Abstract
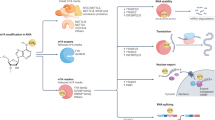
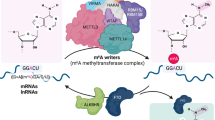
Chemical modifications of ribonucleotides significantly alter the physicochemical properties and functions of RNA. Initially perceived as static and essential marks in ribosomal RNA (rRNA) and transfer RNA (tRNA), recent discoveries unveiled a dynamic landscape of RNA modifications in messenger RNA (mRNA) and other regulatory RNAs. These findings spurred extensive efforts to map the distribution and function of RNA modifications, aiming to elucidate their distribution and functional significance in normal cellular homeostasis and pathological states. Significant dysregulation of RNA modifications is extensively documented in cancers, accentuating the potential of RNA-modifying enzymes as therapeutic targets. However, the essential role of several RNA-modifying enzymes in normal physiological functions raises concerns about potential side effects. A notable example is N-acetyltransferase 10 (NAT10), which is responsible for acetylating cytidines in RNA. While emerging evidence positions NAT10 as an oncogenic factor and a potential target in various cancer types, its essential role in normal cellular processes complicates the development of targeted therapies. This review aims to comprehensively analyze the essential and oncogenic properties of NAT10. We discuss its crucial role in normal cell biology and aging alongside its contribution to cancer development and progression. We advocate for agnostic approaches to disentangling the intertwined essential and oncogenic functions of RNA-modifying enzymes. Such approaches are crucial for understanding the full spectrum of RNA-modifying enzymes and imperative for designing effective and safe therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data shown in this manuscript are publicly available at the following databases: MODOMICS [1], DepMap [125], cBioportal [36], The University of ALabama at Birmingham CANcer data analysis portal (UALCAN) [126], AlphaFold [127], and BioGRID [128]. Data were downloaded from the databases, reanalyzed, and graphed using publicly available R packages. All code is available upon request.
References
Boccaletto P, Stefaniak F, Ray A, Cappannini A, Mukherjee S, Purta E, et al. MODOMICS: a database of RNA modification pathways. 2021 update. Nucleic Acids Res. 2022;50:D231–D5.
Moshitch-Moshkovitz S, Dominissini D, Rechavi G. The epitranscriptome toolbox. Cell. 2022;185:764–76.
Desrosiers R, Friderici K, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci USA. 1974;71:3971–5.
Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–6.
Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell. 2012;149:1635–46.
Shi H, Wei J, He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol Cell. 2019;74:640–50.
Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, et al. The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med. 2017;23:1369–76.
Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millan-Zambrano G, Robson SC, et al. Promoter-bound METTL3 maintains myeloid leukaemia by m(6)A-dependent translation control. Nature. 2017;552:126–31.
Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593:597–601.
Sturgess K, Yankova E, Vijayabaskar MS, Isobe T, Rak J, Kucinski I, et al. Pharmacological inhibition of METTL3 impacts specific haematopoietic lineages. Leukemia. 2023;37:2133–7.
Haruehanroengra P, Zheng YY, Zhou Y, Huang Y, Sheng J. RNA modifications and cancer. RNA Biol. 2020;17:1560–75.
Wang L, Tao Y, Zhai J, Xue M, Zheng C, Hu H. The emerging roles of ac4C acetylation “writer” NAT10 in tumorigenesis: A comprehensive review. Int J Biol Macromol. 2023;254:127789.
Rancati G, Moffat J, Typas A, Pavelka N. Emerging and evolving concepts in gene essentiality. Nat Rev Genet. 2018;19:34–49.
Balmus G, Larrieu D, Barros AC, Collins C, Abrudan M, Demir M, et al. Targeting of NAT10 enhances healthspan in a mouse model of human accelerated aging syndrome. Nat Commun. 2018;9:1700.
Wang M, Cheng R, He H, Han Z, Zhang Y, Wu Q. N(4)-acetylcytidine of Nop2 mRNA is required for the transition of morula-to-blastocyst. Cell Mol Life Sci. 2023;80:307.
Chen L, Wang WJ, Liu Q, Wu YK, Wu YW, Jiang Y, et al. NAT10-mediated N4-acetylcytidine modification is required for meiosis entry and progression in male germ cells. Nucleic Acids Res. 2022;50:10896–913.
