Abstract
Background
Supplementation of formula with bovine milk fat globule membranes has been shown to narrow the gap in immunological and cognitive development between breast-fed and formula-fed infants.
Method
In a double-blinded randomized controlled trial 160 formula-fed infants received an experimental formula (EF), supplemented with bovine milk fat globule membranes, or standard formula until 6 months of age. A breast-fed reference group was recruited. Lipidomic analyses were performed on plasma and erythrocyte membranes at 6 months and on serum at 4 and 12 months of age.
Results
At 6 months of age, we observed a significant separation in the plasma lipidome between the two formula groups, mostly due to differences in concentrations of sphingomyelins (SM), phosphatidylcholines (PC), and ceramides, and in the erythrocyte membrane lipidome, mostly due to SMs, PEs and PCs. Already at 4 months, a separation in the serum lipidome was evident where SMs and PCs contributed. The separation was not detected at 12 months.
Conclusions
The effect of MFGM supplementation on the lipidome is likely part of the mechanisms behind the positive cognitive and immunological effects of feeding the EF previously reported in the same study population.
Similar content being viewed by others
Introduction
Human milk is the optimal nutrition during early infancy, and WHO, UNICEF, and the ESPGHAN Committee on Nutrition all recommend exclusive breastfeeding until 6 month of age.1 If human milk is not available, in developed countries infant formula is the safe alternative even if observational studies have revealed small health disadvantages in formula-fed compared to breast-fed infants. Even after adjustment for socio-economic factors, breast-fed infants have better cognitive development,2,3 and a lower incidence of infections4 compared with formula-fed suggesting that components of human milk, lacking or present at lower concentrations in infant formula, are needed for optimal development of the brain and the immune system. Some of the likely components are present in the milk fat globule membrane (MFGM). Sphingomyelin, gangliosides, sialic acid, cholesterol, and choline are all present in higher/altered concentrations in human milk compared to infant formulas, are components of the MFGM, and have been associated with improved brain function in humans and in animal models.5,6,7,8,9,10,11,12,13,14,15 Further, several of the close to 200 proteins of the MFGM,16 e.g., lactoferrin,17 mucins and butyrophilin,18 and components of the lipid fraction19 have immunological and/or antimicrobial functions. The MFGM fraction has historically been discarded during formula manufacturing when milk fat has been replaced by blends of vegetable oils. Recently different MFGM concentrates from bovine milk have become available and possible to add to infant formulas.
We recently performed a double-blinded randomized controlled trial exploring the effects of feeding infants an experimental formula (EF) supplemented with a bovine MFGM concentrate from <2 to 6 months of age. Infants fed the EF performed better on cognitive testing at 12 months of age20 and had lower incidence of otitis media during the intervention21 compared to infants fed a standard formula (SF).
The aim of the present study was to investigate the lipidome in serum/plasma and erythrocyte membranes of infants fed EF compared to infants fed SF and a breast-fed reference (BFR) group. Our hypothesis was that MFGM supplementation would affect the lipidome in serum/plasma and cell membranes, and thus be a likely contribution to the mechanisms mediating the positive effects of feeding EF on cognitive development and defense against infections.
