 10); and molecular studies (n = 10). Because not all fetuses develop CDH, only those that developed CDH in the nitrofen and nitrofen + ISO-92 litters were included in the analysis, and compared to pups from the control group.
Inhibition of MIF inflammatory active site
Inhibition of MIF was achieved using ISO-92, a molecule based on the (S, R)-3-(4-hydroxyphenyl)-4, 5-dihydro-5-isoxazole acetic acid methyl ester structure, that selectively inhibits MIF inflammatory activity with an IC50 of 550 nM.9 The ISO-92 was synthesized in house at The Feinstein Institute.
Lung volume evaluation by CT imaging (N = 6 pups/group)
At term (E 21.5), fetuses were delivered by cesarean section while the mother was maintained under general anesthesia with isoflurane 1–2% in oxygen at a flow rate of 1–2 l/min. Immediately after delivery and recovery, a whole-body CT scan of each of the rat pup was acquired using an Inveon PET/SPECT/CT scanner (Siemens, Knoxville, TN) using isoflurane 0.5% as needed to eliminate motion. Data were acquired in a high-resolution, small field-of-view mode using a tube voltage of 80 kV and current of 500 μA and no beam filtration. Projection data was reconstructed with a standard filtered back projection algorithm using the Shepp-Logan filter with cutoff at Nyquist into a 1024 × 1024 × 1024 matrix resulting in a 42-micron isotropic voxel size.
Quantification of lung volumes and volumetric rendering of lung volumes from each rat pup were performed using Amira software (FEI, Thermo Fisher Scientific, OR, Version 6.2). The 3D CT data were reduced to 80-micron cubed voxels. The lung tissue was well defined as a low-intensity signal; and by thresholding to this signal a mask-based segmentation was accomplished using the semi-automated feature “edit new label field” in Amira.
Histopathology
Lung morphometry was assessed on fetal rat lungs from each group (N = 6 pups /group). Lung tissues were embedded in paraffin. Serial sections were stained with hematoxylin and eosin. The stained sections were analyzed using a Nikon Eclipse E400 microscope and images were obtained using a Nikon DS-Fi1 camera with 5-megapixel CCD.
Pulmonary alveolar septum thickness was assessed in HE-stained lung sections at a ×400 magnification by averaging 100 measurements per 10 representative fields. Quantitative morphometry was performed by two independent researchers blinded to the treatment strategy. With the assistance of image analysis software (Image Pro-Plus; Media Cybernetics Washington, DC), radial alveolar count was done.
Hemodynamic measurements (N = 10 pups/group)
Right ventricular systolic pressure (RVSP) was assessed within 15 min of birth. A 26-gauge needle connected to the transducer was inserted in to the right ventricle of the heart trans-diaphragmatically. RVSP was assessed and recorded using a computerized hemodynamic recording system (HAEMODYN, Harvard Apparatus, MA) as described previously.9
Vascular remodeling evaluation (N = 10 pups/group)
Pulmonary blood vessel median wall thickness was measured by immunostaining with an anti-α-smooth muscle actin (anti-α-SMA) antibody (Cell signaling Technology, Danvers MA). Vessel wall thickness was measured by ImageJ software. External diameter (ED) and the internal diameter (ID) of 50 medium size pulmonary vessels (with ED of 40–100 μm) per animal was measured by investigators blinded to the sample. Total vascular wall thickness (WT) which is the mean distance between ED and ID was measured and the percentage of wall thickness (WT %) was calculate.9
SFlt-1 was assayed using a commercially available ELISA kit (R&D Systems Inc. Minneapolis, MS), in lung tissue lysates. Briefly, the lung tissue homogenates were diluted up to 10 times in lysis buffer, and the diluted lysates assayed and quantitated as per manufacturer’s protocol. Each set of samples of control, treated with nitrofen, with and without ISO-92 treatment were assayed at least in quadruplicates and quantitated and plotted as indicated.
