Abstract
Objectives
We investigated the diagnostic utility of distal contractile integral (DCI) to esophageal impedance integral (EII) ratio (DCIIR) in high-resolution impedance manometry (HRIM) of ineffective esophageal motility (IEM) in children.
Methods
We performed HRIM in 31 children with dysphagia, odynophagia, chronic vomiting, chest pain, or heartburn sensation. Based on the Chicago classification version 3.0, 20 subjects were diagnosed with IEM, and 11 subjects were normal. We analyzed the EII and DCIIR using MATLAB software.
Results
The DCIIR calculated at the impedance cutoff at 1500 Ω (DCIIR1500) were significantly lower in IEM group than patients with normal motility (P = 0.007). Receiver operating characteristic (ROC) curve analysis showed that a DCIIR1500 < 0.009 mmHg/Ω best predicted IEM in children (P < 0.001). A DCIIR1500 < 0.008 mmHg/Ω is associated with significant body weight loss > 10% within 6 months in children. (P < 0.001).
Conclusions
The calculation of DCIIR1500 may assist the automatic analysis of bolus transit in HRIM study to diagnose IEM in children. An DCIIR1500 < 0.009 mmHg/Ω may assist in the diagnosis of IEM in children, and DCIIR1500 < 0.008 mmHg/Ω correlated with significant body weight loss. The calculation of DCIIR may serve as possible parameters for HRIM.
Similar content being viewed by others
Introduction
Esophageal dysmotility is a rare but troublesome problem in children; however, a prompt diagnosis may lead to adequate timely management.1 Ineffective esophageal motility disorder (IEM) is one of the esophageal hypomotility disorder, and is associated with dysphagia, chronic vomiting, odynophagia, chest pain, or heartburn sensation. 1 Esophageal high-resolution manometry (HRM) with automated calculated parameters, including distal contractile integral (DCI), distal latency (DL), and 4-s integrated relaxation pressure (IRP-4s) to quantify the intraluminal pressure changes of the esophagus, were developed to assist in the diagnosis of esophageal hypomotility and spastic motor disorders in both adults and children.2,3,4
Impedance measurement was demonstrated to be effective in assessing bolus transit patterns in both healthy subjects and patients with esophageal motility disorders.5 With the assembly of impedance channels to the HRM catheter, high-resolution impedance manometry (HRIM) can be used to further assess the efficacy of bolus transit, in conjunction with pressure changes of the esophagus. There is increasing evidence that there is a diagnostic benefit to impedance in HRIM.6,7,8,9 However, the current interpretation of the impedance signal in the assessment of bolus transit of HRIM is largely dependent on the visual interpretation of the impedance plots by physicians.7,8,9,10
Many parameters have been used to assess bolus transit in HRIM measurement in both adults and children.11,12,13,14,15 Automated impedance manometry (AIM) analysis, pressure-flow analysis (PFA), and the esophageal impedance integral (EII) ratio were recently reported to be useful parameters for determining the adequacy of bolus transit in the esophagus.11,12,13,14,15 However, it is difficult to calculate these parameters during swallow events with absent peristalsis, which limit these parameters to be used for the assessment of all kinds of patients, especially in subjects with esophageal hypomotility disorders.11,12,13,14,15
Since subjects with IEM tend to have a relatively small DCI and large EII during HRIM,11,12,13,14,16 we assessed the potential diagnostic utilities of the distal contractile-impedance integral ratio (DCIIR) of HRIM for the diagnosis of IEM in children.
Material and Methods
Study participants
In this case control study, we enrolled 20 IEM children (age, 13.64 ± 1.18 years; 8 males and 12 females) as the study group and another 11 children with normal manometric pattern (age, 15.42 ± 1.03 years; 4 males and 7 females) as the controls in this study. All of them received HRIM at the Departments of Pediatrics and Internal Medicine of National Taiwan University Hospital (NTUH) from October 2014 to Mar 2018. They all presented with clinical symptoms of dysphagia, odynophagia, chronic vomiting, chest pain, or heartburn sensation. In HRIM, the interpretations of the manometric parameters were based on the Chicago Classification version 3.0 criteria with an age adjustment.2,3 All of the study subjects received esophagogastroduodenoscopy (EGD) and a barium contrast study of the upper gastrointestinal tract was performed within six months of the HRIM study to exclude subjects with mucosal lesions and anatomical disorders (e.g., eosinophilic esophagitis, sliding hiatal hernia, volvulus, mid-gut malrotation, annular pancreas, and gastric outlet obstruction). Significant body weight loss was defined as a body weight loss of greater than 10% within six months of symptom onset. Eleven patients in IEM study group met the criteria of significant body weight loss in this cohort. The study protocol was approved by the Institutional Review Board of NTUH.
