Abstract
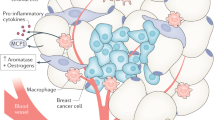
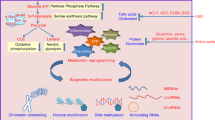
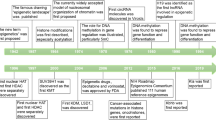
Both breast cancer and obesity can regulate epigenetic changes or be regulated by epigenetic changes. Due to the well-established link between obesity and an increased risk of developing breast cancer, understanding how obesity-mediated epigenetic changes affect breast cancer pathogenesis is critical. Researchers have described how obesity and breast cancer modulate the epigenome individually and synergistically. In this review, the epigenetic alterations that occur in obesity, including DNA methylation, histone, and chromatin modification, accelerated epigenetic age, carcinogenesis, metastasis, and tumor microenvironment modulation, are discussed. Delineating the relationship between obesity and epigenetic regulation is vital to furthering our understanding of breast cancer pathogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Not applicable.
References
Lega IC, Lipscombe LL. Review: diabetes, obesity, and cancer-pathophysiology and clinical implications. Endocr Rev. 2020;41:bnz014.
Harris BHL, Macaulay VM, Harris DA, Klenerman P, Karpe F, Lord SR, et al. Obesity: a perfect storm for carcinogenesis. Cancer Metastasis Rev. 2022;41:491–515.
Zhao Y, Zhang X, Zhao H, Wang J, Zhang Q. CXCL5 secreted from adipose tissue-derived stem cells promotes cancer cell proliferation. Oncol Lett. 2018;15:1403–10.
Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378–97.
Coleman WB. Obesity and the breast cancer methylome. Curr Opin Pharm. 2016;31:104–13.
Prasad M, Rajagopal P, Devarajan N, Veeraraghavan VP, Palanisamy CP, Cui B, et al. A comprehensive review on high -fat diet-induced diabetes mellitus: an epigenetic view. J Nutr Biochem. 2022;107:109037.
Samblas M, Milagro FI, Martínez A. DNA methylation markers in obesity, metabolic syndrome, and weight loss. Epigenetics. 2019;14:421–44.
Alshamsan BI, Suleman K, Agha N, Abdelgawad MI, Alzahrani MJ, Al-Tweigeri T, et al. Association between obesity and the clinical stage of newly diagnosed breast cancer: Experience with 2212 patients. J Clin Oncol. 2020;38:e12602-e.
Thakur C, Qiu Y, Fu Y, Bi Z, Zhang W, Ji H, et al. Epigenetics and environment in breast cancer: New paradigms for anti-cancer therapies. Front Oncol. 2022;12:971288.
Wu Y, Sarkissyan M, Vadgama JV. Epigenetics in breast and prostate cancer. Methods Mol Biol. 2015;1238:425–66.
Liu X, Chen Q, Tsai H-J, Wang G, Hong X, Zhou Y, et al. Maternal preconception body mass index and offspring cord blood DNA methylation: Exploration of early life origins of disease. Environ Mol Mutagen. 2014;55:223–30.
Kühnen P, Handke D, Waterland RA, Hennig BJ, Silver M, Fulford AJ, et al. Interindividual variation in DNA methylation at a putative POMC metastable Epiallele is associated with obesity. Cell Metab. 2016;24:502–9.
Barres R, Kirchner H, Rasmussen M, Yan J, Kantor FR, Krook A, et al. Weight loss after gastric bypass surgery in human obesity remodels promoter methylation. Cell Rep. 2013;3:1020–7.
Milagro FI, Campión J, Cordero P, Goyenechea E, Gómez-Uriz AM, Abete I, et al. A dual epigenomic approach for the search of obesity biomarkers: DNA methylation in relation to diet-induced weight loss. FASEB J. 2011;25:1378–89.
Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–6.