Lin J, Xiang Y, Huang J, Zeng H, Zeng Y, Liu J, et al. NAT10 maintains OGA mRNA stability through ac4C modification in regulating oocyte maturation. Front Endocrinol. 2022;13:907286.
Xiang Y, Zhou C, Zeng Y, Guo Q, Huang J, Wu T, et al. NAT10-mediated N4-acetylcytidine of RNA contributes to post-transcriptional regulation of mouse oocyte maturation in vitro. Front Cell Dev Biol. 2021;9:704341.
Jiang X, Cheng Y, Zhu Y, Xu C, Li Q, Xing X, et al. Maternal NAT10 orchestrates oocyte meiotic cell-cycle progression and maturation in mice. Nat Commun. 2023;14:3729.
Duveau F, Felix MA. Role of pleiotropy in the evolution of a cryptic developmental variation in Caenorhabditis elegans. PLoS Biol. 2012;10:e1001230.
Wang W, Liu H, Wang F, Liu X, Sun Y, Zhao J, et al. N4-acetylation of cytidine in mRNA plays essential roles in plants. Plant Cell. 2023;35:3739–56.
Gramates LS, Agapite J, Attrill H, Calvi BR, Crosby MA, Dos Santos G, et al. FlyBase: a guided tour of highlighted features. Genetics. 2022;220:iyac035.
Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46:D1062–D7.
Hedgespeth BA, Birkenheuer AJ, Friedenberg SG, Olby NJ, Meurs KM. A novel missense mutation of the NAT10 gene in a juvenile Schnauzer dog with chronic respiratory tract infections. J Vet Intern Med. 2021;35:1542–6.
Sharma S, Langhendries JL, Watzinger P, Kotter P, Entian KD, Lafontaine DL. Yeast Kre33 and human NAT10 are conserved 18S rRNA cytosine acetyltransferases that modify tRNAs assisted by the adaptor Tan1/THUMPD1. Nucleic Acids Res. 2015;43:2242–58.
Sharma S, Yang J, van Nues R, Watzinger P, Kotter P, Lafontaine DLJ, et al. Specialized box C/D snoRNPs act as antisense guides to target RNA base acetylation. PLoS Genet. 2017;13:e1006804.
Ikeuchi Y, Kitahara K, Suzuki T. The RNA acetyltransferase driven by ATP hydrolysis synthesizes N4-acetylcytidine of tRNA anticodon. EMBO J. 2008;27:2194–203.
Sas-Chen A, Thomas JM, Matzov D, Taoka M, Nance KD, Nir R, et al. Dynamic RNA acetylation revealed by quantitative cross-evolutionary mapping. Nature. 2020;583:638–43.
Arango D, Sturgill D, Alhusaini N, Dillman AA, Sweet TJ, Hanson G, et al. Acetylation of cytidine in mRNA promotes translation efficiency. Cell. 2018;175:1872–86.e24.
Liu R, Wubulikasimu Z, Cai R, Meng F, Cui Q, Zhou Y, et al. NAT10-mediated N4-acetylcytidine mRNA modification regulates self-renewal in human embryonic stem cells. Nucleic Acids Res. 2023;51:8514–31.
Ge J, Wang Z, Wu J. NAT10-mediated ac(4)C modification promotes ectoderm differentiation of human embryonic stem cells via acetylating NR2F1 mRNA. Cell Prolif. 2023:e13577.
Larrieu D, Britton S, Demir M, Rodriguez R, Jackson SP. Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science. 2014;344:527–32.
Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419.
Tschida BR, Temiz NA, Kuka TP, Lee LA, Riordan JD, Tierrablanca CA, et al. Sleeping beauty insertional mutagenesis in mice identifies drivers of steatosis-associated hepatic tumors. Cancer Res. 2017;77:6576–88.
Tan TZ, Miow QH, Huang RY, Wong MK, Ye J, Lau JA, et al. Functional genomics identifies five distinct molecular subtypes with clinical relevance and pathways for growth control in epithelial ovarian cancer. EMBO Mol Med. 2013;5:1051–66.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4.
Yang C, Wu T, Zhang J, Liu J, Zhao K, Sun W, et al. Prognostic and immunological role of mRNA ac4C regulator NAT10 in pan-cancer: new territory for cancer research? Front Oncol. 2021;11:630417.