HRIM study and data interpretation
Patients were informed to non per os at least 8 h before the HRIM study. The HRIM study was performed using a 4.2-mm-diameter silicone catheter containing 22 closely spaced water-perfused pressure sensors (spaced at 1-cm intervals in the areas measuring the lower esophageal sphincter (LES), and at 2-cm intervals in the areas of the esophageal body) and 12 impedance channels at 2-cm intervals (PART#CE4-1083; Dentsleeve International Ltd., Ontario, Canada). All of the side holes were perfused with distilled water at a rate of 0.15 mL/min using a pneumatic perfusion pump throughout the manometric study, and pressures were recorded with external pressure transducers (Solar GI HRIM water-perfused system, Medical Measurement Systems, Enschede, Netherlands). Patients were asked to perform 10 liquid swallows with 5 mL of saline at 30-s intervals after successful catheter insertion to a depth of 40–50 cm. The pressure and impedance signals of HRIM were recorded at a frequency of 20 Hz and stored on a personal computer. The MMS HRIM software converts recorded signals into digital data, which are displayed as color plots on a Solar GI HRM Compact Pole system (version 9.5, MMS, Solar GI HRIM water-perfused system, Medical Measurement Systems, Enschede, Netherlands). The diagnosis of IEM in our cohort is based on the Chicago Classification version 3.0 criteria with an age adjustment in this study. The contraction vigor is defined as failed once the DCI < 100 mmHg.s.cm, and weak contraction once the DCI value between 100 and 450 mmHg s cm.2,3 IEM is defined as normal IRP4s and more than 50% swallow with failed or weak contraction.2,3
Quantification of EII and the calculation of distal contractile-impedance integral ratio (DCIIR)
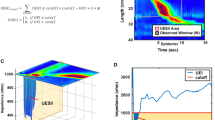
We calculated the EIIcutoff below an impedance cutoff of 1000, 1250, and 1500 Ω (EII100, EII1250 and EII1500, respectively) in an observed time window of 15 s to quantify the signal of bolus transit during the 35-mL saline liquid swallow test (Fig. 1). The EII analysis was conducted using MATLAB software (version 8.6 R2015b; MathWorks).17
a Impedance data below 1500 Ω of this wet swallow is circled in black. b The esophageal impedance integral (EII) below 1500 Ω of this wet swallow was calculated using MATLAB software (version 8.6 R2015b; MathWorks, USA). c The EII1500 was calculated as the integral of the volume drop of impedance below 1500 Ω, where eid is the esophageal impedance data, and the impedance cutoff is 1500 Ω for EII1500. The EII value at less than 1500 Ω in an observed time window after the liquid swallow test to quantify the bolus transit, based on an esophageal impedance data (eid) drop of less than the 1500 Ω impedance cutoff, observation time after wet swallow and length (Ω∙s∙cm)
The EIIcutoff is calculated as follows:
where eid is the esophageal impedance data, d is the distance between two adjacent channels, \(\Delta t\) is the sampling interval, W is the observed window, and I is the indicator function (Fig. 1).
For instance, the EII value at less than 1500 Ω in an observed time window of 15 s after the liquid swallow test to quantify the bolus transit, based on an esophageal impedance data (eid) drop of less than the 1500 Ω impedance cutoff, observation time after wet swallow and length (Ω∙s∙cm).
Previous studies2,11,12,13,14 have shown that subjects with esophageal dysmotility tend to have a low DCI and high EII; therefore, we calculated the DCI to EII (EII1000, EII1250 and EII1500) ratio of each liquid swallow as DCIIR (DCIIR1000, DCIIR1250 and DCIIR1500) in this study.
