Abstract
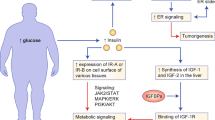
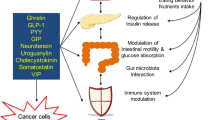
This Review focuses on the mechanistic evidence for a link between obesity, dysregulated cellular metabolism and breast cancer. Strong evidence now links obesity with the development of 13 different types of cancer, including oestrogen receptor-positive breast cancer in postmenopausal women. A number of local and systemic changes are hypothesized to support this relationship, including increased circulating levels of insulin and glucose as well as adipose tissue-derived oestrogens, adipokines and inflammatory mediators. Metabolic pathways of energy production and utilization are dysregulated in tumour cells and this dysregulation is a newly accepted hallmark of cancer. Dysregulated metabolism is also hypothesized to be a feature of non-neoplastic cells in the tumour microenvironment. Obesity-associated factors regulate metabolic pathways in both breast cancer cells and cells in the breast microenvironment, which provides a molecular link between obesity and breast cancer. Consequently, interventions that target these pathways might provide a benefit in postmenopausal women and individuals with obesity, a population at high risk of breast cancer.
Key points
-
Strong evidence links obesity to the development of 13 types of cancer, including oestrogen receptor-positive breast cancer in postmenopausal women.
-
Metabolic pathways involving PI3K–AKT, HIF1α, LKB1–AMPK and p53 are key regulators of breast cancer cell metabolism and growth.
-
Obesity-associated factors drive metabolic alterations in both breast cancer cells and cells of the breast microenvironment that support tumour growth.
-
Therapies that target metabolic pathways might prove effective at treating and preventing breast cancer via effects on cancer cells, the tumour microenvironment and whole-body metabolism.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Smittenaar, C. R., Petersen, K. A., Stewart, K. & Moitt, N. Cancer incidence and mortality projections in the UK until 2035. Br. J. Cancer 115, 1147–1155 (2016).
Rosenberg, P. S., Barker, K. A. & Anderson, W. F. Estrogen receptor status and the future burden of invasive and in situ breast cancers in the United States. J. Natl Cancer Inst. 107, djv159 (2015).
Heer, E. et al. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob. Health 8, e1027–e1037 (2020).
WHO. Obesity and Overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (2020).
Hales, C. M., Carroll, M. D., Fryar, C. D. & Ogden, C. L. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief no. 360 (National Center for Health Statistics, 2020).
OECD. The heavy burden of obesity: the economics of prevention. (OECD, 2019).
Lauby-Secretan, B. et al. Body fatness and cancer — viewpoint of the IARC Working Group. N. Engl. J. Med. 375, 794–798 (2016).
Neuhouser, M. L. et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the Women’s Health Initiative randomized clinical trials. JAMA Oncol. 1, 611–621 (2015).
Chan, D. S. M. et al. World Cancer Research Fund International: Continuous Update Project-systematic literature review and meta-analysis of observational cohort studies on physical activity, sedentary behavior, adiposity, and weight change and breast cancer risk. Cancer Causes Control 30, 1183–1200 (2019).
Munsell, M. F., Sprague, B. L., Berry, D. A., Chisholm, G. & Trentham-Dietz, A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol. Rev. 36, 114–136 (2014).
Corvera, S. & Gealekman, O. Adipose tissue angiogenesis: impact on obesity and type-2 diabetes. Biochim. Biophys. Acta 1842, 463–472 (2014).
Engin, A. The Pathogenesis of obesity-associated adipose tissue inflammation. Adv. Exp. Med. Biol. 960, 221–245 (2017).
Friedman, J. M. Leptin and the endocrine control of energy balance. Nat. Metab. 1, 754–764 (2019).
Howlader, N. et al. SEER Cancer Statistics Review, 1975–2017 https://seer.cancer.gov/csr/1975_2017/ (2020).
Burger, H. G., Hale, G. E., Robertson, D. M. & Dennerstein, L. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum. Reprod. Update 13, 559–565 (2007).