Zhang X, Liu J, Yan S, Huang K, Bai Y, Zheng S. High expression of N-acetyltransferase 10: a novel independent prognostic marker of worse outcome in patients with hepatocellular carcinoma. Int J Clin Exp Pathol. 2015;8:14765–71.
Li Q, Liu X, Jin K, Lu M, Zhang C, Du X, et al. NAT10 is upregulated in hepatocellular carcinoma and enhances mutant p53 activity. BMC Cancer. 2017;17:605.
Liang P, Hu R, Liu Z, Miao M, Jiang H, Li C. NAT10 upregulation indicates a poor prognosis in acute myeloid leukemia. Curr Probl Cancer. 2020;44:100491.
Wu J, Zhu H, Wu J, Chen W, Guan X. Inhibition of N-acetyltransferase 10 using remodelin attenuates doxorubicin resistance by reversing the epithelial-mesenchymal transition in breast cancer. Am J Transl Res. 2018;10:256–64.
Liu HY, Liu YY, Yang F, Zhang L, Zhang FL, Hu X, et al. Acetylation of MORC2 by NAT10 regulates cell-cycle checkpoint control and resistance to DNA-damaging chemotherapy and radiotherapy in breast cancer. Nucleic Acids Res. 2020;48:3638–56.
Pan Z, Bao Y, Hu M, Zhu Y, Tan C, Fan L, et al. Role of NAT10-mediated ac4C-modified HSP90AA1 RNA acetylation in ER stress-mediated metastasis and lenvatinib resistance in hepatocellular carcinoma. Cell Death Discov. 2023;9:56.
Wei W, Zhang S, Han H, Wang X, Zheng S, Wang Z, et al. NAT10-mediated ac4C tRNA modification promotes EGFR mRNA translation and gefitinib resistance in cancer. Cell Rep. 2023;42:112810.
Xie R, Cheng L, Huang M, Huang L, Chen Z, Zhang Q, et al. NAT10 drives cisplatin chemoresistance by enhancing ac4C-associated DNA repair in bladder cancer. Cancer Res. 2023;83:1666–83.
Cao Y, Yao M, Wu Y, Ma N, Liu H, Zhang B. N-acetyltransferase 10 promotes micronuclei formation to activate the senescence-associated secretory phenotype machinery in colorectal cancer cells. Transl Oncol. 2020;13:100783.
Guerrero Llobet S, Bhattacharya A, Everts M, Kok K, van der Vegt B, Fehrmann RSN, et al. An mRNA expression-based signature for oncogene-induced replication-stress. Oncogene. 2022;41:1216–24.
Liu H, Ling Y, Gong Y, Sun Y, Hou L, Zhang B. DNA damage induces N-acetyltransferase NAT10 gene expression through transcriptional activation. Mol Cell Biochem. 2007;300:249–58.
Tan Y, Zheng J, Liu X, Lu M, Zhang C, Xing B, et al. Loss of nucleolar localization of NAT10 promotes cell migration and invasion in hepatocellular carcinoma. Biochem Biophys Res Commun. 2018;499:1032–8.
Zhang H, Hou W, Wang HL, Liu HJ, Jia XY, Zheng XZ, et al. GSK-3beta-regulated N-acetyltransferase 10 is involved in colorectal cancer invasion. Clin Cancer Res. 2014;20:4717–29.
Shen Q, Zheng X, McNutt MA, Guang L, Sun Y, Wang J, et al. NAT10, a nucleolar protein, localizes to the midbody and regulates cytokinesis and acetylation of microtubules. Exp Cell Res. 2009;315:1653–67.
Zheng J, Tan Y, Liu X, Zhang C, Su K, Jiang Y, et al. NAT10 regulates mitotic cell fate by acetylating Eg5 to control bipolar spindle assembly and chromosome segregation. Cell Death Differ. 2022;29:846–60.
Zi J, Han Q, Gu S, McGrath M, Kane S, Song C, et al. Targeting NAT10 induces apoptosis associated with enhancing endoplasmic reticulum stress in acute myeloid leukemia cells. Front Oncol. 2020;10:598107.
Zhang Y, Jing Y, Wang Y, Tang J, Zhu X, Jin WL, et al. NAT10 promotes gastric cancer metastasis via N4-acetylated COL5A1. Signal Transduct Target Ther. 2021;6:173.