Painter RC, Osmond C, Gluckman P, Hanson M, Phillips DI, Roseboom TJ. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG. 2008;115:1243–9.
Tobi EW, Goeman JJ, Monajemi R, Gu H, Putter H, Zhang Y, et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat Commun. 2014;5:5592.
Dunford AR, Sangster JM. Maternal and paternal periconceptional nutrition as an indicator of offspring metabolic syndrome risk in later life through epigenetic imprinting: A systematic review. Diabetes Metab Syndr. 2017;11:S655-s62.
Hardikar AA, Satoor SN, Karandikar MS, Joglekar MV, Puranik AS, Wong W, et al. Multigenerational undernutrition increases susceptibility to obesity and diabetes that is not reversed after dietary recuperation. Cell Metab. 2015;22:312–9.
Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8:e55387.
Molina-Serrano D, Kyriakou D, Kirmizis A. Histone modifications as an intersection between diet and longevity. Front Genet. 2019;10:192.
Shimada M, Mochizuki K, Goda T. Feeding rats dietary resistant starch reduces both the binding of ChREBP and the acetylation of Histones on the Thrsp gene in the Jejunum. J Agric Food Chem. 2011;59:1464–9.
Zhang X, Zhou D, Strakovsky R, Zhang Y, Pan YX. Hepatic cellular senescence pathway genes are induced through histone modifications in a diet-induced obese rat model. Am J Physiol Gastrointest Liver Physiol. 2012;302:G558–64.
Nie L, Shuai L, Zhu M, Liu P, Xie ZF, Jiang S, et al. The landscape of histone modifications in a high-fat Diet-Induced Obese (DIO) mouse model. Mol Cell Proteom. 2017;16:1324–34.
Siersbæk M, Varticovski L, Yang S, Baek S, Nielsen R, Mandrup S, et al. High fat diet-induced changes of mouse hepatic transcription and enhancer activity can be reversed by subsequent weight loss. Sci Rep. 2017;7:40220.
Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3:662–73.
Schones DE, Leung A, Natarajan R. Chromatin modifications associated with diabetes and obesity. Arterioscler Thromb Vasc Biol. 2015;35:1557–61.
Pogribny IP, Tryndyak VP, Bagnyukova TV, Melnyk S, Montgomery B, Ross SA, et al. Hepatic epigenetic phenotype predetermines individual susceptibility to hepatic steatosis in mice fed a lipogenic methyl-deficient diet. J Hepatol. 2009;51:176–86.
Qin Y, Grimm SA, Roberts JD, Chrysovergis K, Wade PA. Alterations in promoter interaction landscape and transcriptional network underlying metabolic adaptation to diet. Nat Commun. 2020;11:962.
Widiker S, Karst S, Wagener A, Brockmann GA. High-fat diet leads to a decreased methylation of the Mc4r gene in the obese BFMI and the lean B6 mouse lines. J Appl Genet. 2010;51:193–7.
Leung A, Parks BW, Du J, Trac C, Setten R, Chen Y, et al. Open chromatin profiling in mice livers reveals unique chromatin variations induced by high fat diet. J Biol Chem. 2014;289:23557–67.
Tekola-Ayele F. Invited commentary: epigenetic clocks and obesity-towards the next frontier using integrative approaches and early-life models. Am J Epidemiol. 2021;190:994–7.
Christensen BC, Houseman EA, Marsit CJ, Zheng S, Wrensch MR, Wiemels JL, et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet. 2009;5:e1000602.
Taormina G, Mirisola MG. Longevity: epigenetic and biomolecular aspects. Biomol Concepts. 2015;6:105–17.
Nevalainen T, Kananen L, Marttila S, Jylhävä J, Mononen N, Kähönen M, et al. Obesity accelerates epigenetic aging in middle-aged but not in elderly individuals. Clin Epigenetics. 2017;9:20.
Fontana L, Hu FB. Optimal body weight for health and longevity: bridging basic, clinical, and population research. Aging Cell. 2014;13:391–400.
Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21–33.
Bosello O, Vanzo A. Obesity paradox and aging. Eat Weight Disord. 2021;26:27–35.
Chapman IM. Obesity paradox during aging. Interdiscip Top Gerontol. 2010;37:20–36.
Franzago M, Pilenzi L, Di Rado S, Vitacolonna E, Stuppia L. The epigenetic aging, obesity, and lifestyle. Front Cell Dev Biol. 2022;10:985274.
Foster CA, Barker-Kamps M, Goering M, Patki A, Tiwari HK, Mrug S. Epigenetic age acceleration correlates with BMI in young adults. Aging. 2023;15:513–23.
Bentley RA, Ross CN, O’Brien MJ. Obesity, metabolism, and aging: a multiscalar approach. Prog Mol Biol Transl Sci. 2018;155:25–42.
Abbas G, Salman A, Rahman SU, Ateeq MK, Usman M, Sajid S, et al. Aging mechanisms: Linking oxidative stress, obesity and inflammation. Matrix Sci Med. 2017;1:30–3.
Suliga E. Visceral adipose tissue in children and adolescents: a review. Nutr Res Rev. 2009;22:137–47.
Lloyd LJ, Langley-Evans SC, McMullen S. Childhood obesity and risk of the adult metabolic syndrome: a systematic review. Int J Obes. 2012;36:1–11.
Smith LA, Craven DM, Rainey MA, Cozzo AJ, Carson MS, Glenny EM, et al. Separate and combined effects of advanced age and obesity on mammary adipose inflammation, immunosuppression and tumor progression in mouse models of triple negative breast cancer. Front Oncol. 2023;12:1031174.
de Toro-Martin J, Guenard F, Tchernof A, Hould FS, Lebel S, Julien F, et al. Body mass index is associated with epigenetic age acceleration in the visceral adipose tissue of subjects with severe obesity. Clin Epigenetics. 2019;11:172.
Etzel L, Hastings WJ, Hall MA, Heim CM, Meaney MJ, Noll JG, et al. Obesity and accelerated epigenetic aging in a high-risk cohort of children. Sci Rep. 2022;12:8328.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52.
Zwamborn RAJ, Slieker RC, Mulder PCA, Zoetemelk I, Verschuren L, Suchiman HED, et al. Prolonged high-fat diet induces gradual and fat depot-specific DNA methylation changes in adult mice. Sci Rep. 2017;7:43261.
Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019;20:2358.
Petrus P, Bialesova L, Checa A, Kerr A, Naz S, Bäckdahl J, et al. Adipocyte expression of SLC19A1 Links DNA hypermethylation to adipose tissue inflammation and insulin resistance. J Clin Endocrinol Metab. 2018;103:710–21.
Parrillo L, Costa V, Raciti GA, Longo M, Spinelli R, Esposito R, et al. Hoxa5 undergoes dynamic DNA methylation and transcriptional repression in the adipose tissue of mice exposed to high-fat diet. Int J Obes. 2016;40:929–37.
Cruceriu D, Baldasici O, Balacescu O, Berindan-Neagoe I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: molecular insights and therapeutic approaches. Cell Oncol. 2020;43:1–18.
Castellano-Castillo D, Moreno-Indias I, Sanchez-Alcoholado L, Ramos-Molina B, Alcaide-Torres J, Morcillo S, et al. Altered Adipose tissue DNA methylation status in metabolic syndrome: relationships between global DNA methylation and specific methylation at adipogenic, lipid metabolism and inflammatory candidate genes and metabolic variables. J Clin Med. 2019;8:87.
Donovan MG, Wren SN, Cenker M, Selmin OI, Romagnolo DF. Dietary fat and obesity as modulators of breast cancer risk: Focus on DNA methylation. Br J Pharm. 2020;177:1331–50.
de Heredia FP, Gómez-Martínez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332–8.