Methods
Sample collection and formulas
As described previously,20 160 formula-fed infants, 80 boys and 80 girls, were randomized to receive either the EF or SF from inclusion until 6 months of age. Eighty breast-fed infants, 40 boys and 40 girls, were recruited as a reference group. Inclusion criteria were age <2 months, gestational age 37–42 weeks, birth weight 2500–4500 g, and no chronic illness. Infants were either exclusively formula-fed or exclusively breast-fed at inclusion. BabySemp (Semper AB, Sundbyberg, Sweden) was used as SF and the EF was modified from this by lowering the energy density from 66 to 60 kcal/100 mL, the protein concentration from 1.27 to 1.20 g/100 mL and by supplementation with a bovine MFGM fraction (Lacprodan MFGM-10; Arla Foods ingredients, Viby, Denmark), contributing 4% (wt/wt) MFGM protein of the total protein content. In both formulas long-chain polyunsaturated fatty acids were added to the same concentration (arachidonic acid (20:4n-6, ARA) 15 mg/100 ml and docosahexaenoic acid (22:6n-3, DHA) 9 mg/100 ml). Infants in the EF group totally compensated for the lower energy and protein contents of the EF formula by larger ingested volumes resulting in identical total energy and protein intakes for the EF and SF groups and only marginally higher total fat intake for the EF group.20 The intervention was blinded for the study staff and parents until all infants had passed the study visit at 12 months of age. Blood samples were collected at 4, 6, and 12 months of age and were obtained >2 h after the latest meal. The study was approved by the Regional Ethical Review Board in Umeå. Complete oral and written information was given to the parents/caregivers, and written consent was obtained from parents/caregivers of all infants before inclusion. This study was registered with number NCT00624689 at clinicaltrials.gov. A study flow chart is presented in Fig. 1. A CONSORT checklist is available as supplemental material (Supplemental Table S1).
Sample preparation
Venous blood was collected in EDTA and SST tubes. Serum at 4 and 12 months were obtained by centrifugation at 1300× g for 10 min and frozen. At 6 months, plasma and erythrocytes were separated by centrifugation at 2000× g for 10 min at 4 °C. After removal of plasma, the erythrocyte pellet was washed three times with cold 0.15 M NaCl-1 mM EDTA, pH 7.4. The erythrocytes were resuspended in the EDTA-NaCl-solution and 10 µl DL-ɑ-tocopherol (1.6 mg/ml ethanol) was added. Samples were stored at –80 °C. The plasma, erythrocyte and serum samples lipid extraction was according to Folch et al.22
To 20 µl of plasma 110 µl of CHCl3:MeOH (2:1 v/v) including internal standard solution was added. The samples were shaken for 2 min, and allowed to stand for 30 min at room temperature before centrifugation (14000 rpm, 3 min, 4 °C). A volume of 50 µl of the lower phase was collected and mixed with 70 µl of CHCl3:MeOH (2:1 v/v). Extracts were stored at –80 °C.
To 10 µL of serum 10 µL of 0.9% NaCl, 40 µL of CHCl3:MeOH (2:1, v/v), and 80 µL of an 3.5 µg mL−1 internal standard solution was added. The samples were vortexed and allowed to stand on ice for 30 min after which they were centrifuged (9400× g, 3 min, 4 °C). A volume of 60 µL from the lower layer of each sample was transferred to a glass vial with an insert and 60 µL of CHCl3:MeOH (2:1, v/v) was added. The samples were randomized before analysis.
To 20 µl of erythrocytes, 30 µl of 0.15 M NaCl and 250 µl of CHCl3:MeOH (2:1 v/v), including internal standards, were added. The samples were shaken for 2 min, and allowed to stand for 30 min at room temperature before centrifugation (14000 rpm, 3 min, 4 °C). After centrifugation 120 µl of the lower phase was collected and this ready-to-use extract was stored at –80 °C.
Lipidomic analyses and data processing
Plasma at 6 months of age
The following compounds were used as internal standards: Phosphatidic acid (PA) (17:0/17:0), Phosphatidylethanolamine (PE) (17:0/17:0), Phosphatidylglycerol (PG) (17:0/17:0), Monoacylglycerol (MG) (17:0/0:0/0:0), Diacylglycerol (DG) (17:0/0:0/17:0), Triacylglycerol (TG) (17:0/17:0/17:0), Phosphatidylcholine (PC) (19:0/19:0), and Lyso-PC(17:0/0:0), TG(16:0/16:0/16:0)-13C3. QC-samples (Quality control sample, mix of extract from the samples), dilution series of QC and LIMA-samples (pooled plasma samples from the lab) were used as a quality check. The samples were analyzed using ultra-high performance liquid chromatography-quadrupole time-of-flight/mass spectrometry (UHPLC-QTOF/MS) system (Agilent Technologies, Santa Clara, CA). Lipids were separated on a ACQUITY UPLC® CSH C18 column (2.1 × 50 mm, 1.7 µm) (Waters, Milford, MA) using a method modified from,23 and detected by an 6540 Q-TOF mass spectrometer in positive ion mode. Targeted MS data processing was performed using Agilent MassHunter ProFinder B.06 software (Agilent Technologies). In-house libraries with masses and internal retention times were used for lipid annotation according to lipid class and the total number of carbons and double bond. For some lipids, each specific fatty acid tail was detected and is in those cases presented. An analytic effect in intensity of detection was observed and data were therefore normalized using the internal standards.