Stanczyk, F. Z., Jurow, J. & Hsing, A. W. Limitations of direct immunoassays for measuring circulating estradiol levels in postmenopausal women and men in epidemiologic studies. Cancer Epidemiol. Biomarkers Prev. 19, 903–906 (2010).
McTiernan, A. et al. Relation of BMI and physical activity to sex hormones in postmenopausal women. Obesity 14, 1662–1677 (2006).
Brown, K. A. et al. Menopause Is a determinant of breast aromatase expression and its associations with BMI, inflammation, and systemic markers. J. Clin. Endocrinol. Metab. 102, 1692–1701 (2017).
Zahid, H. et al. Leptin regulation of the p53-HIF1α/PKM2-aromatase axis in breast adipose stromal cells: a novel mechanism for the obesity-breast cancer link. Int. J. Obes. 42, 711–720 (2018).
Morris, P. G. et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev. Res. 4, 1021–1029 (2011).
Misso, M. L. et al. Adipose aromatase gene expression is greater in older women and is unaffected by postmenopausal estrogen therapy. Menopause 12, 210–215 (2005).
Miller, W. R. & O’Neill, J. The importance of local synthesis of estrogen within the breast. Steroids 50, 537–548 (1987).
Docanto, M. M. et al. Ghrelin and des-acyl ghrelin inhibit aromatase expression and activity in human adipose stromal cells: suppression of cAMP as a possible mechanism. Breast Cancer Res. Treat. 147, 193–201 (2014).
Au, C. C. et al. Des-acyl ghrelin inhibits the capacity of macrophages to stimulate the expression of aromatase in breast adipose stromal cells. J. Steroid Biochem. Mol. Biol. 170, 49–53 (2017).
Au, C. C. et al. Three-dimensional growth of breast cancer cells potentiates the anti-tumor effects of unacylated ghrelin and AZP-531. eLife 9, e56913 (2020).
Au, C. C., Furness, J. B. & Brown, K. A. Ghrelin and breast cancer: emerging roles in obesity, estrogen regulation, and cancer. Front. Oncol. 6, 265 (2016).
Macis, D., Guerrieri-Gonzaga, A. & Gandini, S. Circulating adiponectin and breast cancer risk: a systematic review and meta-analysis. Int. J. Epidemiol. 43, 1226–1236 (2014).
Wairagu, P. M. et al. Insulin priming effect on estradiol-induced breast cancer metabolism and growth. Cancer Biol. Ther. 16, 484–492 (2015).
Naimo, G. D., Gelsomino, L., Catalano, S., Mauro, L. & Ando, S. Interfering role of ERα on adiponectin action in breast cancer. Front. Endocrinol. 11, 66 (2020).
Ando, S., Naimo, G. D., Gelsomino, L., Catalano, S. & Mauro, L. Novel insights into adiponectin action in breast cancer: evidence of its mechanistic effects mediated by ERα expression. Obes. Rev. 21, e13004 (2020).
Bhardwaj, P. et al. Estrogens and breast cancer: mechanisms involved in obesity-related development, growth and progression. J. Steroid. Biochem. Mol. Biol. 189, 161–170 (2019).
Zhu, J. & Thompson, C. B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell. Biol. 20, 436–450 (2019).
Herzig, S. & Shaw, R. J. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell. Biol. 19, 121–135 (2018).
Hoxhaj, G. & Manning, B. D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 20, 74–88 (2020).
Goncalves, M. D., Hopkins, B. D. & Cantley, L. C. Phosphatidylinositol 3-kinase, growth disorders, and cancer. N. Engl. J. Med. 379, 2052–2062 (2018).
Lawrence, M. S. et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501 (2014).
Elstrom, R. L. et al. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 64, 3892–3899 (2004).
Jaldin-Fincati, J. R., Pavarotti, M., Frendo-Cumbo, S., Bilan, P. J. & Klip, A. Update on GLUT4 vesicle traffic: a cornerstone of insulin action. Trends Endocrinol. Metab. 28, 597–611 (2017).
Wang, X., Simpson, E. R. & Brown, K. A. p53: protection against tumor growth beyond effects on cell cycle and apoptosis. Cancer Res. 75, 5001–5007 (2015).