Pradhan A. Obesity, metabolic Syndrome, and Type 2 Diabetes: Inflammatory basis of glucose metabolic disorders. Nutr Rev. 2007;65:S152–S6.
Izquierdo AG, Crujeiras AB. Obesity-related epigenetic changes after Bariatric surgery. Front Endocrinol. 2019;10:232.
Guénard F, Tchernof A, Deshaies Y, Cianflone K, Kral JG, Marceau P, et al. Methylation and expression of immune and inflammatory genes in the offspring of bariatric bypass surgery patients. J Obes. 2013;2013:492170.
Berglind D, Müller P, Willmer M, Sinha I, Tynelius P, Näslund E, et al. Differential methylation in inflammation and type 2 diabetes genes in siblings born before and after maternal bariatric surgery. Obesity. 2016;24:250–61.
Crujeiras AB, Diaz-Lagares A, Sandoval J, Milagro FI, Navas-Carretero S, Carreira MC, et al. DNA methylation map in circulating leukocytes mirrors subcutaneous adipose tissue methylation pattern: a genome-wide analysis from non-obese and obese patients. Sci Rep. 2017;7:41903.
Ali MM, Naquiallah D, Qureshi M, Mirza MI, Hassan C, Masrur M, et al. DNA methylation profile of genes involved in inflammation and autoimmunity correlates with vascular function in morbidly obese adults. Epigenetics. 2022;17:93–109.
Doumatey AP, He WJ, Gaye A, Lei L, Zhou J, Gibbons GH, et al. Circulating MiR-374a-5p is a potential modulator of the inflammatory process in obesity. Sci Rep. 2018;8:7680.
Landrier JF, Derghal A, Mounien L. MicroRNAs in obesity and related metabolic disorders. Cells. 2019;8:859.
Chatterjee TK, Idelman G, Blanco V, Blomkalns AL, Piegore MG Jr, Weintraub DS, et al. Histone deacetylase 9 is a negative regulator of adipogenic differentiation. J Biol Chem. 2011;286:27836–47.
Wang S, Lin Y, Gao L, Yang Z, Lin J, Ren S, et al. PPAR-γ integrates obesity and adipocyte clock through epigenetic regulation of Bmal1. Theranostics. 2022;12:1589–606.
Kim AY, Park YJ, Pan X, Shin KC, Kwak SH, Bassas AF, et al. Obesity-induced DNA hypermethylation of the adiponectin gene mediates insulin resistance. Nat Commun. 2015;6:7585.
Dolinoy DC. The agouti mouse model: an epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr Rev. 2008;66:S7–11.
Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23:314–8.
Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–72.
Zheng Y, Joyce BT, Colicino E, Liu L, Zhang W, Dai Q, et al. Blood epigenetic age may predict cancer incidence and mortality. EBioMedicine. 2016;5:68–73.
Xu Z, Sandler DP, Taylor JA. Blood DNA methylation and breast cancer: a prospective case-cohort analysis in the sister study. J Natl Cancer Inst. 2020;112:87–94.
de Almeida BP, Apolónio JD, Binnie A, Castelo-Branco P. Roadmap of DNA methylation in breast cancer identifies novel prognostic biomarkers. BMC Cancer. 2019;19:219.
Xing M, Yang Y, Huang J, Fang Y, Jin Y, Li L, et al. TFPI inhibits breast cancer progression by suppressing ERK/p38 MAPK signaling pathway. Genes Genomics. 2022;44:801–12.
Saelee P, Pongtheerat T. APC promoter hypermethylation as a prognostic marker in breast cancer patients. Asian Pac J Cancer Prev. 2020;21:3627–32.
Lin RK, Su CM, Lin SY, Thi Anh Thu L, Liew PL, Chen JY, et al. Hypermethylation of TMEM240 predicts poor hormone therapy response and disease progression in breast cancer. Mol Med. 2022;28:67.