Serum at 4 and 12 months of age
The following compounds were used as internal standards: PA(17:0/17:0), PG(17:0/17:0), PE(17:0/17:0), TG(19:0/19:0/19:0), PC(14:0)-d13, PC(17:0/17:0), TG(16:0/16:0/16:0)-13C3, Cer(d18:1/17:0), Sphingomyelin (SM) (d18:1/17:0), Lyso-PC(17:0/0:0), TG(8:0/8:0/8:0)-13C3, PC(16:0-d31/18:1), Cholesteryl ester (CE) (17:0), Phosphatidylserine (PS) (17:0/17:0), and TG(15:0/15:0/15:0). The lipidomics analyses were performed on a UHPLC-QTOF/MS system (Agilent Technologies) in positive ion mode using a previously described method.24 Data processing was performed using MZmine 2.17 including detection and alignment of peaks, peak integration, normalization, and peak identification.25 Data imputation of missing values was performed with half of the rows minimum. Lipids were annotated according to lipid class, total number of carbon and double bonds. For some lipids each specific fatty acid tail was detected and is in those cases presented. As a batch effect was observed, i.e., lipid concentrations varied among batches, the data were normalized using internal standards.
Erythrocyte membranes at 6 months of age
Two internal standards were used, TG(16:0/16:0/16:0)-13C3 (in positive mode) and Cer(d18:1/16:0-d31) (in positive and negative mode). QC-samples and dilution series of QC was used as a quality check. Samples were analyzed by UHPLC-QTOF/MS (Agilent 6550) in positive and negative ion mode. For analysis details and data processing (in ProFinder B.08) see above for plasma at 6 months. There was a concentration effect, i.e., a variation in total lipid concentration among samples, and the data were normalized using the total sum of lipids.
Statistics
The multivariate analyses at 6 months of age were based on identified lipids and at 4 and 12 months on both identified and unidentified lipids. To detect any outliers, principal component analysis (PCA) was performed. Strong outliers were identified by Hotelling’s T2. Orthogonal projections to latent structures discriminant analysis (OPLS-DA) were performed to detect any class variation in the lipid profile between the groups. To validate the model cross-validation, permutation test and CV-ANOVA were used. Significance level was set to CV-ANOVA p < 0.001.26 Multivariate analyses were performed using SIMCA (SIMCA-P + 14.1, Umetrics, Umeå, Sweden). Differences in relative concentrations of specific lipids were analysed using the two-sided t-test (SPSS statistics 25) of log transformed relative concentrations and reported as unadjusted p-values. To highlight specific lipids of interest, a combination of OPLS-DA loadings, variable importance in the projection (VIP) and the two-sided t-test were used. Significant levels were set to VIP > 1 and unadjusted p < 0.001.
Results
Plasma lipidome at 6 months of age
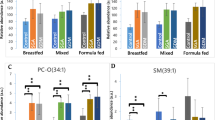
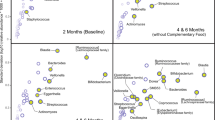
Of the 220 infants still in the study at 6 months of age, lipidomic analyses of plasma were performed in 213 infants (73 EF, 70 SF, 70 BFR) (Fig. 1). No outliers were detected. There was a clear separation between the EF and SF groups in the OPLS-DA (Fig. 2). The separation was mostly due to SM, PC, Cer, and TG. Most of the phospholipids (PL) and TG species important for the separation were higher in relative concentration in the EF group compared to the SF group. Individual lipids with the highest VIP values were SM, PC, and Cer species (Table 1).