Feng, Z. et al. The regulation of AMPK β1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 67, 3043–3053 (2007).
Zeng, P. Y. & Berger, S. L. LKB1 is recruited to the p21/WAF1 promoter by p53 to mediate transcriptional activation. Cancer Res. 66, 10701–10708 (2006).
Zhang, Y. et al. LKB1 deficiency-induced metabolic reprogramming in tumorigenesis and non-neoplastic diseases. Mol. Metab. 44, 101131 (2021).
Hardie, D. G. & Alessi, D. R. LKB1 and AMPK and the cancer-metabolism link — ten years after. BMC Biol. 11, 36 (2013).
Hawley, S. A. et al. Complexes between the LKB1 tumor suppressor, STRAD α/β and MO25 α/β are upstream kinases in the AMP-activated protein kinase cascade. J. Biol. 2, 28 (2003).
Woods, A. et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr. Biol. 13, 2004–2008 (2003).
Shaw, R. J. et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl Acad. Sci. USA 101, 3329–3335 (2004).
Hadad, S. M. et al. Histological evaluation of AMPK signalling in primary breast cancer. BMC Cancer 9, 307 (2009).
Zadra, G., Batista, J. L. & Loda, M. Dissecting the dual role of AMPK in cancer: from experimental to human studies. Mol. Cancer Res. 13, 1059–1072 (2015).
Hardie, D. G. The LKB1-AMPK pathway — friend or foe in cancer? Cancer Cell 23, 131–132 (2013).
Barthel, A. et al. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J. Biol. Chem. 274, 20281–20286 (1999).
Adekola, K., Rosen, S. T. & Shanmugam, M. Glucose transporters in cancer metabolism. Curr. Opin. Oncol. 24, 650–654 (2012).
Waldhart, A. N. et al. Phosphorylation of TXNIP by AKT mediates acute influx of glucose in response to insulin. Cell Rep. 19, 2005–2013 (2017).
Wu, N. et al. AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Mol. Cell 49, 1167–1175 (2013).
Parikh, H. et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 4, e158 (2007).
Volinsky, N. et al. Signalling mechanisms regulating phenotypic changes in breast cancer cells. Biosci. Rep. 35, e00178 (2015).
Iqbal, M. A. et al. Insulin enhances metabolic capacities of cancer cells by dual regulation of glycolytic enzyme pyruvate kinase M2. Mol. Cancer 12, 72 (2013).
Bousquenaud, M., Fico, F., Solinas, G., Ruegg, C. & Santamaria-Martinez, A. Obesity promotes the expansion of metastasis-initiating cells in breast cancer. Breast Cancer Res. 20, 104 (2018).
Lai, Q. et al. Positive correlation between the expression of hEag1 and HIF-1α in breast cancers: an observational study. BMJ Open 4, e005049 (2014).
Drabovich, A. P., Pavlou, M. P., Dimitromanolakis, A. & Diamandis, E. P. Quantitative analysis of energy metabolic pathways in MCF-7 breast cancer cells by selected reaction monitoring assay. Mol. Cell Proteom. 11, 422–434 (2012).
O’Mahony, F., Razandi, M., Pedram, A., Harvey, B. J. & Levin, E. R. Estrogen modulates metabolic pathway adaptation to available glucose in breast cancer cells. Mol. Endocrinol. 26, 2058–2070 (2012).
Imbert-Fernandez, Y. et al. Estradiol stimulates glucose metabolism via 6-phosphofructo-2-kinase (PFKFB3). J. Biol. Chem. 289, 9440–9448 (2014).
Brown, K. A. et al. LKB1 expression is inhibited by estradiol-17β in MCF-7 cells. J. Steroid. Biochem. Mol. Biol. 127, 439–443 (2011).
Ko, B. H., Paik, J. Y., Jung, K. H. & Lee, K. H. 17β-estradiol augments 18F-FDG uptake and glycolysis of T47D breast cancer cells via membrane-initiated rapid PI3K-Akt activation. J. Nucl. Med. 51, 1740–174 (2010).
