Abstract
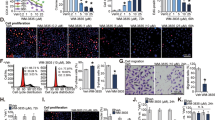
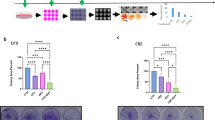
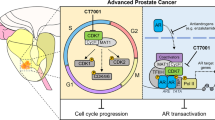
WEE1 and CHEK1 (CHK1) kinases are critical regulators of the G2/M cell cycle checkpoint and DNA damage response pathways. The WEE1 inhibitor AZD1775 and the CHK1 inhibitor SRA737 are in clinical trials for various cancers, but have not been thoroughly examined in prostate cancer, particularly castration-resistant (CRPC) and neuroendocrine prostate cancers (NEPC). Our data demonstrated elevated WEE1 and CHK1 expressions in CRPC and NEPC cell lines and patient samples. AZD1775 resulted in rapid and potent cell killing with comparable IC50s across different prostate cancer cell lines, while SRA737 displayed time-dependent progressive cell killing with 10- to 20-fold differences in IC50s. Notably, their combination synergistically reduced the viability of all CRPC cell lines and tumor spheroids in a concentration- and time-dependent manner. Importantly, in a transgenic mouse model of NEPC, both agents alone or in combination suppressed tumor growth, improved overall survival, and reduced the incidence of distant metastases, with SRA737 exhibiting remarkable single agent anticancer activity. Mechanistically, SRA737 synergized with AZD1775 by blocking AZD1775-induced feedback activation of CHK1 in prostate cancer cells, resulting in increased mitotic entry and accumulation of DNA damage. In summary, this preclinical study shows that CHK1 inhibitor SRA737 alone and its combination with AZD1775 offer potential effective treatments for CRPC and NEPC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data generated in this study are available in the article and its supplementary files. Raw and derived data supporting the findings of this study, but not listed in detail, are available from the corresponding author upon request. Expression profile data analyzed in this study were obtained from the Cancer Genome Atlas, including the NEPC dataset [17] (all accessible via https://portal.gdc.cancer.gov/), and publicly available data from the SU2C/PCF Dream Team [36].
References
Elbaek CR, Petrosius V, Sorensen CS. WEE1 kinase limits CDK activities to safeguard DNA replication and mitotic entry. Mutat Res. 2020;819-820:111694.
Qiu Z, Oleinick NL, Zhang J. ATR/CHK1 inhibitors and cancer therapy. Radiother Oncol. 2018;126:450–64.
Visconti R, Della Monica R, Grieco D. Cell cycle checkpoint in cancer: a therapeutically targetable double-edged sword. J Exp Clin Cancer Res. 2016;35:153.
Ghelli Luserna di Rora A, Cerchione C, Martinelli G, Simonetti G. A WEE1 family business: regulation of mitosis, cancer progression, and therapeutic target. J Hematol Oncol. 2020;13:126.
Mak JP, Man WY, Chow JP, Ma HT, Poon RY. Pharmacological inactivation of CHK1 and WEE1 induces mitotic catastrophe in nasopharyngeal carcinoma cells. Oncotarget. 2015;6:21074–84.
Corella AN, Cabiliza Ordonio MVA, Coleman I, Lucas JM, Kaipainen A, Nguyen HM, et al. Identification of therapeutic vulnerabilities in small-cell neuroendocrine prostate cancer. Clin Cancer Res. 2020;26:1667–77.
Ku BM, Bae YH, Koh J, Sun JM, Lee SH, Ahn JS, et al. Mutational status of TP53 defines the efficacy of Wee1 inhibitor AZD1775 in KRAS-mutant non-small cell lung cancer. Oncotarget. 2017;8:67526–37.
Hirai H, Iwasawa Y, Okada M, Arai T, Nishibata T, Kobayashi M, et al. Small-molecule inhibition of Wee1 kinase by MK-1775 selectively sensitizes p53-deficient tumor cells to DNA-damaging agents. Mol Cancer Ther. 2009;8:2992–3000.