Molecular studies (N = 10 pups/group)
Phospho-eNOS, Arginase 1and 2 (Arg-1, Arg-2), Tie2, Angiopoietin 1 (Ang-I), VEGF and MIF expression were measured using western blot analysis. Briefly, snap frozen lungs were homogenized in RIPA buffer containing 50 mM Tris·Cl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 0.25% deoxycholate, 0.1% SDS, 1 × protease inhibitor cocktail (Cocktail Set I, Calbiochem), 1 mM PMSF, and 0.2 mM sodium orthovanadate. The protein concentration was measured in the lysates using a Bio-Rad Protein Assay kit (Pierce, Rockford, IL). Total protein lysates (50 μg/lane) were loaded on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane using a Bio-Rad miniblot apparatus. Membranes were blotted with anti-phospho- eNOS antibody (Cell Signaling Technology, Danvers MA) Arg-1&2 (Santa Cruz Biotechnology Inc., Dallas, TX), Tie/TEK (Lifespan BioSciences NC, Seattle WA), VEGF (DAKO, Glostrup, Denmark), and anti-MIF (R&D systems, Minneapolis, MN), followed by secondary anti-rabbit-HRP antibody (Santa Cruz Biotechnology, CA). Blots were developed using a chemiluminescence detection kit (Pierce, Rockford, IL) and exposed to X-ray film (Eastman Kodak, Rochester, NY). Equal protein loading and protein transfer were confirmed by immunoblot analysis for the determination of β-actin/GAPDH protein (Santa Cruz Biotechnology Inc. Dallas, TX) on the same (stripped) blots. Immunoblots were quantitated using Image J software and values were normalized and represented as a ratio of β-actin/GAPDH protein expression.
Statistical analysis
Values in the figures are reported as mean ± SD of samples taken at least in triplicates. A one-way ANOVA with ‘Bonferroni’s Multiple Comparison Test’, post hoc test was performed using Graph Pad Prism version 5 for Windows, (Graph Pad Software, La Jolla, CA), and P values < 0.05 were considered significant.
Results
Nitrofen administration was associated with the development of CDH in about 60% of the neonates as expected. Inhibition of MIF activity antenatally did not affect the body weight of newborns (mean weight of nitrofen group 5.64 + 0.11 g vs ISO-92 group 5.84 + 0.50 g).
CT scans of the CDH group revealed lung bases characterized by an irregular surface when compared to both control and treated CDH (nitrofen + ISO92) groups, in which the lung base surface was smooth (Fig. 1a–c). Static lung volumetric studies as defined by the CT scan showed a significant reduction of total lung volumes and lung volume-to-body weight ratios, among the non-treated CDH group compared to controls (P < 0.05). In the ISO-92-treated CDH group, the lung volume, as well as the lung volume-to-total body weight ratio, were significantly higher than the non-treated group (P < 0.05) but not significantly different from control pups (P > 0.05) (Fig. 1d, e) suggesting better lung development with ISO92. Corresponding histopathology of neonate lungs, confirmed better lung development, increased angiogenesis and alveolarization, decreased alveolar septum thickness, and improved radial alveolar count in neonates treated with nitrofen + ISO-92 compared to nitrofen-only group (Fig. 2).