Kim, S., Taylor, J. A., Milne, G. L. & Sandler, D. P. Association between urinary prostaglandin E2 metabolite and breast cancer risk: a prospective, case-cohort study of postmenopausal women. Cancer Prev. Res. 6, 511–518 (2013).
Heikkila, K. et al. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes Control. 20, 15–26 (2009).
Wang, D. & DuBois, R. N. Urinary PGE-M: a promising cancer biomarker. Cancer Prev. Res. 6, 507–510 (2013).
George, R. J., Sturmoski, M. A., Anant, S. & Houchen, C. W. EP4 mediates PGE2 dependent cell survival through the PI3 kinase/AKT pathway. Prostaglandins Other Lipid Mediat. 83, 112–120 (2007).
Badache, A. & Hynes, N. E. Interleukin 6 inhibits proliferation and, in cooperation with an epidermal growth factor receptor autocrine loop, increases migration of T47D breast cancer cells. Cancer Res. 61, 383–391 (2001).
Gui, Y. et al. The association between obesity related adipokines and risk of breast cancer: a meta-analysis. Oncotarget 8, 75389–75399 (2017).
Blanquer-Rossello, M. D. M., Oliver, J., Sastre-Serra, J., Valle, A. & Roca, P. Leptin regulates energy metabolism in MCF-7 breast cancer cells. Int. J. Biochem. Cell Biol. 72, 18–26 (2016).
El-Masry, O. S., Al-Sakkaf, K., Brown, B. L. & Dobson, P. R. Differential crosstalk between the AMPK and PI3K/Akt pathways in breast cancer cells of differing genotypes: leptin inhibits the effectiveness of AMPK activation. Oncol. Rep. 34, 1675–1680 (2015).
Frankenberry, K. A., Skinner, H., Somasundar, P., McFadden, D. W. & Vona-Davis, L. C. Leptin receptor expression and cell signaling in breast cancer. Int. J. Oncol. 28, 985–993 (2006).
Wei, L. et al. Leptin promotes epithelial-mesenchymal transition of breast cancer via the upregulation of pyruvate kinase M2. J. Exp. Clin. Cancer Res. 35, 166 (2016).
Gonzalez-Perez, R. R. et al. Leptin upregulates VEGF in breast cancer via canonic and non-canonical signalling pathways and NFκB/HIF-1α activation. Cell Signal 22, 1350–1362 (2010).
Bartella, V. et al. Insulin-dependent leptin expression in breast cancer cells. Cancer Res. 68, 4919–4927 (2008).
Cascio, S. et al. Mechanism of leptin expression in breast cancer cells: role of hypoxia-inducible factor-1α. Oncogene 27, 540–547 (2008).
Miyoshi, Y. et al. Association of serum adiponectin levels with breast cancer risk. Clin. Cancer Res. 9, 5699–5704 (2003).
Dos Santos, E. et al. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol. Rep. 20, 971–977 (2008).
Wang, Y. et al. Adiponectin modulates the glycogen synthase kinase-3β/β-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 66, 11462–11470 (2006).
Dieudonne, M. N. et al. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem. Biophys. Res. Commun. 345, 271–279 (2006).
Mao, X. et al. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat. Cell Biol. 8, 516–523 (2006).
Taliaferro-Smith, L. et al. LKB1 is required for adiponectin-mediated modulation of AMPK-S6K axis and inhibition of migration and invasion of breast cancer cells. Oncogene 28, 2621–2633 (2009).
Wu, Q. et al. Cancer-associated adipocytes: key players in breast cancer progression. J. Hematol. Oncol. 12, 95 (2019).
Wang, Y. Y. et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2, e87489 (2017).
Hoy, A. J., Balaban, S. & Saunders, D. N. Adipocyte-tumor cell metabolic crosstalk in breast cancer. Trends Mol. Med. 23, 381–392 (2017).
Balaban, S. et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 5, 1 (2017).
Madak-Erdogan, Z. et al. Free fatty acids rewire cancer metabolism in obesity-associated breast cancer via estrogen receptor and mTOR signaling. Cancer Res. 79, 2494–2510 (2019).