Deng M, Zhang L, Zheng W, Chen J, Du N, Li M, et al. Helicobacter pylori-induced NAT10 stabilizes MDM2 mRNA via RNA acetylation to facilitate gastric cancer progression. J Exp Clin Cancer Res. 2023;42:9.
Tao W, Tian G, Xu S, Li J, Zhang Z, Li J. NAT10 as a potential prognostic biomarker and therapeutic target for HNSCC. Cancer Cell Int. 2021;21:413.
Liu S, Liu W, Ding Z, Yang X, Jiang Y, Wu Y, et al. Identification and validation of a novel tumor driver gene signature for diagnosis and prognosis of head and neck squamous cell carcinoma. Front Mol Biosci. 2022;9:912620.
Wang G, Zhang M, Zhang Y, Xie Y, Zou J, Zhong J, et al. NAT10-mediated mRNA N4-acetylcytidine modification promotes bladder cancer progression. Clin Transl Med. 2022;12:e738.
Ma N, Liu H, Wu Y, Yao M, Zhang B. Inhibition of N-acetyltransferase 10 suppresses the progression of prostate cancer through regulation of DNA replication. Int J Mol Sci. 2022;23:6573.
Zong G, Wang X, Guo X, Zhao Q, Wang C, Shen S, et al. NAT10-mediated AXL mRNA N4-acetylcytidine modification promotes pancreatic carcinoma progression. Exp Cell Res. 2023;428:113620.
Xu D, Huang K, Chen Y, Yang F, Xia C, Yang H. Immune response and drug therapy based on ac4C-modified gene in pancreatic cancer typing. Front Immunol. 2023;14:1133166.
Zhang Y, Deng Z, Sun S, Xie S, Jiang M, Chen B, et al. NAT10 acetylates BCL-XL mRNA to promote the proliferation of multiple myeloma cells through PI3K-AKT pathway. Front Oncol. 2022;12:967811.
Wei R, Cui X, Min J, Lin Z, Zhou Y, Guo M, et al. NAT10 promotes cell proliferation by acetylating CEP170 mRNA to enhance translation efficiency in multiple myeloma. Acta Pharm Sin B. 2022;12:3313–25.
Liao L, He Y, Li SJ, Yu XM, Liu ZC, Liang YY, et al. Lysine 2-hydroxyisobutyrylation of NAT10 promotes cancer metastasis in an ac4C-dependent manner. Cell Res. 2023;33:355–71.
Yu XM, Li SJ, Yao ZT, Xu JJ, Zheng CC, Liu ZC, et al. N4-acetylcytidine modification of lncRNA CTC-490G23.2 promotes cancer metastasis through interacting with PTBP1 to increase CD44 alternative splicing. Oncogene. 2023;42:1101–16.
Li Z, Li D, Yang T, Yao C. NAT10 promotes the tumorigenesis and progression of laryngeal squamous cell carcinoma through ac4C modification of FOXM1 mRNA. Cancer Biol Ther. 2023;24:2274143.
Shang X, Peng Y, Wang Y, Feng Z, Li M, Peng Z, et al. Profile analysis of N4-acetylcytidine (ac4C) on mRNA of human lung adenocarcinoma and paired adjacent non-tumor tissues. Biochim Biophys Acta Gen Subj. 2023;1867:130498.
Chen X, Hao Y, Liu Y, Zhong S, You Y, Ao K, et al. NAT10/ac4C/FOXP1 promotes malignant progression and facilitates immunosuppression by reprogramming glycolytic metabolism in cervical cancer. Adv Sci. 2023;10:e2302705.
Long Y, Ren Y, Wei Q, Mobet Y, Liu Y, Zhao H, et al. NAT10-mediated RNA acetylation enhances HNRNPUL1 mRNA stability to contribute cervical cancer progression. Int J Med Sci. 2023;20:1079–90.
Zhang W, Gao J, Fan L, Wang J, He B, Wang Y, et al. ac4C acetylation regulates mRNA stability and translation efficiency in osteosarcoma. Heliyon. 2023;9:e17103.
Zheng X, Wang Q, Zhou Y, Zhang D, Geng Y, Hu W, et al. N-acetyltransferase 10 promotes colon cancer progression by inhibiting ferroptosis through N4-acetylation and stabilization of ferroptosis suppressor protein 1 (FSP1) mRNA. Cancer Commun. 2022;42:1347–66.