CT scan of three studied groups. CT images of the three groups a normal, b nitrofen, and c nitrofen + ISO-92; nitrofen group showed irregular lung surface compared to the treated group (nitrofen + ISO-92) and control. d Lung volume of three Studied Groups. There was a significant reduction of total lung volume (nitrofen group) as compared to the treated group (nitrofen + ISO-92) and control group. e Lung volume-to-body weight ratio and showed a significant reduction in nitrofen group compared to other groups. (N = 6 pups/group. Data are presented as mean + SD; *P < 0.05)
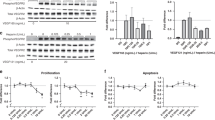
Histological studies of neonates rat pups in the three studied groups a normal, b nitrofen, and c nitrofen + ISO-92, showed better lung development, angiogenesis and alveolarization in treated groups with ISO-92 (c) as compared to the non-treated group (b) (nitrofen group). N = 6 pups/group; images with ×20 power. Lung morphometric studies in all three studied groups showed a significant decrease in septum thickness (d) and a significant improve of alveolar count (e), in ISO-92-treated group vs non treated group. (N = 6 pups/group. Data are presented as mean + SD; *P < 0.05). MIF inhibition by ISO-92 does not affect MIF expression or secretion in pup lung homogenates. MIF was not significantly different between non-treated group (nitrofen only) vs. treated group (nitrofen + ISO-92) (f, g). (N = 10 pups/group. Data are presented as mean + SD; *P < 0.05). MIF/CD74 ligand/receptor complex assay among the three studied group showed a significant enhanced expression in CDH pups, not treated with ISO-92 in comparison to control group. Treated group with ISO-92 showed a significant reduction of MIF/CD74 ligand ratio in comparison to not treated group (h). (N = 10 pups/group. Data are presented as mean + SD; *P < 0.05)
To explore the MIF/CD74 (ligand/receptor) complex in the lungs of fetuses with nitrofen-induced CDH, we assayed lung tissue lysate in all three groups (control, nitrofen-only group, and nitrofen with ISO-92 group) for MIF and CD74. Our results show the enhanced expression of CD74 in fetuses with CDH in both nitrofen-only and nitrofen with ISO-92 groups as compared to the control group (P < 0.05; Fig. 2h), but treated CDH group with ISO-92 showed a significant reduction of CD74 expression compared to non-treated CDH group (P < 0.05; Fig. 2h). ISO-92 is an inhibitor of the MIF inflammatory active site and did not affect the MIF expression or secretion. MIF expression and accumulation in lung homogenates was not significantly different when compared with the nitrofen group and the nitrofen + ISO-92-treated group (Fig. 2f, g).
Neonates with CDH (nitrofen only group), developed significant pulmonary hypertension as shown by higher RVSP, in comparison to the control group (21.5 + 1.1 mm Hg vs. 14.2 + 0.9 mm Hg; P < 0.05; Fig. 3). Pups with CDH in the nitrofen + ISO 92 group, had RVSP (15.1 + 0.56 mm Hg), which were close to the RVSP of controls and significantly lower than the nitrofen-only group (P < 0.05; Fig. 3a). Vascular wall thickness was also significantly higher in the nitrofen group vs. nitrofen + ISO-92-treated group (72.3 + 4.7 μm vs. 48.4 + 2.96 μm; P < 0.05; Fig. 3b).
Hemodynamic and vascular remodeling measurement in three studied groups. a Non-treated CDH group (nitrofen-only) develop significant pulmonary hypertension and high right ventricular systolic pressure (RVSP) in comparison to control. Treated group (nitrofen + ISO-92) showed significant low RVSP as compared to non-treated group. (N = 10 pups/group. Data are presented as mean + SD; *P < 0.05). b Vascular wall thickness of pulmonary blood vessel in non-treated CDH pups (nitrofen only) shows significant increase in vascular wall thickness compared to treated CDH Pups (nitrofen + ISO-92), which shows significant decrease in wall thickness (72.3 + 4.7 vs. 48.4 + 2.96). (N = 10 pups/group. Data are presented as mean + SD; *P < 0.05)
ISO-92 inhibits the activity of circulating extracellular MIF. As a result of MIF activity inhibition, there was a concurrent reduction of sFlt1 and a significant increase of p-eNOS and VEGF expression in neonate rats with CDH treated with nitrofen + ISO92 in comparison to the nitrofen only group (P < 0.05; Fig. 4a–e)
Effect of MIF inhibition on sFlt-1and p-eNOS activity in three studied groups. a (Western blot) and b (p-eNOS to GAPDH ratio): p-eNOS expression was significantly improved by MIF inhibition in CDH pup-treated group (nitrofen + ISO-92) as compared to non-treated group (nitrofen only). (N = 10 pups/group. Data are presented as mean + SD; *P < 0.05). c MIF inhibition by ISO-92 in CDH-treated pups (nitrofen + ISO-92) inhibits the activity of circulating extracellular MIF. As a result there was a concurrent significant reduction of sFlt-1 was seen in treated pups. (N = 10 pups/group. Data are presented as mean + SD; *P < 0.05). d (Western blot) and e (VEGF to Actin ratio): MIF inhibition with ISO-92 enhances the expression of VEGF in treated pup as compared to non-treated nitrofen-only group (N = 10 pups/group. Data are presented as mean + SD; *P < 0.05)
To further investigate the underlying mechanism(s) that are involved in improving lung development in our model, several relevant molecules and pathways were characterize. Our data show that the significant increase of p-eNOS expression was associated with a significant reduction of both arginase 1 and 2, and angiopoietin-1 (Ang-1) in the group treated with nitrofen + ISO-92, compared to the nitrofen-only group (P < 0.05; Fig. 5). Another mechanism/pathway which has significant impact on endothelial cell migration and proliferation and subsequently on angiogenesis, was the tyrosine kinase endothelial receptor system (TIE2/TEK). Our data show a significant reduction of TIE2 expression in neonates with CDH and treated with nitrofen-only in comparison to the control group and the CDH group treated with nitrofen + ISO92 (P < 0.05; Fig. 5).