Martinez-Outschoorn, U., Sotgia, F. & Lisanti, M. P. Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function. Semin. Oncol. 41, 195–216 (2014).
Docanto, M. M., Ham, S., Corbould, A. & Brown, K. A. Obesity-associated inflammatory cytokines and prostaglandin E2 stimulate glucose transporter mRNA expression and glucose uptake in primary human adipose stromal cells. J. Interferon Cytokine Res. 35, 600–605 (2015).
DeClerck, Y. A. Desmoplasia: a response or a niche? Cancer Discov. 2, 772–774 (2012).
de Kruijf, E. M. et al. Tumor-stroma ratio in the primary tumor is a prognostic factor in early breast cancer patients, especially in triple-negative carcinoma patients. Breast Cancer Res. Treat. 125, 687–696 (2011).
Vachon, C. M. et al. Aromatase immunoreactivity is increased in mammographically dense regions of the breast. Breast Cancer Res. Treat. 125, 243–252 (2011).
Huo, C. W. et al. High mammographic density is associated with an increase in stromal collagen and immune cells within the mammary epithelium. Breast Cancer Res. 17, 79 (2015).
van Harmelen, V. et al. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int. J. Obes. Relat. Metab. Disord. 27, 889–895 (2003).
Brown, K. A. et al. Subcellular localization of cyclic AMP-responsive element binding protein-regulated transcription coactivator 2 provides a link between obesity and breast cancer in postmenopausal women. Cancer Res. 69, 5392–5399 (2009).
Samarajeewa, N. U. et al. HIF-1α stimulates aromatase expression driven by prostaglandin E2 in breast adipose stroma. Breast Cancer Res. 15, R30 (2013).
Wang, X. et al. Prostaglandin E2 inhibits p53 in human breast adipose stromal cells: a novel mechanism for the regulation of aromatase in obesity and breast cancer. Cancer Res. 75, 645–655 (2015).
Chow, J. D., Simpson, E. R. & Boon, W. C. Alternative 5´-untranslated first exons of the mouse Cyp19A1 (aromatase) gene. J. Steroid Biochem. Mol. Biol. 115, 115–125 (2009).
Ackerman, G. E., Smith, M. E., Mendelson, C. R., MacDonald, P. C. & Simpson, E. R. Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J. Clin. Endocrinol. Metab. 53, 412–417 (1981).
Zhao, H. et al. A humanized pattern of aromatase expression is associated with mammary hyperplasia in mice. Endocrinology 153, 2701–2713 (2012).
Samarajeewa, N. U., Docanto, M. M., Simpson, E. R. & Brown, K. A. CREB-regulated transcription co-activator family stimulates promoter II-driven aromatase expression in preadipocytes. Horm. Cancer 4, 233–241 (2013).
Ham, S. et al. Overexpression of aromatase associated with loss of heterozygosity of the STK11 gene accounts for prepubertal gynecomastia in boys with Peutz-Jeghers syndrome. J. Clin. Endocrinol. Metab. 98, E1979–E1987 (2013).
Shaw, R. J. et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 (2005).
Brown, K. A., Hunger, N. I., Docanto, M. & Simpson, E. R. Metformin inhibits aromatase expression in human breast adipose stromal cells via stimulation of AMP-activated protein kinase. Breast Cancer Res. Treat. 123, 591–596 (2010).
Samarajeewa, N. U., Ham, S., Yang, F., Simpson, E. R. & Brown, K. A. Promoter-specific effects of metformin on aromatase transcript expression. Steroids 76, 768–771 (2011).
O’Neill, L. A., Kishton, R. J. & Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 16, 553–565 (2016).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Kratz, M. et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 20, 614–625 (2014).
Xu, X. et al. Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell Metab. 18, 816–830 (2013).
Yang, H. et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J. Immunol. 185, 1836–1845 (2010).
Lu, J., Zhao, J., Meng, H. & Zhang, X. Adipose tissue-resident immune cells in obesity and type 2 diabetes. Front. Immunol. 10, 1173 (2019).
Wang, Z. et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 25, 141–151 (2019).