Statistical analysis
The STATA (version 14; StataCorp LP, TX) and MedCalc (version 18.2.1; MedCalc Software, Ostend, Belgium) software packages were used for statistical analyses. Student’s t-test with unequal variance was used to assess differences in the mean and the 95% CIs of continuous variables between the two groups. Fisher’s exact test or chi-squared test was used to determine differences in incidence between the groups. Receiver operating characteristic (ROC) curve analysis provided the cutoff for the prediction and was used to calculate the area under the curve (AUC). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of each diagnostic method were also calculated. A logistic regression was also applied in the analysis. The primary end point of this analysis is the diagnosis of IEM, and the secondary end point of this analysis in significant body weight loss > 10% within 6 months. A P-value < 0.05 was regarded as indicative of statistical significance.
Results
General characteristics
These 31 children (age, 14.27 ± 0.85 years; 95% confidence interval (CI), 12.53–16.00 years; 12 males and 19 females) who received HRIM during the study period were recruited for this analysis. Twenty children (64.52%) were diagnosed with IEM (age, 13.64 ± 1.18 years; 95% CI, 11.16–16.12 years; 8 males and 12 females). There is no difference in gender, age, and the esophageal length between IEM subjects and controls (Table 1). The DCI is significantly lower in IEM subjects than controls (239.08 ± 43.11 vs. 1084.11 ± 143.21 mmHg.s.cm; 95% CI, 148.84–329.31 vs. 765.01–1403.21 mmHg.s.cm; P = 0.0001). The contractile front velocity (CFV), DL, and IRP4s are not different between IEM and control subjects (Table 1).
The prevalence of significant body weight loss the disease diagnosis is significantly higher in IEM group than controls (55% vs. 0%, P = 0.002). None were diagnosed with sliding hiatal hernia, upper gastrointestinal tract obstruction, eosinophilic esophagitis, or mid-gut gastric volvulus by EGD and barium contrast study.
IEM children had a lower DCI and DCIIR1000-1500 than the control subjects (Table 1). There was no obvious difference in the length from the inlet of upper esophageal sphincter to the outlet of lower esophageal sphincter between these 2 groups (26.31 ± 0.74 vs. 26.22 ± 0.78, P = 0.93). There was no difference in age or gender between the IEM and control subjects in this study (Table 1).
Among these 310 wet swallow tests in these 31 study subjects, 121 swallows with DCI > 450 mmHg.s.cm were regarded as intact contraction and another 189 swallows with DCI < 450 mmHg.s.cm were graded as failed and weak contraction. The EII1500 is significantly higher in ineffective contraction swallows (failed and weak contraction) than intact contraction swallows (777379 ± 245687.3 vs. 71675.2 ± 4070.76, P = 0.026). The phenomena is also consistent in EII1250 and EII1000 (P = 0.027 and 0.027, respectively) (Supplementary Table 1).
Predictors of IEM disorder in children
The ROC analysis results for the best prediction cutoff of DCIIR1000-1500 are shown in Table 2. The DCIIR1500 achieved better prediction than DCIIR1000 and DCIIR1250 in this model (Table 2). The cutoff at < 0.009 mmHg/Ω of DCIIR1500 achieved 90.9% sensitivity, 95% specificity, and an AUC of 0.96 (Table 2). The PPV, NPV, and diagnostic accuracy for the prediction of esophageal hypomotility at DCIIR1500 < 0.009 mmHg/Ω cutoff were 95%, 90.91%, and 93.55%, respectively. The DCIIR1500 < 0.009 mmHg/Ω is predictive of IEM in this cohort (Odds ratio = 190, 95% CI = 10.71-3369.95, P < 0.001).
Predictors of significant body weight loss in children
Eleven children were reported to have 10% body weight loss within six months of symptom onset, and all of them were diagnosed as IEM. The prevalence of significant body weight loss was significantly higher in IEM group than the control group (55% vs. 0%, P = 0.002).