Western blot studies. a, d Blot films for arginase 1 and 2, TIE2/TEK and angioprotein 1 assays in three studied groups. b, c Neonates with CDH showed a significant reduction of both arginase 1 and 2, among treated group (nitrofen + ISO-92) compared to non-treated group (nitrofen-only). e Our data showed a significant reduction of TIE2 activation in neonates with CDH in non-treated group (nitrofen only) in comparison to both control group and treated group (nitrofen + ISO92). f Inhibition of MIF in treated group with ISO-92 showed significant reduction in angioprotein 1 as compared to non-treated nitrofen-only group. (N = 10 pups/group. Data are presented as mean + SD; *P < 0.05)
Discussion
Congenital diaphragmatic hernia is a devastating cause of respiratory failure and death in the newborn. The associated PPHN and lung hypoplasia are the main factors responsible for poor health, morbidity, and death. Despite all the technical and pharmaceutical advances in other areas of medicine, treating babies with CDH remains a significant clinical challenge. Our study shows that antenatal treatment of pregnant rats, with the MIF inhibitor ISO-92, in a CDH rat model improves lung development, and subsequently attenuates the severity of postnatal pulmonary hypertension. The improvement in lung development in the ISO-92 treated pups was confirmed by histology, lung morphometry and static lung volumetrics as defined by in vivo CT scanning. The more mature lung growth and development in the ISO-92 treated CDH rat pups was associated with a significant decrease in postnatal pulmonary hypertension severity (as indicated by decreased right ventricular systolic pressure), decreased thickness of the alveolar septum and vessel wall thickness with improved radial alveolar counts in CDH pups treated with ISO-92. Furthermore, there was a significant increase in p-eNOS expression, a significantly higher expression of angiogenesis markers (VEGF, TIE2) and a decrease in SFlt-1. Collectively the trajectories of these biomarkers confirm improved angiogenesis and subsequent lung growth and lung development with MIF inhibition treatment.
Neonates with CDH have a combination of pulmonary hypoplasia, pulmonary hypertension and reduced surfactant.16 The rat nitrofen model has been used as a model of CDH since the early 1970s, and mimics the human clinical condition by inducing lung hypoplasia and persistent pulmonary hypoplasia. The mechanism by which nitrofen induces the diaphragmatic defect and lung hypoplasia is not fully understood.17 Dams treated with nitrofen and did not develop CDH lung, showed hypoplastic lung pathology with variable degrees.2 However, the herbicide also affects overall fetal growth, suggesting potential systemic effects.