O’Neill, L. A. & Hardie, D. G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493, 346–355 (2013).
Galic, S. et al. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J. Clin. Invest. 121, 4903–4915 (2011).
Boutens, L. et al. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia 61, 942–953 (2018).
Choe, S. S. et al. Macrophage HIF-2α ameliorates adipose tissue inflammation and insulin resistance in obesity. Diabetes 63, 3359–3371 (2014).
Frauwirth, K. A. et al. The CD28 signaling pathway regulates glucose metabolism. Immunity 16, 769–777 (2002).
Vasan, K., Werner, M. & Chandel, N. S. Mitochondrial metabolism as a target for cancer therapy. Cell. Metab. 32, 341–352 (2020).
Cai, H., Everett, R. S. & Thakker, D. R. Efficacious dose of metformin for breast cancer therapy is determined by cation transporter expression in tumours. Br. J. Pharmacol. 176, 2724–2735 (2019).
Wheaton, W. W. et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. eLife 3, e02242 (2014).
Evans, J. M., Donnelly, L. A., Emslie-Smith, A. M., Alessi, D. R. & Morris, A. D. Metformin and reduced risk of cancer in diabetic patients. BMJ 330, 1304–1305 (2005).
Brown, K. A., Samarajeewa, N. U. & Simpson, E. R. Endocrine-related cancers and the role of AMPK. Mol. Cell Endocrinol. 366, 170–179 (2013).
Algire, C. et al. Diet and tumor LKB1 expression interact to determine sensitivity to anti-neoplastic effects of metformin in vivo. Oncogene 30, 1174–1182 (2011).
Grossmann, M. E., Yang, D. Q., Guo, Z., Potter, D. A. & Cleary, M. P. Metformin treatment for the prevention and/or treatment of breast/mammary tumorigenesis. Curr. Pharmacol. Rep. 1, 312–323 (2015).
Samuel, S. M., Varghese, E., Kubatka, P., Triggle, C. R. & Busselberg, D. Metformin: the answer to cancer in “a flower? Current knowledge and future prospects of metformin as an anti-cancer agent in breast cancer. Biomolecules 9, 846 (2019).
Goodwin, P. J. et al. Effect of metformin vs placebo on and metabolic factors in NCIC CTG MA.32. J. Natl Cancer Inst. 107, djv006 (2015).
Pimentel, I. et al. The effect of metformin vs placebo on sex hormones in CCTG MA.32. J. Natl Cancer Inst. https://doi.org/10.1093/jnci/djaa082 (2020).
Millis, S. Z., Ikeda, S., Reddy, S., Gatalica, Z. & Kurzrock, R. Landscape of phosphatidylinositol-3-kinase pathway alterations across 19784 diverse solid tumors. JAMA Oncol. 2, 1565–1573 (2016).
Hopkins, B. D. et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499–503 (2018).
Garrido-Castro, A. C. et al. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 22, 120 (2020).
Eliassen, A. H., Colditz, G. A., Rosner, B., Willett, W. C. & Hankinson, S. E. Adult weight change and risk of postmenopausal breast cancer. JAMA 296, 193–201 (2006).
de Roon, M. et al. Effect of exercise and/or reduced calorie dietary interventions on breast cancer-related endogenous sex hormones in healthy postmenopausal women. Breast Cancer Res. 20, 81 (2018).
Haw, J. S. et al. Long-term sustainability of diabetes prevention approaches: a systematic review and meta-analysis of randomized clinical trials. JAMA Intern. Med. 177, 1808–1817 (2017).
Imayama, I. et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: a randomized controlled trial. Cancer Res. 72, 2314–2326 (2012).
Iyengar, N. M. & Jones, L. W. Development of exercise as interception therapy for cancer: a review. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.2585 (2019).
McTiernan, A. et al. Physical activity in cancer prevention and survival: a systematic review. Med. Sci. Sports Exerc. 51, 1252–1261 (2019).
Ligibel, J. A. et al. Randomized phase III trial evaluating the role of weight loss in adjuvant treatment of overweight and obese women with early breast cancer (Alliance A011401): study design. NPJ Breast Cancer 3, 37 (2017).