Subjects with significant body weight loss (n = 11) were noted to have a significantly lower DCIIR1250 (0.006 ± 0.002 vs. 0.021 ± 0.004 mmHg/Ω, P = 0.002) and DCIIR1500 (0.003 ± 0.001 vs. 0.016 ± 0.004 mmHg/Ω, P = 0.007) than those without significant body weight loss (n = 20). The DCIIR1000 was not different between subjects with significant body weight loss (n = 11) and others (n = 20) (P = 0.18).
ROC analysis was applied to calculate the predictive value of DCIIR1000, DCIIR1250, and DCIIR1500 for significant body weight loss in children (Table 3). In this study cohort, the DCIIR1500 achieved better prediction of significant body weight loss than DCIIR1000 and DCIIR1250 (Table 3). The DCIIR1500 cutoff < 0.008 mmHg/Ω achieved 100% sensitivity, 60% specificity, and an AUC of 0.82 in the study cohort. The PPV, NPV, and diagnostic accuracy for the prediction of significant body weight loss at DCIIR1500 < 0.008 mmHg/Ω cutoff were 57.89%, 100%, and 74.19%, respectively. The DCIIR1500 < 0.008 mmHg/Ω is predictive of significant body weight loss > 10% within 6 months in this cohort (risk difference = 0.58, 95% CI = 0.36-0.80, P = 0.001).
Discussion
There is increasing evidence supporting the clinical utility of impedance signals in the data interpretation of multichannel intraluminal impedance-pH (MII-pH) monitoring in children.18,19,20,21,22 However, the majority of these studies focused on the utility in diagnosing gastroesophageal reflux signals, instead of bolus transit.18,19,20,21,22 Impedance signals were shown to be effective in assessing bolus transit during swallow studies in humans, but the majority of HRIM studies depended on the visual interpretation of physicians.5,6,7,8,9,10,23 The presence of impaired bolus transit is associated with persistent low impedance after esophageal contraction of each swallow event, which may be indicated by larger EII and smaller DCIIR in our calculation.5,6,7,8,9,10
Generalized automatic analysis systems for interpreting bolus transit and clearance assessed by impedance remain lacking, and there are increasing studies trying to identify applicable parameters to assist in the assessment of impedance signals in HRIM in recent years.11,12,13,14,15,16 Our study firstly combined the signals from both manometers and impedance interpretation in HRIM by calculation of the DCI to EII ratio, which is easy to apply to all types of esophageal peristalsis and bolus clearance patterns during liquid swallow tests of HRIM. We further validated the diagnostic utility for pediatric IEM and significant body weight loss > 10% within 6 months in children.
Recent studies demonstrated the diagnostic benefit of PFA in dysphagic adults and children with deglutitive aspiration.11,24,25 The AIM analysis was also shown to detect esophageal motor dysfunction in patients with non-obstructive dysphagia with normal manometry, and was predictive for dysphagia after fundoplication.26,27 However, the calculation of PFA and AIM (including peak pressure, PeakP; intra-bolus pressure, IBP; pressure at the time of nadir impedance, PNadImp; IBP slop; time interval between nadir esophageal impedance and peak esophageal pressure, TNadImp-PeakP; the impedance ratio of nadir impedance to the impedance at peak pressure, IR; and pressure-flow index, PFI) in subjects with absent esophageal peristalsis during the liquid swallow test of HRIM is very difficult.
The EII ratio after and before esophageal contraction during the liquid swallow test was shown to be correlated with the amount of barium retained in the esophagus under fluoroscopy.12 The EII ratio after and before esophageal contraction was demonstrated to have a higher sensitivity and specificity than timed-barium esophagram bolus transit assessments in a recent study on achalasia patients.28 However, the calculation of the EII ratio still depends on the presence of esophageal contraction. Hence, the calculation of the EII ratio in the liquid swallow with absent peristalsis, such as type I achalasia or some IEM patients, is very difficult. The lack of calculated values in dysphagic patients with absent peristalsis may limit the clinical utility of automatic analysis systems of impedance (PFA and EII ratio) for bolus transit and clearance.