Serum lipidome at 4 and 12 months of age
Analyses were performed on serum samples from a randomly selected subgroup (n = 90; 30 SF, 30 EF, and 30 BFR) at 4 and 12 months of age (Fig. 1). At 4 and 12 months of age, one sample was excluded due to analytical problems. No outliers were detected. At 4 months, there was a clear separation between the EF and SF groups in the OPLS-DA. The separation was transient and not detected at 12 months, 6 months after the intervention ended (Fig. 3). The group separation at 4 months of age was mostly due to SM and PC, whereas PE and TG contributed less. Most of the PLs important for the separation were higher in the EF group compared to the SF group. Individual lipids with the highest VIP values were SM and PC species (Table 1).
OPLS-DA plot of serum lipidomics at 4 (circles) and 12 (triangles) months for the SF (green) and EF (blue) groups. OPLS-DA (2 + 4, R2X (cum) 0.588, R2Y (cum) 0,667, Q2 (cum) 0,414) CV-ANOVA < 0.001 (uv-scaled), 484 lipids, both identified and unidentified, were included in the model. There was a clear separation between the SF and EF group at 4 months but not at 12 months of age
Erythrocyte membrane lipidome at 6 months of age
Lipidomic analyses on erythrocyte membranes were performed in 213 infants (70 SF, 73 EF, 70 BFR) at 6 months of age (Fig. 1). No outliers were detected. There was a clear separation between the two formula groups in the OPLS-DA (Fig. 4). Lipids important for the separation were mostly SM, PE, and PC species. Relative concentrations of most of the PLs important for the separation were higher in the EF group (Table 1).
OPLS-DA plot of erythrocyte membrane lipidomics at 6 months for the SF (green circles) and EF (blue circles) groups. OPLS-DA (1 + 1, R2X (cum) 0.238, R2Y (cum) 1, Q2 (cum) 0.585) CV-ANOVA < 0.001 (uv-scaled), 112 lipids are included in the model. There was a clear separation between the formula groups
Discussion
We found significant differences in the serum/plasma lipidome at 4 and 6 months of age in infants fed the EF compared to infants fed SF. This separation was also detected in erythrocyte membranes at 6 months, but did not remain in sera collected at 12 months of age, 6 months after the end of the intervention. SM and PC species contributed most to the separation in serum/plasma and erythrocyte membranes at 4 and 6 months of age. At 6 months Cer also contributed to the separation in plasma. Most PCs important for the separation were higher in the EF group as opposed to Cer and SM species having a more heterogeneous pattern. In the erythrocyte membrane, PEs also contributed to the separation and were higher in the EF group. These separations are reasonably caused by differences in lipid intake between the two formula groups. We have previously presented, in the same study population, that the EF group had moderately higher total serum cholesterol concentration than the SF group at 6 months of age27 due to higher cholesterol intake during the intervention. The conspicuous difference in the lipidomic profile between the EF and SF groups at 6 months of age in the present study is unlikely caused by the higher total fat intake in the EF group, which was mainly caused by a higher TG intake, but is rather explained by a clear difference in fat quality due to the MFGM supplementation of the EF as the lipids important for the separation between the EF and SF groups are polar lipids present at high concentrations in the MFGM.