Ashcraft, K. A., Peace, R. M., Betof, A. S., Dewhirst, M. W. & Jones, L. W. Efficacy and mechanisms of aerobic exercise on cancer initiation, progression, and metastasis: a critical systematic review of in vivo preclinical data. Cancer Res. 76, 4032–4050 (2016).
Theriau, C. F., Shpilberg, Y., Riddell, M. C. & Connor, M. K. Voluntary physical activity abolishes the proliferative tumor growth microenvironment created by adipose tissue in animals fed a high fat diet. J. Appl. Physiol. 121, 139–153 (2016).
Qin, Y. et al. Weight loss reduces basal-like breast cancer through kinome reprogramming. Cancer Cell Int. 16, 26 (2016).
Swami, S. et al. Vitamin D mitigates the adverse effects of obesity on breast cancer in mice. Endocr. Relat. Cancer 23, 251–264 (2016).
Hanker, A. B., Kaklamani, V. & Arteaga, C. L. Challenges for the clinical development of PI3K inhibitors: strategies to improve their impact in solid tumors. Cancer Discov. 9, 482–491 (2019).
Ligibel, J. A. & Winer, E. P. Aromatase inhibition in obese women: how much is enough? J. Clin. Oncol. 30, 2940–2942 (2012).
Brown, K. A., Andreopoulou, E. & Andreopoulou, P. Endocrine therapy-related endocrinopathies - biology, prevalence and implications for the management of breast cancer. Oncol. Hematol. Rev. 16, 17–22 (2020).
van den Berg, M. M. et al. Weight change during chemotherapy in breast cancer patients: a meta-analysis. BMC Cancer 17, 259 (2017).
Brown, J. C. & Ligibel, J. A. Lifestyle interventions for breast cancer prevention. Curr. Breast Cancer Rep. 10, 202–208 (2018).
Tsatsoulis, A. & Paschou, S. A. Metabolically healthy obesity: criteria, epidemiology, controversies, and consequences. Curr. Obes. Rep. 9, 109–120 (2020).
Batsis, J. A. et al. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999-2004. Int. J. Obes. 40, 761–767 (2016).
Sahakyan, K. R. et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann. Intern. Med. 163, 827–835 (2015).
Grier, T., Canham-Chervak, M., Sharp, M. & Jones, B. H. Does body mass index misclassify physically active young men? Prev. Med. Rep. 2, 483–487 (2015).
Heymsfield, S. B., Peterson, C. M., Thomas, D. M., Heo, M. & Schuna, J. M. Jr Why are there race/ethnic differences in adult body mass index-adiposity relationships? A quantitative critical review. Obes. Rev. 17, 262–275 (2016).
Banack, H. R., Wactawski-Wende, J., Hovey, K. M. & Stokes, A. Is BMI a valid measure of obesity in postmenopausal women? Menopause 25, 307–313 (2018).
Acknowledgements
Research work by K.A.B. is supported by NIH grant R01 CA215797 and the Anne Moore Breast Cancer Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Peer review information
Nature Reviews Endocrinology thanks the (anonymous) reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brown, K.A. Metabolic pathways in obesity-related breast cancer. Nat Rev Endocrinol 17, 350–363 (2021). https://doi.org/10.1038/s41574-021-00487-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-021-00487-0
This article is cited by
-
FTO is a major genetic link between breast cancer, obesity, and diabetes
Breast Cancer Research and Treatment (2024)
-
Association of systemic inflammation with the obesity paradox in cancer: results from multi-cohort studies
Inflammation Research (2024)
-
Multi-Trait Body Shape Phenotypes and Breast Cancer Risk in Postmenopausal Women: A Causal Mediation Analysis in the UK Biobank Cohort
Journal of Epidemiology and Global Health (2024)
-
Comprehensive analysis of a cuproptosis-related ceRNA network implicates a potential endocrine therapy resistance mechanism in ER-positive breast cancer
BMC Medical Genomics (2023)
-
Bodywide ecological interventions on cancer
Nature Medicine (2023)