By adapting the concept of PFA and the EII ratio to illustrate the esophageal motility function, combining signals from both manometers and impedance channels, we calculated the DCI to the overall EII at different impedance thresholds as DCIIR1000-1500. The DCIIR 1500 was demonstrated to have better diagnostic accuracy than DCIIR1000 and DCIIR1250 in this study. A recent study that conducted impedance analysis at a threshold of 1500 Ω provided more accurate information for the detection of liquid residue and supported our findings.29 The calculation of DCIIR1500 may be easily applied to all kinds of esophageal peristalsis or bolus clearance patterns during the liquid swallow test of HRIM. We further demonstrated that DCIIR1500 at a cutoff of < 0.009 mmHg/Ω was predictive of esophageal hypomotility in children. Furthermore, DCIIR1500 at a cutoff of < 0.008 mmHg/Ω was associated with significant body weight loss in this cohort.
A limitation of this study was the lack of symptom-free healthy control children, but it was unethical to perform invasive procedures in symptom-free healthy children. Another limitation was the relatively small sample size of patients for analysis. Given that statistical significance was achieved in such a small sample size, the significance should also exist in a larger population.
Conclusion
We developed a novel parameter, DCIIR1500, which combines the signal from both manometry and impedance of HRIM, for the diagnosis of IEM by HRIM in children with dysphagia, odynophagia, and chest pain. This novel parameter is easy to apply to all kinds of esophageal peristalsis and bolus clearance patterns during the liquid swallow test in HRIM, and may be applied to the automated analysis system to combine both the pressure and impedance signals in the future.
References
Rosen R, et al. An ANMS-NASPGHAN consensus document on esophageal and antroduodenal manometry in children. Neurogastroenterol Motil. 30, e13239 (2018).
Kahrilas, P. J. et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol. Motil. 27, 160–174 (2015).
Singendonk, M. M. et al. Applying the Chicago Classification criteria of esophageal motility to a pediatric cohort: effects of patient age and size. Neurogastroenterol. Motil. 26, 1333–1341 (2014).
Singendonk MMJ, et al. Intra- and interrater reliability of the Chicago Classification of achalasia subtypes in pediatric high-resolution esophageal manometry (HRM) recordings. Neurogastroenterol Motil. 29, e13113 (2017).
Bogte, A. et al. Assessment of bolus transit with intraluminal impedance measurement in patients with esophageal motility disorders. Neurogastroenterol. Motil. 27, 1446–1452 (2015).
Tutuian, R. et al. Esophageal function testing with combined multichannel intraluminal impedance and manometry: multicenter study in healthy volunteers. Clin. Gastroenterol. Hepatol. 1, 174–182 (2003).
Tutuian, R. & Castell, D. O. Clarification of the esophageal function defect in patients with manometric ineffective esophageal motility: studies using combined impedance-manometry. Clin. Gastroenterol. Hepatol. 2, 230–236 (2004).
Blonski, W. et al. An analysis of distal esophageal impedance in individuals with and without esophageal motility abnormalities. J. Clin. Gastroenterol. 42, 776–781 (2008).
Tutuian, R. & Castell, D. O. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: study in 350 patients. Am. J. Gastroenterol. 99, 1011–1019 (2004).
Tseng, P. H. et al. Normative values and factors affecting water-perfused esophageal highresolution impedance manometry for a Chinese population. Neurogastroenterol. Motil. 30, e13265 (2018).
Rommel, N. et al. Automated impedance manometry analysis as a method to assess esophageal function. Neurogastroenterol. Motil. 26, 636–645 (2014).
Lin, Z. et al. Parameters for quantifying bolus retention with high-resolution impedance manometry. Neurogastroenterol. Motil. 26, 929–936 (2014).
Singendonk, M. M. J. et al. Novel pressure-impedance parameters for evaluating esophageal function in pediatric achalasia. J. Pediatr. Gastroenterol. Nutr. 66, 37–42 (2018).
Rommel, N. et al. The potential benefits of applying recent advances in esophageal motility testing in patients with esophageal atresia. Front. Pediatr. 5, 137 (2017).
Singendonk, M. M. et al. Pressure-flow characteristics of normal and disordered esophageal motor patterns. J. Pediatr. 166, 690–696 (2015).
Ghosh, S. K. et al. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G988–G997 (2006).
Wu, J. F., Lu, C. H., Yang, C. H. & Tsai, I. J. Diagnostic role of anal sphincter relaxation integral in high-resolution anorectal manometry for Hirschsprung disease in infants. J. Pediatr. 194, 136–141 (2018).