Type of feeding, i.e., breastfeeding or formula-feeding, and type of fat consumed affect blood lipid profiles in infants. In a prospective observational cohort study, breast-fed, compared to formula-fed infants, had a different lipid profile and three lipid molecular species, PC (35:2), SM (36:2) and SM (39:1), were suggested to be used collectively as biomarkers indicating whether infants were formula- or breast-fed.28 In the present study these lipids had high VIP values and showed the same pattern when comparing the SF and BFR groups at 4 months, supporting the role of these lipids as biomarkers of SF feeding vs breastfeeding. However, the pattern of these lipids differ between the EF and SF groups indicating that these biomarkers might not be valid when comparing infants fed MFGM supplemented formula with breast-fed infants.
A previous study showed that feeding preterm infants sphingomyelin-fortified formula resulted in higher SM concentrations and more favorable neurodevelopment,15 and feeding piglets a formula supplemented with PL and gangliosides resulted in improved spatial learning and growth of brain tissue.29 Several differences in the lipidome between the EF and SF groups in our study are interesting in relation to our previous finding that the EF group had improved cognitive development compared to the SF group at 12 months of age.20 Higher relative concentrations of PCs and some SMs contributed to the separation between the groups, both in plasma, serum, and erythrocyte membrane. The concentrations of several Cer, a metabolite of SMs and other sphingolipids30 were affected indicating that the metabolism of sphingolipids was altered. Cognitive function reflects brain development from fetal life until the time of measurement. A positive effect on brain development at any time before 12 months of age may theoretically explain the positive effect on cognitive function seen in the EF group at this time point. Thus, the clear lipidomic differences between the EF and SF groups during the intervention period could be relevant for the effect on cognitive function even if the effect on the lipidome was transient and not present at 12 months of age.
The erythrocyte membrane lipidome, which is less affected by the recent dietary intake and rather reflects long-term lipid intake,31 was clearly different between the EF and SF groups. The separation was mostly due to SM, PC, and PE species. A dietary effect on the erythrocyte membrane lipidome is likely a proxy for effects on cell membranes in other tissues. Extrapolating lipidomic observations to the central nervous system must however be made with caution, since the transport of lipids and fatty acids over the blood-brain barrier (BBB) is not fully understood. Two different transport mechanisms have been implicated, i.e., passive diffusion or transporter proteins.32 Animal and in vitro studies have addressed the uptake and metabolism of DHA over the BBB. In a recent report, fatty acid transport protein 1 (FATP 1), localized in the basal membrane of brain microvessels, was suggested to contribute to the transport of DHA, taurine, and biotin into the brain.33 DHA attached to Lyso-PC seems to be the preferred form of DHA taken up by the brain34 and might be mediated by the transporter Mfsd2a.35 These studies indicate that not only the concentration of a fatty acid is of importance, but also in which form, esterified or non-esterified, and if esterified, to which lipid affect its transport across the BBB. The association between the lipidome in blood and central nervous system is not fully understood. However, in a Finnish study, erythrocytes, retinal cells, and cells from the optic nerve from nine humans were collected. Several lipids of the erythrocytes correlated with lipids in ocular tissue.36
With respect to immunological effects, it is known that PLs play an important role in modulating gut inflammation,37 and also affect the inflammatory response in the lungs during pulmonary infection.38 Sphingolipid metabolites, Cer, and sphingosine-1-phosphate, regulate immune cell trafficking and epithelial integrity and therefore are important in inflammation and inflammatory diseases.30 Changes in SM and its metabolite Cer affect T-cell response, both in early activation steps and effector functions.39 Our intervention did affect the lipidome of erythrocyte membranes, where alteration of SMs contributed, thus suggesting that the lipid composition of cell membranes of immune cells are affected similarly. We also found an effect on SM and Cer in plasma at 6 months of age. An improved cell function due to the altered lipid composition of the cell membrane is a likely part of the mechanism behind the positive effect on the immune system previously reported in the same study population.21
A strength of the current study is the double-blinded randomized design and the longitudinal aspect. Besides the analyses of plasma and erythrocyte membranes at 6 months of age, we analyzed the serum lipidome both at 4 months of age, when the intervention was at its maximum, and at 12 months of age, 6 months after the intervention. This made it possible to study both direct and possible long-term effects on the lipidomes. Another strength was the low drop-out rate.