Jin C, Wang T, Zhang D, Yang P, Zhang C, Peng W, et al. Acetyltransferase NAT10 regulates the Wnt/beta-catenin signaling pathway to promote colorectal cancer progression via ac(4)C acetylation of KIF23 mRNA. J Exp Clin Cancer Res. 2022;41:345.
Zhang H, Shan W, Yang Z, Zhang Y, Wang M, Gao L, et al. NAT10 mediated mRNA acetylation modification patterns associated with colon cancer progression and microsatellite status. Epigenetics. 2023;18:2188667.
Liu Z, Liu X, Li Y, Ren P, Zhang C, Wang L, et al. miR-6716-5p promotes metastasis of colorectal cancer through downregulating NAT10 expression. Cancer Manag Res. 2019;11:5317–32.
Liu X, Tan Y, Zhang C, Zhang Y, Zhang L, Ren P, et al. NAT10 regulates p53 activation through acetylating p53 at K120 and ubiquitinating Mdm2. EMBO Rep. 2016;17:349–66.
Lv J, Liu H, Wang Q, Tang Z, Hou L, Zhang B. Molecular cloning of a novel human gene encoding histone acetyltransferase-like protein involved in transcriptional activation of hTERT. Biochem Biophys Res Commun. 2003;311:506–13.
Chi YH, Haller K, Peloponese JM Jr, Jeang KT. Histone acetyltransferase hALP and nuclear membrane protein hsSUN1 function in de-condensation of mitotic chromosomes. J Biol Chem. 2007;282:27447–58.
Zou YJ, Shan MM, Wan X, Liu JC, Zhang KH, Ju JQ, et al. Kinesin KIF15 regulates tubulin acetylation and spindle assembly checkpoint in mouse oocyte meiosis. Cell Mol Life Sci. 2022;79:422.
Wang T, Zou Y, Huang N, Teng J, Chen J. CCDC84 acetylation oscillation regulates centrosome duplication by modulating HsSAS-6 degradation. Cell Rep. 2019;29:2078–91.e5.
Zhang L, Li DQ. MORC2 regulates DNA damage response through a PARP1-dependent pathway. Nucleic Acids Res. 2019;47:8502–20.
Kong R, Zhang L, Hu L, Peng Q, Han W, Du X, et al. hALP, a novel transcriptional U three protein (t-UTP), activates RNA polymerase I transcription by binding and acetylating the upstream binding factor (UBF). J Biol Chem. 2011;286:7139–48.
Liu X, Cai S, Zhang C, Liu Z, Luo J, Xing B, et al. Deacetylation of NAT10 by Sirt1 promotes the transition from rRNA biogenesis to autophagy upon energy stress. Nucleic Acids Res. 2018;46:9601–16.
Cai S, Liu X, Zhang C, Xing B, Du X. Autoacetylation of NAT10 is critical for its function in rRNA transcription activation. Biochem Biophys Res Commun. 2017;483:624–9.
Chimnaronk S, Suzuki T, Manita T, Ikeuchi Y, Yao M, Suzuki T, et al. RNA helicase module in an acetyltransferase that modifies a specific tRNA anticodon. EMBO J. 2009;28:1362–73.
Thomas G, Gordon J, Rogg H. N4-Acetylcytidine. A previously unidentified labile component of the small subunit of eukaryotic ribosomes. J Biol Chem. 1978;253:1101–5.
Ito S, Akamatsu Y, Noma A, Kimura S, Miyauchi K, Ikeuchi Y, et al. A single acetylation of 18 S rRNA is essential for biogenesis of the small ribosomal subunit in Saccharomyces cerevisiae. J Biol Chem. 2014;289:26201–12.
Ito S, Horikawa S, Suzuki T, Kawauchi H, Tanaka Y, Suzuki T, et al. Human NAT10 is an ATP-dependent RNA acetyltransferase responsible for N4-acetylcytidine formation in 18 S ribosomal RNA (rRNA). J Biol Chem. 2014;289:35724–30.
Tardu M, Jones JD, Kennedy RT, Lin Q, Koutmou KS. Identification and quantification of modified nucleosides in Saccharomyces cerevisiae mRNAs. ACS Chem Biol. 2019;14:1403–9.