While the underlying etiology of CDH remains unclear, and oxidative stress may, in part, play a role, however, hypoxia as such is not believed to be a cause of CDH. Other studies focusing on lung development among CDH models, showed that both NO and VEGF promote airway branching in normal and CDH lung explants.18,19 Further, inhaled NO prolongs survival in live CDH rat pups.20 A recent study identified 186 known mRNA and 100 miRNA which were differentially expressed in nitrofen induced hypoplastic lungs and could be a player in the pathogenesis of CDH.21 There are multiple known relevant stimuli for extracellular MIF accumulation; not just hypoxia; such as, NF-κB inactivation,22 C5a,23 thrombin24 and nitric oxide.8 MIF expression is 10-fold higher in newborns than in children and adults (11), suggesting that while newborns may utilize these higher concentrations to cope with an immunosuppressive environment, the higher concentrations may also prove detrimental. It should be noted that the MIF inhibitor (ISO-92) inhibits the inflammatory activity of MIF, but not its production, or the antioxidant activity of the molecule. The advantage of ISO-92 is that it inhibits the immune-active hydrophobic pocket of extracellular MIF, and does not enter the cell. Since there is a large storage of intracellular MIF, once administration of the inhibitor ceases, MIF could in principle be replaced from intracellular storages. Thus, while the inhibitor could be used pre-delivery when the fetus is at severe risk, once stopped at term, there would be sufficient active circulating MIF to balance the immunosuppressive environment. Clearly, since MIF could represent a potential attractive target for both CDH and immune-modulating adjunctive therapies for neonatal sepsis, long term impact of temporary inhibition of MIF activity during mid-gestation to term on both vital organ development and function, would need to be addressed in future studies.
MIF expression is enhanced in hypoxia through HIF-1α25 and subsequently has a positive feedback effect on HIF-1α accumulation.26 MIF can increase the cell proliferation of fibroblasts,27 endothelial cells,28 and vascular smooth muscle cells29 and lead to enhanced vascular wall thickness and pulmonary hypertension.9 CDH, and the associated hypoxic environment, leads to increase vascular wall thickness and high RVSP which are the two main components of pulmonary hypertension. ISO92 is a novel inhibitor of the proinflammatory cytokine MIF.30 ISO92 is a fluorinated derivative of the commercially available MIF inhibitor and has a significantly higher affinity for the protein.9 Its role as an MIF inhibitor in models of pulmonary hypertension induced by hypoxia was studied by our collaborators.9 In our study, blocking the inflammatory active site of MIF by ISO-92, in utero, in the CDH rat neonate model, lead to decreased pulmonary cellularity, vascular wall thickness and decreased RVSP.
Poor vascular growth in the CDH rat model is associated with poor lung growth. The exact mechanism of defective angiogenesis associated with CDH is not fully understood. However, VEGF is one of the most important factors involved in early angiogenesis, initial endothelial cell proliferation and differentiation. The Angiopoietin-Tie2 system along with VEGF plays a regulatory role in vasculogenesis and angiogenesis. Angiopoietin binds to the endothelial receptor tyrosine Tie2 and promotes endothelial cell migration and survival.31 On the other hand, sFlt-1 (soluble sFlt-1) is a potent anti-angiogenic factor that binds extracellularly to VEGF,32 and is higher in animal models of PPHN.33,34 Our study shows that inhibiting the MIF activity in the rat CDH model was associated with higher expression of VEGF and Tie2 receptor and showed normalization of Sflt-1. These molecular changes were associated with a significant improvement in pulmonary angiogenesis which was also accompanied by improvement of lung development as shown by in vivo CT and post-mortem histological studies (Figs. 1 and 2). CT scans of the neonate’s lungs showed irregular surface and smaller lung volumes among neonates with CDH after birth. Specifically, in the group treated with ISO-92, there was a significant increase of both lung volumes and the ratio of lung volume-to-body weight in comparison to neonates with untreated CDH (Fig. 1).
In addition to being a potent vascular vasodilator, nitric oxide (NO) enhances angiogenesis by activating endothelial cell growth and tube formation.35 There are few conflicting studies/case report which describe enhanced expression of p-eNOS in PPHN/CDH newborn,36,37 which may be explained by p-eNOS level being upregulated by prior treatment including mechanical ventilation, inhaled nitric oxide and/or oxygen exposure. In our study treating neonatal rats with CDH with ISO-92, was associated with a significant increase of p-eNOS expression, which increases NO production. This was also associated with decreases in both arginase1 and 2 expression (Fig. 5). Arginase is a urea cycle enzyme that competes with eNOS and inhibits NO synthesis via a common substrate, l-arginine. In our study, among neonates with CDH, both arginase 1 and 2 enzymes were overexpressed significantly in comparison to healthy neonates. As a result of this significant increase, both NO production and its bioavailability were significantly compromised in neonates with CDH. We demonstrated that treating pregnant adult rats with ISO-92 after inducing CDH on day 8–9 of gestation, significantly decreased both arginase1 and 2 expression (Arg-1 and Arg-II) and increased NO production. Accordingly, we postulate that inhibition of both arg 1&2, could be the mechanism, through which inhibition of MIF activity lead to increase NO bioavailability in utero thereby improving both pulmonary angiogenesis and lung development. Our novel findings imply that we have identified a new pathway involved in the pathogenesis of CDH and thus offer a new pathway and therapeutic target to study in future preclinical and clinical CDH research studies.