All analyses were performed on an intention-to-treat basis. As previously described,20 a majority of the parents introduced taste portions of solid food between 4 and 6 months of age in accord with the Swedish National Food Agency recommendations. Some parents stopped the study formula due to various symptoms, and breastfeeding was partly or totally stopped for some of the infants in the BFR group. This dilution of the intervention is a limitation of the study but gives an estimate of the effect on the population level.
The blood samples were stored at –80 °C for 3–9 years until analysis. As previously described, the lipid components are stable during long-term storage at −80 °C,40 although some effect has been shown on the concentration of LPC.41 Since the formula-fed infants were recruited randomly the in between group separation should not be affected by the storage time.
Since we are using a multivariate analysis method and our method is limited to identify class of lipid and number of carbons and bonds, the results regarding specific lipids should be interpreted with caution. The main purpose of the analysis was to detect potential differences in the lipidomic profile, not to identify specific lipids. To confirm and validate specific lipids of interest, and to match specific lipids between the MFGM fraction and blood samples, a targeted method should be used.
The intervention of the study is complex including both supplementation with a bovine MFGM concentrate and adjustments of macronutrients as previously described.20 Any effect of the intervention may be due to a number of different mechanisms and it is not possible to separate one part of the intervention from another. The complex intervention is a limitation for the interpretation of mechanisms behind the functional outcomes, which can only be speculative.
In conclusion, we found that feeding infants a formula supplemented with a bovine MFGM fraction clearly affects their serum/plasma and erythrocyte membrane lipidomes. Infants fed the EF had higher concentrations of many PCs and altered concentrations of SMs. It is reasonable that these changes reflect or are part of the mechanisms mediating the positive effects on cognitive development and immune defense previously shown in the study population. However, to verify this, further studies are needed to confirm the observed effects of MFGM supplemented infant formula on infant lipid metabolism and to study the mechanism behind the functional outcomes in dept.
References
Agostoni, C. et al. Breast-feeding: a commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 49, 112–125 (2009).
Anderson, J. W., Johnstone, B. M. & Remley, D. T. Breast-feeding and cognitive development: a meta-analysis. Am. J. Clin. Nutr. 70, 525–535 (1999).
Horta, B. L., Loret de Mola, C. & Victora, C. G. Breastfeeding and intelligence: a systematic review and meta-analysis. Acta Paediatr. 104, 14–19 (2015).
Ip, S., Chung, M., Raman, G., Trikalinos, T. A. & Lau, J. A summary of the Agency for Healthcare Research and Quality’s evidence report on breastfeeding in developed countries. Breastfeed. Med. 4(Suppl 1), S17–S30 (2009).
Oshida, K. et al. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatr. Res. 53, 589–593 (2003).
Zeisel, S. H., Char, D. & Sheard, N. F. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J. Nutr. 116, 50–58 (1986).
Gurnida, D. A., Rowan, A. M., Idjradinata, P., Muchtadi, D. & Sekarwana, N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum. Dev. 88, 595–601 (2012).
Wang, B. Molecular mechanism underlying sialic acid as an essential nutrient for brain development and cognition. Adv. Nutr. 3, 465s–472s (2012).
Wang, B., McVeagh, P., Petocz, P. & Brand-Miller, J. Brain ganglioside and glycoprotein sialic acid in breastfed compared with formula-fed infants. Am. J. Clin. Nutr. 78, 1024–1029 (2003).
Claumarchirant, L., Matencio, E., Sanchez-Siles, L. M., Alegria, A. & Lagarda, M. J. Sterol composition in infant formulas and estimated intake. J. Agric. Food Chem. 63, 7245–7251 (2015).
Haque, Z. U. & Mozaffar, Z. Importance of dietary cholesterol for the maturation of mouse brain myelin. Biosci. Biotechnol. Biochem. 56, 1351–1354 (1992).