Tsai K, Jaguva Vasudevan AA, Martinez Campos C, Emery A, Swanstrom R, Cullen BR. Acetylation of cytidine residues boosts HIV-1 gene expression by increasing viral RNA stability. Cell Host Microbe. 2020;28:306–12.e6.
Bortolin-Cavaille ML, Quillien A, Thalalla Gamage S, Thomas JM, Sas-Chen A, Sharma S, et al. Probing small ribosomal subunit RNA helix 45 acetylation across eukaryotic evolution. Nucleic Acids Res. 2022;50:6284–99.
Thalalla Gamage S, Bortolin-Cavaille ML, Link C, Bryson K, Sas-Chen A, Schwartz S, et al. Antisense pairing and SNORD13 structure guide RNA cytidine acetylation. RNA. 2022;28:1582–96.
Taniguchi T, Miyauchi K, Sakaguchi Y, Yamashita S, Soma A, Tomita K, et al. Acetate-dependent tRNA acetylation required for decoding fidelity in protein synthesis. Nat Chem Biol. 2018;14:1010–20.
Svobodova Kovarikova A, Stixova L, Kovarik A, Bartova E. PARP-dependent and NAT10-independent acetylation of N4-cytidine in RNA appears in UV-damaged chromatin. Epigenetics Chromatin. 2023;16:26.
Huang B, Johansson MJ, Bystrom AS. An early step in wobble uridine tRNA modification requires the Elongator complex. RNA. 2005;11:424–36.
Bose DA, Donahue G, Reinberg D, Shiekhattar R, Bonasio R, Berger SL. RNA binding to CBP stimulates histone acetylation and transcription. Cell. 2017;168:135–49.e22.
Furman D, Chang J, Lartigue L, Bolen CR, Haddad F, Gaudilliere B, et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat Med. 2017;23:174–84.
Van Rompay AR, Norda A, Linden K, Johansson M, Karlsson A. Phosphorylation of uridine and cytidine nucleoside analogs by two human uridine-cytidine kinases. Mol Pharm. 2001;59:1181–6.
Pelletier J, Thomas G, Volarevic S. Ribosome biogenesis in cancer: new players and therapeutic avenues. Nat Rev Cancer. 2018;18:51–63.
Cheng J, Bassler J, Fischer P, Lau B, Kellner N, Kunze R, et al. Thermophile 90S Pre-ribosome structures reveal the reverse order of co-transcriptional 18S rRNA subdomain integration. Mol Cell. 2019;75:1256–69.e7.
Arango D, Sturgill D, Yang R, Kanai T, Bauer P, Roy J, et al. Direct epitranscriptomic regulation of mammalian translation initiation through N4-acetylcytidine. Mol Cell. 2022;82:2797–814.e11.
Arango D, Sturgill D, Oberdoerffer S. Immunoprecipitation and sequencing of acetylated RNA. Bio Protoc. 2019;9:e3278.
Thalalla Gamage S, Sas-Chen A, Schwartz S, Meier JL. Quantitative nucleotide resolution profiling of RNA cytidine acetylation by ac4C-seq. Nat Protoc. 2021;16:2286–307.
Sturgill D, Arango D, Oberdoerffer S. Protocol for base resolution mapping of ac4C using RedaC:T-seq. STAR Protoc. 2022;3:101858.
Yan S, Lu Z, Yang W, Xu J, Wang Y, Xiong W, et al. Antibody-free fluorine-assisted metabolic sequencing of RNA N(4)-acetylcytidine. J Am Chem Soc. 2023;145:22232–42.
Leung AK, Gerlich D, Miller G, Lyon C, Lam YW, Lleres D, et al. Quantitative kinetic analysis of nucleolar breakdown and reassembly during mitosis in live human cells. J Cell Biol. 2004;166:787–800.
Safieddine A, Coleno E, Salloum S, Imbert A, Traboulsi AM, Kwon OS, et al. A choreography of centrosomal mRNAs reveals a conserved localization mechanism involving active polysome transport. Nat Commun. 2021;12:1352.
Park S, Dahn R, Kurt E, Presle A, VanDenHeuvel K, Moravec C, et al. The mammalian midbody and midbody remnant are assembly sites for RNA and localized translation. Dev Cell. 2023;58:1917–32.e6.
Lu X, Liu L. Genome stability from the perspective of telomere length. Trends Genet. 2024;40:175–86.