Postnatal CDH treatments have shown limited utility. Thus, there is an increasing demand and urgency to focus on prenatal treatment of the disease to improve overall survival and outcomes. Prenatal surgical approaches have shown some early promising results.38 However, prenatal surgery is associated with a significant increased risk of preterm delivery, chorioamnionitis and fetal death. Non-invasive prenatal medical therapy with inherent less risk, is clearly a more desirable approach. However, only a few medical therapies have been used prenatally for the treatment of CDH including dexamethasone and sildenafil,7 sildenafil alone,2 simvastatin,39 and thyrotropin releasing hormone (TRH) with dexamethasone,40 with variable outcomes.
Our main study aim was to develop a prenatal interventional therapy to promote lung development among fetuses with CDH. We focused on inhibiting MIF activity in a CDH embryo model, to promote lung development, vascular remodeling and attenuate the degree of pulmonary hypertension at birth in CDH pups, and to further understand the molecular mechanisms underlying the MIF inhibition intervention. Our study shows that antenatal inhibition of MIF in a CDH neonate rat model, has a remarkable, positive impact on lung development (alveolarization and angiogenesis). We further believe that the significant enhancement of lung growth and development in our model, is the main source of the reduced pulmonary vascular resistance we observed in the treated neonates after birth as evidenced by normalization of the right ventricular systolic pressure and right ventricular wall thickness. We therefore suggest that this new therapeutic approach will positively impact the both fetal lung development and neonatal lung function before and after birth, respectively.
Limitation of our study
It is well known that the majority (60%) of the rat pups in dams treated with nitrofen do develop CDH lungs, a fraction of the pups does not. The pups that do not develop CDH are characterized by variable degrees of hypoplastic lung pathology but do not have a diaphragmatic defect. As our goal was to investigate the effect of ISO-92 on CDH only, we focused our analysis on the pups who developed the full clinical picture relevant to neonatal CDH disease. However, in all studies (including ours) where the nitrofen-induced CDH model is used as a test bed, one cannot exclude the possibility that the overall ‘treatment’ effect might be confounded by this variable. Furthermore, caution should be exercised in making generalization as disease pathogenesis may be different in survivor vs non-survivors. Future efforts focused on developing more robust animal models of CDH would help solve this problem.
Conclusion
Prenatal inhibition of MIF activity in a pregnant rat model of fetal CDH improves both angiogenesis and lung development in neonates with CDH. We believe that if implemented clinically this strategy may reduce the morbidity and mortality of this devastating condition. However further studies are needed to explore the full mechanism of action and safety prior to recommending it as an effective antenatal approach to improve lung growth in CDH.
Change history
06 March 2019
In the original version of this article, the name of the author “Kamesh Ayasolla” was incorrectly given as “Kamesh Ayyasola”. This has now been corrected to “Kamesh Ayasolla” in both the PDF and HTML versions of the article.
References
CDH Statistics. National and International Statistics for Congenital Diaphragmatic Hernia (Cherubs Congenital Diaphragmatic Hernia Support, Awareness and Research www.cherubscdh.org. 2014).
Luong, C. et al. Antenatal sildenafil treatment attenuates pulmonary hypertension in experimental congenital diaphragmatic hernia. Circulation 123, 2120–2131 (2011).
Goonasekera, C. et al. Mortality following congenital diaphragmatic hernia repair: the role of anesthesia. Paediatr. Anaesth. 26, 1197–1201 (2016).
Thebaud, B., Mercier, J. C. & Dinh-Xuan, A. T. Congenital diaphragmatic hernia. A cause of persistent pulmonary hypertension of the newborn which lacks an effective therapy. Biol. Neonate. 74, 323–336 (1998).