Elias, P. K., Elias, M. F., D’Agostino, R. B., Sullivan, L. M. & Wolf, P. A. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom. Med. 67, 24–30 (2005).
Zeisel, S. H. The fetal origins of memory: the role of dietary choline in optimal brain development. J. Pediatr. 149, S131–S136 (2006).
Koletzko, B. Human milk lipids. Ann. Nutr. Metab. 69(Suppl 2), 28–40 (2016).
Tanaka, K. et al. The pilot study: sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain. Dev. 35, 45–52 (2013).
Liao, Y., Alvarado, R., Phinney, B. & Lonnerdal, B. Proteomic characterization of human milk fat globule membrane proteins during a 12 month lactation period. J. Proteome Res. 10, 3530–3541 (2011).
King, J. C. et al. A double-blind, placebo-controlled, pilot study of bovine lactoferrin supplementation in bottle-fed infants. J. Pediatr. Gastroenterol. Nutr. 44, 245–251 (2007).
Liu, B. & Newburg, D. S. Human milk glycoproteins protect infants against human pathogens. Breastfeed. Med. 8, 354–362 (2013).
Fuller, K. L., Kuhlenschmidt, T. B., Kuhlenschmidt, M. S., Jimenez-Flores, R. & Donovan, S. M. Milk fat globule membrane isolated from buttermilk or whey cream and their lipid components inhibit infectivity of rotavirus in vitro. J. Dairy Sci. 96, 3488–3497 (2013).
Timby, N., Domellof, E., Hernell, O., Lonnerdal, B. & Domellof, M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am. J. Clin. Nutr. 99, 860–868 (2014).
Timby, N. et al. Infections in infants fed formula supplemented with bovine milk fat globule membranes. J. Pediatr. Gastroenterol. Nutr. 60, 384–389 (2015).
Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 (1957).
Nygren, H., Seppanen-Laakso, T., Castillo, S., Hyotylainen, T. & Oresic, M. Liquid chromatography-mass spectrometry (LC-MS)-based lipidomics for studies of body fluids and tissues. Methods Mol. Biol. 708, 247–257 (2011).
O’Gorman, A. et al. Identification of a plasma signature of psychotic disorder in children and adolescents from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort. Transl. Psychiatry 7, e1240 (2017).
Pluskal, T., Castillo, S., Villar-Briones, A. & Oresic, M. MZmine 2: modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinforma. 11, 395 (2010).
Wheelock, A. M. & Wheelock, C. E. Trials and tribulations of ‘omics data analysis: assessing quality of SIMCA-based multivariate models using examples from pulmonary medicine. Mol. Biosyst. 9, 2589–2596 (2013).
Timby, N., Lönnerdal, B., Hernell, O. & Domellöf, M. Cardiovascular risk markers until 12 mo of age in infants fed a formula supplemented with bovine milk fat globule membranes. Pediatr. Res. 76(4), 394–400 (2014).
Acharjee, A. et al. The translation of lipid profiles to nutritional biomarkers in the study of infant metabolism. Metabolomics 13, 25 (2017).
Liu, H. et al. Early supplementation of phospholipids and gangliosides affects brain and cognitive development in neonatal piglets. J. Nutr. 144, 1903–1909 (2014).
Maceyka, M. & Spiegel, S. Sphingolipid metabolites in inflammatory disease. Nature 510, 58–67 (2014).
Arab, L. Biomarkers of fat and fatty acid intake. J. Nutr. 133(Suppl 3), 925s–932s (2003).
Mitchell, R. W., On, N. H., Del Bigio, M. R., Miller, D. W. & Hatch, G. M. Fatty acid transport protein expression in human brain and potential role in fatty acid transport across human brain microvessel endothelial cells. J. Neurochem. 117, 735–746 (2011).
Ochiai, Y. et al. The blood-brain barrier fatty acid transport protein 1 (FATP1/SLC27A1) supplies docosahexaenoic acid to the brain, and insulin facilitates transport. J. Neurochem. 141, 400–412 (2017).