Fu D, Collins K. Purification of human telomerase complexes identifies factors involved in telomerase biogenesis and telomere length regulation. Mol Cell. 2007;28:773–85.
Kim HS, Parker DJ, Hardiman MM, Munkacsy E, Jiang N, Rogers AN, et al. Early-adulthood spike in protein translation drives aging via juvenile hormone/germline signaling. Nat Commun. 2023;14:5021.
Buchwalter A, Hetzer MW. Nucleolar expansion and elevated protein translation in premature aging. Nat Commun. 2017;8:328.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Dalhat MH, Mohammed MRS, Alkhatabi HA, Rehan M, Ahmad A, Choudhry H, et al. NAT10: An RNA cytidine transferase regulates fatty acid metabolism in cancer cells. Clin Transl Med. 2022;12:e1045.
Dalhat MH, Choudhry H, Khan MI. NAT10, an RNA cytidine acetyltransferase, regulates ferroptosis in cancer cells. Antioxidants. 2023;12:1116.
Dalhat MH, Mohammed MRS, Ahmad A, Khan MI, Choudhry H. Remodelin, a N-acetyltransferase 10 (NAT10) inhibitor, alters mitochondrial lipid metabolism in cancer cells. J Cell Biochem. 2021;122:1936–45.
Yang Q, Lei X, He J, Peng Y, Zhang Y, Ling R, et al. N4-acetylcytidine drives glycolysis addiction in gastric cancer via NAT10/SEPT9/HIF-1alpha positive feedback loop. Adv Sci. 2023;10:e2300898.
Koundouros N, Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer. 2020;122:4–22.
Pope LE, Dixon SJ. Regulation of ferroptosis by lipid metabolism. Trends Cell Biol. 2023;33:1077–87.
Liu HY, Liu YY, Zhang YL, Ning Y, Zhang FL, Li DQ. Poly(ADP-ribosyl)ation of acetyltransferase NAT10 by PARP1 is required for its nucleoplasmic translocation and function in response to DNA damage. Cell Commun Signal. 2022;20:127.
Shrimp JH, Jing Y, Gamage ST, Nelson KM, Han J, Bryson KM, et al. Remodelin is a cryptic assay interference chemotype that does not inhibit NAT10-dependent cytidine acetylation. ACS Med Chem Lett. 2021;12:887–92.
Dalhat MH, Altayb HN, Khan MI, Choudhry H. Structural insights of human N-acetyltransferase 10 and identification of its potential novel inhibitors. Sci Rep. 2021;11:6051.
Shubina MY, Musinova YR, Sheval EV. Proliferation, cancer, and aging-novel functions of the nucleolar methyltransferase fibrillarin? Cell Biol Int. 2018;42:1463–6.
Garus A, Autexier C. Dyskerin: an essential pseudouridine synthase with multifaceted roles in ribosome biogenesis, splicing, and telomere maintenance. RNA. 2021;27:1441–58.
Guo J, Zhu P, Ye Z, Wang M, Yang H, Huang S, et al. YRDC mediates the resistance of lenvatinib in hepatocarcinoma cells via modulating the translation of KRAS. Front Pharmacol. 2021;12:744578.
DepMap. DepMap 19Q4 Public. figshare. In: Broad, editor. 2019.
Chandrashekar DS, Karthikeyan SK, Korla PK, Patel H, Shovon AR, Athar M, et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia. 2022;25:18–27.
Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–9.
Oughtred R, Rust J, Chang C, Breitkreutz BJ, Stark C, Willems A, et al. The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 2021;30:187–200.
Acknowledgements
This work was supported by the National Cancer Institute (NCI) grant R00CA245035, the American Association for Cancer Research (AACR) award 22-20-01-ARAN, the V Scholar Award V2022-016, and the American Cancer Society Institutional Research Grant IRG-21-144-27.
Author information
Authors and Affiliations
Contributions
M.H.D, S.N. and D.A. wrote the first draft. D.A. structured, wrote, and corrected all the sections in the paper. H.S. and D.A. analyzed publicly available data and prepared the figures. M.H.D., S.N, H.S. and D.A. revised the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dalhat, M.H., Narayan, S., Serio, H. et al. Dissecting the oncogenic properties of essential RNA-modifying enzymes: a focus on NAT10. Oncogene 43, 1077–1086 (2024). https://doi.org/10.1038/s41388-024-02975-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-024-02975-9