Liu H, Xie HQ, Zhao Y, Zhang W, Zhang Y. DNA methylation-mediated down-regulation of TMEM130 promotes cell migration in breast cancer. Acta Histochem. 2021;123:151814.
Ansar M, Thu LTA, Hung CS, Su CM, Huang MH, Liao LM, et al. Promoter hypomethylation and overexpression of TSTD1 mediate poor treatment response in breast cancer. Front Oncol. 2022;12:1004261.
Muhammad JS, Guimei M, Jayakumar MN, Shafarin J, Janeeh AS, AbuJabal R, et al. Estrogen-induced hypomethylation and overexpression of YAP1 facilitate breast cancer cell growth and survival. Neoplasia. 2021;23:68–79.
Dong X, Yang Y, Yuan Q, Hou J, Wu G. High expression of CEMIP correlates poor prognosis and the tumur microenvironment in breast cancer as a promisingly prognostic biomarker. Front Genet. 2021;12:768140.
Shao F, Yang X, Wang W, Wang J, Guo W, Feng X, et al. Associations of PGK1 promoter hypomethylation and PGK1-mediated PDHK1 phosphorylation with cancer stage and prognosis: a TCGA pan-cancer analysis. Cancer Commun (Lond). 2019;39:54.
Luo L, Fu S, Du W, He LN, Zhang X, Wang Y, et al. LRRC3B and its promoter hypomethylation status predicts response to anti-PD-1 based immunotherapy. Front Immunol. 2023;14:959868.
Stefansson OA, Moran S, Gomez A, Sayols S, Arribas-Jorba C, Sandoval J, et al. A DNA methylation-based definition of biologically distinct breast cancer subtypes. Mol Oncol. 2015;9:555–68.
Rahman MM, Brane AC, Tollefsbol TO. MicroRNAs and epigenetics strategies to reverse breast cancer. Cells. 2019;8:1214.
Pham TMQ, Phan TH, Jasmine TX, Tran TTT, Huynh LAK, Vo TL, et al. Multimodal analysis of genome-wide methylation, copy number aberrations, and end motif signatures enhances detection of early-stage breast cancer. Front Oncol. 2023;13:1127086.
Zhao B, Lv Y. A biomechanical view of epigenetic tumor regulation. J Biol Phys. 2023;49:283–307.
Stowers RS, Shcherbina A, Israeli J, Gruber JJ, Chang J, Nam S, et al. Matrix stiffness induces a tumorigenic phenotype in mammary epithelium through changes in chromatin accessibility. Nat Biomed Eng. 2019;3:1009–19.
Audia JE, Campbell RM. Histone modifications and cancer. Cold Spring Harb Perspect Biol. 2016;8:a019521.
Li D, Zhao W, Zhang X, Lv H, Li C, Sun L. NEFM DNA methylation correlates with immune infiltration and survival in breast cancer. Clin Epigenetics. 2021;13:112.
Xu P, Xiong W, Lin Y, Fan L, Pan H, Li Y. Histone deacetylase 2 knockout suppresses immune escape of triple-negative breast cancer cells via downregulating PD-L1 expression. Cell Death Dis. 2021;12:779.
Sarkar T, Dhar S, Chakraborty D, Pati S, Bose S, Panda AK, et al. FOXP3/HAT1 axis controls treg infiltration in the tumor microenvironment by inducing CCR4 Expression in breast cancer. Front Immunol. 2022;13:740588.
Sasidharan Nair V, El Salhat H, Taha RZ, John A, Ali BR, Elkord E. DNA methylation and repressive H3K9 and H3K27 trimethylation in the promoter regions of PD-1, CTLA-4, TIM-3, LAG-3, TIGIT, and PD-L1 genes in human primary breast cancer. Clin Epigenetics. 2018;10:78.