Gien, J. & Kinsella, J. P. Management of pulmonary hypertension in infants with congenital diaphragmatic hernia. J. Perinatol. 36(Suppl 2), S28–S31 (2016).
Dillon, E., Renwick, M. & Wright, C. Congenital diaphragmatic herniation: antenatal detection and outcome. Br. J. Radiol. 73, 360–365 (2000).
Burgos, C. M. et al. Improved pulmonary function in the nitrofen model of congenital diaphragmatic hernia following prenatal maternal dexamethasone and/or sildenafil. Pediatr. Res. 80, 577–585 (2016).
Zicari, A. et al. Macrophage migration inhibitory factor-nitric oxide interaction in human fetal membranes at term pregnancy. J. Soc. Gynecol. Investig. 13, 263–270 (2006).
Zhang, Y. et al. Macrophage migration inhibitory factor mediates hypoxia-induced pulmonary hypertension. Mol. Med. 18, 215–223 (2012).
Roger, T. et al. High expression levels of macrophage migration inhibitory factor sustain the innate immune responses of neonates. Proc. Natl Acad. Sci. USA 113, E997–E1005 (2016).
Nguyen, M. T. et al. The cytokine macrophage migration inhibitory factor reduces pro-oxidative stress-induced apoptosis. J. Immunol. 170, 3337–3347 (2003).
Wang, L. et al. Arginase inhibition enhances angiogenesis in endothelial cells exposed to hypoxia. Microvasc. Res. 98, 1–8 (2015).
Thebaud, B. et al. Vitamin A decreases the incidence and severity of nitrofen-induced congenital diaphragmatic hernia in rats. Am. J. Physiol. 277(2 Pt 1), L423–L429 (1999).
Wilcox, D. T. et al. Animal models in congenital diaphragmatic hernia. Clin. Perinatol. 23, 813–822 (1996).
Dingemann, J. et al. Expression of the Wilm’s tumor gene WT1 during diaphragmatic development in the nitrofen model for congenital diaphragmatic hernia. Pediatr. Surg. Int. 27, 159–163 (2011).
Naeye, R. L. et al. Unsuspected pulmonary vascular abnormalities associated with diaphragmatic hernia. Pediatrics 58, 902–906 (1976).
Greer, J. J., Babiuk, R. P. & Thebaud, B. Etiology of congenital diaphragmatic hernia: the retinoid hypothesis. Pediatr. Res. 53, 726–730 (2003).
Muehlethaler, V. et al. Impaired VEGF and nitric oxide signaling after nitrofen exposure in rat fetal lung explants. Am. J. Physiol. Lung Cell. Mol. Physiol. 294, L110–L120 (2008).
Shinkai, M. et al. Effect of nitric oxide on the development of nitrofen-induced fetal hypoplastic lung explants. J. Pediatr. Surg. 40, 17–21 (2005).
Mann, O. et al. Effect of prenatal glucocorticoids and postnatal nitric oxide inhalation on survival of newborn rats with nitrofen-induced congenital diaphragmatic hernia. J. Pediatr. Surg. 37, 730–734 (2002).
Mahood, T. H. et al. The transcriptome of nitrofen-induced pulmonary hypoplasia in the rat model of congenital diaphragmatic hernia. Pediatr. Res. 79, 766–775 (2016).
Cho, M. L. et al. NF-kappaB inhibition leads to increased synthesis and secretion of MIF in human CD4+T cells. Immunol. Lett. 123, 21–30 (2009).
Riedemann, N. C. et al. Regulatory role of C5a on macrophage migration inhibitory factor release from neutrophils. J. Immunol. 173, 1355–1359 (2004).
Vera, P. L. et al. Thrombin induces macrophage migration inhibitory factor release and upregulation in urothelium: a possible contribution to bladder inflammation. PLoS One 5, e15904 (2010).
Baugh, J. A. et al. Dual regulation of macrophage migration inhibitory factor (MIF) expression in hypoxia by CREB and HIF-1. Biochem. Biophys. Res. Commun. 347, 895–903 (2006).