Singendonk, M. M., Benninga, M. A. & van Wijk, M. P. Reflux monitoring in children. Neurogastroenterol. Motil. 28, 1452–1459 (2016).
Wu, J. F. et al. Combined multichannel intraluminal impedance and pH monitoring assists the diagnosis of sliding hiatal hernia in children with gastroesophageal reflux disease. J. Gastroenterol. 48, 1242–1248 (2013).
Chiu, J. Y., Wu, J. F. & Ni, Y. H. Correlation between gastroesophageal reflux disease questionnaire and erosive esophagitis in school-aged children receiving endoscopy. Pediatr. Neonatol. 55, 439–443 (2014).
Liu, Y. W. et al. The correlation between endoscopic reflux esophagitis and combined multichannel intraluminal impedance-pH monitoring in children. Pediatr. Neonatol. 57, 385–389 (2016).
Hojsak, I. et al. The role of combined 24-h multichannel intraluminal impedance-pH monitoring in the evaluation of children with gastrointestinal symptoms suggesting gastro-esophageal reflux disease. Neurogastroenterol. Motil. 28, 1488–1493 (2016).
Tutuian, R. et al. Multichannel intraluminal impedance in esophageal function testing and gastroesophageal reflux monitoring. J. Clin. Gastroenterol. 37, 206–215 (2003).
Rommel, N. et al. Objective assessment of swallow function in children with suspected aspiration using pharyngeal automated impedance manometry. J. Pediatr. Gastroenterol. Nutr. 58, 789–794 (2014).
Omari, T. I. et al. An impedance-manometry based method for non-radiological detection of pharyngeal postswallow residue. Neurogastroenterol. Motil. 24, e277–e284 (2012).
Nguyen, N. Q. et al. Automated impedance-manometry analysis detects esophageal motor dysfunction in patients who have non-obstructive dysphagia with normal manometry. Neurogastroenterol. Motil. 25, 238–245 (2013).
Myers, J. C. et al. Susceptibility to dysphagia after fundoplication revealed by novel automated impedance manometry analysis. Neurogastroenterol. Motil. 24, 812–e393 (2012).
Carlson, D. A. et al. Improved assessment of bolus clearance in patients with achalasia using high-resolution impedance manometry. Clin. Gastroenterol. Hepatol. 16, 672–680 (2018).
Lee, T. H. et al. Impedance analysis using high-resolution impedance manometry facilitates assessment of pharyngeal residue in patients with oropharyngeal dysphagia. J. Neurogastroenterol. Motil. 20, 362–370 (2014).
Acknowledgements
This work is supported by Donation Grant FD105012 and a Grant from National Taiwan University Hospital (NTUH 107 P06).
Author information
Authors and Affiliations
Contributions
J-.F.W. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. J-.F.W. wrote the manuscript. J-.F.W.: study concept and design. J-.F.W., C.C., P-.H.T., I-.J.T., Y-.C.L., and C-.H.Y.: contribution to study design. J.-.F.W., C.C., Y-.C.L., and P-.H.T.: acquisition of data, and analysis and interpretation of data. J-.F.W.: drafting of the manuscript. J-.F.W., C.C., P-.H.T., Y-.C.L., I-.J.T., and C-.H.Y.: critical revision of the manuscript for important intellectual content. J-.F.W.: statistical analysis. C-.H.Y., C.C., Y-.C.L.,: administrative, technical support. J-.F.W., C-.H.Y., and I-.J.T.: obtained funding. J-.F.W. and C-.H.Y.: study supervision. J-.F.W., CC, C-.H.Y., Y-.C.L., P-.H.T., and I-.J.T.: decision to submit the paper for publication. All authors has seen and approved the submission of this version of the manuscript and takes full responsibility for the manuscript. All authors had final approval of the final version to be published, and agreement to be accountable for all aspects of the work in ensuring that questions related the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wu, JF., Chung, C., Tseng, PH. et al. Distal contractile to impedance integral ratio assist the diagnosis of pediatric ineffective esophageal motility disorder. Pediatr Res 84, 849–853 (2018). https://doi.org/10.1038/s41390-018-0197-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0197-3