Qin Y, Vasilatos SN, Chen L, Wu H, Cao Z, Fu Y, et al. Inhibition of histone lysine-specific demethylase 1 elicits breast tumor immunity and enhances antitumor efficacy of immune checkpoint blockade. Oncogene. 2019;38:390–405.
Hong J, Lee JH, Zhang Z, Wu Y, Yang M, Liao Y, et al. PRC2-mediated epigenetic suppression of Type I IFN-STAT2 signaling impairs antitumor immunity in luminal breast cancer. Cancer Res. 2022;82:4624–40.
Wang YF, Yu L, Hu ZL, Fang YF, Shen YY, Song MF, et al. Regulation of CCL2 by EZH2 affects tumor-associated macrophages polarization and infiltration in breast cancer. Cell Death Dis. 2022;13:748.
Huang S, Wang Z, Zhou J, Huang J, Zhou L, Luo J, et al. EZH2 inhibitor GSK126 suppresses antitumor immunity by driving production of myeloid-derived suppressor cells. Cancer Res. 2019;79:2009–20.
Crujeiras AB, Diaz-Lagares A, Stefansson OA, Macias-Gonzalez M, Sandoval J, Cueva J, et al. Obesity and menopause modify the epigenomic profile of breast cancer. Endocr Relat Cancer. 2017;24:351–63.
Daraei A, Izadi P, Khorasani G, Nafissi N, Naghizadeh MM, Younosi N, et al. Epigenetic changes of the ESR1 gene in breast tissue of healthy women: a missing link with breast cancer risk factors? Genet Test Mol Biomark. 2017;21:464–70.
Hair BY, Troester MA, Edmiston SN, Parrish EA, Robinson WR, Wu MC, et al. Body mass index is associated with gene methylation in estrogen receptor-positive breast tumors. Cancer Epidemiol Biomark Prev. 2015;24:580–6.
Bowers LW, Doerstling SS, Shamsunder MG, Lineberger CG, Rossi EL, Montgomery SA, et al. Reversing the genomic, epigenetic, and triple-negative breast cancer–enhancing effects of obesity. Cancer Prev Res. 2022;15:581–94.
Brock CK, Hebert KL, Artiles M, Wright MK, Cheng T, Windsor GO, et al. A role for adipocytes and adipose stem cells in the breast tumor microenvironment and regenerative medicine. Front Physiol. 2021;12:751239.
Rossi EL, de Angel RE, Bowers LW, Khatib SA, Smith LA, Van Buren E, et al. Obesity-associated alterations in inflammation, epigenetics, and mammary tumor growth persist in formerly obese mice. Cancer Prev Res. 2016;9:339–48.
Chen M, Li S, Arora I, Yi N, Sharma M, Li Z, et al. Maternal soybean diet on prevention of obesity-related breast cancer through early-life gut microbiome and epigenetic regulation. J Nutr Biochem. 2022;110:109119.
Horvath S. Erratum to: DNA methylation age of human tissues and cell types. Genome Biol. 2015;16:96.
Olsson LT, Walens A, Hamilton AM, Benefield HC, Fleming JM, Carey LA, et al. Obesity and breast cancer metastasis across genomic subtypes. Cancer Epidemiol, Biomark Prev. 2022;31:1944–51.
Barone I, Giordano C, Bonofiglio D, Ando S, Catalano S. The weight of obesity in breast cancer progression and metastasis: Clinical and molecular perspectives. Semin Cancer Biol. 2020;60:274–84.
Osman MA, Hennessy BT. Obesity correlation with metastases development and response to first-line metastatic chemotherapy in breast cancer. Clin Med Insights: Oncol. 2015;9:CMO.S32812.
Devericks EN, Carson MS, McCullough LE, Coleman MF, Hursting SD. The obesity-breast cancer link: a multidisciplinary perspective. Cancer Metastasis Rev. 2022;41:607–25.
Bousquenaud M, Fico F, Solinas G, Rüegg C, Santamaria-Martínez A. Obesity promotes the expansion of metastasis-initiating cells in breast cancer. Breast Cancer Res. 2018;20:104.