Winner, M. et al. Amplification of tumor hypoxic responses by macrophage migration inhibitory factor-dependent hypoxia-inducible factor stabilization. Cancer Res. 67, 186–193 (2007).
Xi, Z. D., Xie, C. Y. & Xi, Y. B. Macrophage migration inhibitory factor enhances lipopolysaccharide-induced fibroblast proliferation by inducing toll-like receptor 4. BMC Musculoskelet. Disord. 17, 43 (2016).
Le Hiress, M. et al. Proinflammatory signature of the dysfunctional endothelium in pulmonary hypertension. Role of the macrophage migration inhibitory factor/CD74 complex. Am. J. Respir. Crit. Care. Med. 192, 983–997 (2015).
Fu, H. et al. Hypoxia stimulates the expression of macrophage migration inhibitory factor in human vascular smooth muscle cells via HIF-1alpha dependent pathway. BMC Cell. Biol. 11, 66 (2010).
Yousef, A.-A. Inventor modified macrophage migration inhibitory factor inhibitors. US patent application publication No. 2009-0137647 A1. May 28, 2009.
Fukuhara, S. et al. Angiopoietin-1/Tie2 receptor signaling in vascular quiescence and angiogenesis. Histol. Histopathol. 25, 387–396 (2010).
Ikeda, T. et al. Hypoxia down-regulates sFlt-1 (sVEGFR-1) expression in human microvascular endothelial cells by a mechanism involving mRNA alternative processing. Biochem. J. 436, 399–407 (2011).
Tiede, S. L. et al. New potential diagnostic biomarkers for pulmonary hypertension. Eur. Respir. J. 46, 1390–1396 (2015).
Ataga, K. I. et al. Association of soluble fms-like tyrosine kinase-1 with pulmonary hypertension and haemolysis in sickle cell disease. Br. J. Haematol. 152, 485–491 (2011).
Acker, S. N. et al. Pulmonary artery endothelial cell dysfunction and decreased populations of highly proliferative endothelial cells in experimental congenital diaphragmatic hernia. Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L943–L952 (2013).
Hoehn, T. et al. Endothelial nitric oxide synthase (NOS) is upregulated in rapid progressive pulmonary hypertension of the newborn. Intensive Care Med. 29, 1757–1762 (2003).
Sood, B. G. et al. Expression of eNOS in the lungs of neonates with pulmonary hypertension. Exp. Mol. Pathol. 90, 9–12 (2011).
Grivell, R.M., C. Andersen, and J.M. Dodd, Prenatal interventions for congenital diaphragmatic hernia for improving outcomes. Cochrane Database Syst Rev, 2015: CD008925.
Makanga, M. et al. Prevention of pulmonary hypoplasia and pulmonary vascular remodeling by antenatal simvastatin treatment in nitrofen-induced congenital diaphragmatic hernia. Am. J. Physiol. Lung Cell. Mol. Physiol. 308, L672–L682 (2015).
Ijsselstijn, H. et al. Prenatal hormones alter antioxidant enzymes and lung histology in rats with congenital diaphragmatic hernia. Am. J. Physiol. 272(6 Pt 1), L1059–L1065 (1997).
Acknowledgements
The study received funding from Neonatal/Perinatal division of Pediatrics at Cohen Children Medical Center of New York and Lilling Family Neonatal Research Laboratory, The Feinstein Institute for Medical Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Perveen, S., Ayasolla, K., Zagloul, N. et al. MIF inhibition enhances pulmonary angiogenesis and lung development in congenital diaphragmatic hernia. Pediatr Res 85, 711–718 (2019). https://doi.org/10.1038/s41390-019-0335-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-019-0335-6
This article is cited by
-
Congenital diaphragmatic hernia: phosphodiesterase-5 and Arginase inhibitors prevent pulmonary vascular hypoplasia in rat lungs
Pediatric Research (2024)
-
Establishment of tissue-resident immune populations in the fetus
Seminars in Immunopathology (2022)
-
Emerging antenatal therapies for congenital diaphragmatic hernia-induced pulmonary hypertension in preclinical models
Pediatric Research (2021)