Lagarde, M. et al. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 16, 201–204 (2001).
Nguyen, L. N. et al. Mfsd2a is a transporter for the essential omega-3 fatty acid docosahexaenoic acid. Nature 509, 503–506 (2014).
Acar, N. et al. Lipid composition of the human eye: are red blood cells a good mirror of retinal and optic nerve fatty acids? PLoS ONE 7, e35102 (2012).
Sturm, A. & Dignass, A. U. Modulation of gastrointestinal wound repair and inflammation by phospholipids. Biochim. Biophys. Acta 1582, 282–288 (2002).
Shaikh, S. R., Fessler, M. B. & Gowdy, K. M. Role for phospholipid acyl chains and cholesterol in pulmonary infections and inflammation. J. Leukoc. Biol. 100, 985–997 (2016).
Beyersdorf, N. & Muller, N. Sphingomyelin breakdown in T cells: role in activation, effector functions and immunoregulation. Biol. Chem. 396, 749–758 (2015).
Matthan, N. R., Ip, B., Resteghini, N., Ausman, L. M. & Lichtenstein, A. H. Long-term fatty acid stability in human serum cholesteryl ester, triglyceride, and phospholipid fractions. J. Lipid Res. 51, 2826–2832 (2010).
Hyotylainen, T. & Oresic, M. Optimizing the lipidomics workflow for clinical studies—practical considerations. Anal. Bioanal. Chem. 407, 4973–4993 (2015).
Acknowledgements
We are grateful to the participating infants and their parents. We thank the research nurses Carina Forslund and Camilla Steinvall Lindberg, as well as the medical laboratory technologists Carina Lagerqvist for their dedicated field- and laboratory work. We also thank Hans Stenlund, Maria Ahnlund, Jonas Gullberg, and Annika Johansson at Swedish Metabolomics Centre (Cooperation between Umeå University and Swedish University of Agricultural Sciences, Umeå, Sweden) for plasma and erythrocyte membrane lipidomic analyses, Anette Untermann for technical assistance in the serum lipidomic analyses performed at the Steno Diabetes Center Copenhagen (Copenhagen, Denmark) and BILS (Bioinformatics for life sciences Sweden, currently NBIS) for support through Rui Climaco Pinto (based at CLiC -Computational life science cluster, Umeå University, Umeå, Sweden) for statistical advice. T.G. and N.T. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The study was funded by grants from Sweden's Innovation Agency (Vinova), Semper/Hero and Västerbotten County Council.
Author information
Authors and Affiliations
Contributions
Substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; T.G., T.S.D., L.A., M.D., O.H., T.H., M.K., B.L., M.O. and N.T. Drafting the article or revising it critically for important intellectual content; T.G., T.S.D., L.A., M.D., O.H., T.H., M.K., B.L., M.O. and N.T. Final approval of the version to be published T.G., T.S.D., L.A., M.D., O.H., T.H., M.K., B.L., M.O. and N.T.
Corresponding author
Ethics declarations
Competing interests
O.H. and B.L. are members of Hero and Semper scientific advisory boards. T.G., T.S.D., L.A., M.D., T.H., M.K., M.O. and N.T. declare no conflicts of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Grip, T., Dyrlund, T.S., Ahonen, L. et al. Serum, plasma and erythrocyte membrane lipidomes in infants fed formula supplemented with bovine milk fat globule membranes. Pediatr Res 84, 726–732 (2018). https://doi.org/10.1038/s41390-018-0130-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0130-9
This article is cited by
-
Metabolic phenotype of breast-fed infants, and infants fed standard formula or bovine MFGM supplemented formula: a randomized controlled trial
Scientific Reports (2019)
-
Fecal microbiome and metabolome of infants fed bovine MFGM supplemented formula or standard formula with breast-fed infants as reference: a randomized controlled trial
Scientific Reports (2019)