Arcaro K, Atasayan O, Bayrak OF, Cicekdal MB, Cleary MP, Dogan S, et al. Effects of two types of energy restriction on methylation levels of adiponectin receptor 1 and leptin receptor overlapping transcript in a mouse mammary tumour virus-transforming growth factor-α breast cancer mouse model. Br J Nutr. 2021;125:1–9.
Hjort L, Jørgensen SW, Gillberg L, Hall E, Brøns C, Frystyk J, et al. 36 h fasting of young men influences adipose tissue DNA methylation of LEP and ADIPOQ in a birth weight-dependent manner. Clin Epigenetics. 2017;9:40.
Houde AA, Légaré C, Biron S, Lescelleur O, Biertho L, Marceau S, et al. Leptin and adiponectin DNA methylation levels in adipose tissues and blood cells are associated with BMI, waist girth and LDL-cholesterol levels in severely obese men and women. BMC Med Genet. 2015;16:29.
Taroeno-Hariadi KW, Hardianti MS, Sinorita H, Aryandono T. Obesity, leptin, and deregulation of microRNA in lipid metabolisms: their contribution to breast cancer prognosis. Diabetol Metab Syndr. 2021;13:10.
Arguelles AO, Meruvu S, Bowman JD, Choudhury M. Are epigenetic drugs for diabetes and obesity at our door step? Drug Discov Today. 2016;21:499–509.
Mahmoud AM. An overview of epigenetics in obesity: the role of lifestyle and therapeutic interventions. Int J Mol Sci. 2022;23:1341.
Cabrera SM, Colvin SC, Tersey SA, Maier B, Nadler JL, Mirmira RG. Effects of combination therapy with dipeptidyl peptidase-IV and histone deacetylase inhibitors in the non-obese diabetic mouse model of type 1 diabetes. Clin Exp Immunol. 2013;172:375–82.
Xie XY, Kong PR, Wu JF, Li Y, Li YX. Curcumin attenuates lipolysis stimulated by tumor necrosis factor-α or isoproterenol in 3T3-L1 adipocytes. Phytomedicine. 2012;20:3–8.
Sandhu R, Rivenbark AG, Coleman WB. Enhancement of chemotherapeutic efficacy in hypermethylator breast cancer cells through targeted and pharmacologic inhibition of DNMT3b. Breast Cancer Res Treat. 2012;131:385–99.
Crujeiras AB, Díaz-Lagares A, Carreira MC, Amil M, Casanueva FF. Oxidative stress associated to dysfunctional adipose tissue: a potential link between obesity, type 2 diabetes mellitus and breast cancer. Free Radic Res. 2013;47:243–56.
Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–95.
Acknowledgements
This work is partially funded through a NACA agreement with the USDA Project #6054-41000-112-000D and La-CATs U54 GM104940. This work is also funded through the LSUHSC School of Medicine Research Enhancement grants and Fred G. Brazda Foundation to SKA. All figures were created with BioRender.com. We appreciate Elizabeth C. Martin for assistance with editing. We are grateful to Krewe de Pink, an organization of breast cancer survivors, their families, and community members based in New Orleans who are devoted to supporting local breast cancer research. We also want to acknowledge and thank the patients who donate breast cancer tissue.
Author information
Authors and Affiliations
Contributions
SKA conceived the idea, MEB executed the idea, CBL wrote several sections and coordinated with others, and SKA and MEB finalized the article. CBL, JK, MCB, MLH, CAA, NMC, ECM, CR, KLH, MK, JAB, VTH, BMCB, and BAB wrote parts of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lagarde, C.B., Kavalakatt, J., Benz, M.C. et al. Obesity-associated epigenetic alterations and the obesity-breast cancer axis. Oncogene 43, 763–775 (2024). https://doi.org/10.1038/s41388-024-02954-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-024-02954-0