Kolb EA, Houghton PJ, Kurmasheva RT, Mosse YP, Maris JM, Erickson SW, et al. Preclinical evaluation of the combination of AZD1775 and irinotecan against selected pediatric solid tumors: a Pediatric Preclinical Testing Consortium report. Pediatr Blood Cancer. 2020;67:e28098.
Richer AL, Cala JM, O’Brien K, Carson VM, Inge LJ, Whitsett TG. WEE1 kinase inhibitor AZD1775 has preclinical efficacy in LKB1-deficient non-small cell lung cancer. Cancer Res. 2017;77:4663–72.
Matheson CJ, Venkataraman S, Amani V, Harris PS, Backos DS, Donson AM, et al. A WEE1 inhibitor analog of AZD1775 maintains synergy with cisplatin and demonstrates reduced single-agent cytotoxicity in medulloblastoma cells. ACS Chem Biol. 2016;11:921–30.
Patties I, Kallendrusch S, Bohme L, Kendzia E, Oppermann H, Gaunitz F, et al. The Chk1 inhibitor SAR-020106 sensitizes human glioblastoma cells to irradiation, to temozolomide, and to decitabine treatment. J Exp Clin Cancer Res. 2019;38:420.
Doerr F, George J, Schmitt A, Beleggia F, Rehkamper T, Hermann S, et al. Targeting a non-oncogene addiction to the ATR/CHK1 axis for the treatment of small cell lung cancer. Sci Rep. 2017;7:15511.
Nyquist MD, Corella A, Coleman I, De Sarkar N, Kaipainen A, Ha G, et al. Combined TP53 and RB1 loss promotes prostate cancer resistance to a spectrum of therapeutics and confers vulnerability to replication stress. Cell Rep. 2020;31:107669.
Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, et al. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate. 2011;71:1668–79.
Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28.
Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22:298–305.
Kristeleit R, Plummer R, Jones R, Carter L, Blagden S, Sarker D, et al. A phase 1/2 trial of SRA737 (a Chk1 inhibitor) administered orally in patients with advanced cancer. Br J Cancer. 2023;129:38–45.
Gorecki L, Andrs M, Korabecny J. Clinical candidates targeting the ATR-CHK1-WEE1 axis in cancer. Cancers. 2021;13:795.
Booth L, Roberts J, Poklepovic A, Dent P. The CHK1 inhibitor SRA737 synergizes with PARP1 inhibitors to kill carcinoma cells. Cancer Biol Ther. 2018;19:786–96.
Zhu Y, Liu C, Nadiminty N, Lou W, Tummala R, Evans CP, et al. Inhibition of ABCB1 expression overcomes acquired docetaxel resistance in prostate cancer. Mol Cancer Ther. 2013;12:1829–36.
Liu C, Lou W, Zhu Y, Nadiminty N, Schwartz CT, Evans CP, et al. Niclosamide inhibits androgen receptor variants expression and overcomes enzalutamide resistance in castration-resistant prostate cancer. Clin Cancer Res. 2014;20:3198–210.
Liu C, Lou W, Zhu Y, Yang JC, Nadiminty N, Gaikwad NW, et al. Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Res. 2015;75:1413–22.
Leung-Pineda V, Ryan CE, Piwnica-Worms H. Phosphorylation of Chk1 by ATR is antagonized by a Chk1-regulated protein phosphatase 2A circuit. Mol Cell Biol. 2006;26:7529–38.
Bukhari AB, Lewis CW, Pearce JJ, Luong D, Chan GK, Gamper AM. Inhibiting Wee1 and ATR kinases produces tumor-selective synthetic lethality and suppresses metastasis. J Clin Invest. 2019;129:1329–44.
Gingrich JR, Barrios RJ, Kattan MW, Nahm HS, Finegold MJ, Greenberg NM. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 1997;57:4687–91.
Deneka AY, Einarson MB, Bennett J, Nikonova AS, Elmekawy M, Zhou Y, et al. Synthetic lethal targeting of mitotic checkpoints in HPV-negative head and neck cancer. Cancers. 2020;12:306.
Carrassa L, Chila R, Lupi M, Ricci F, Celenza C, Mazzoletti M, et al. Combined inhibition of Chk1 and Wee1: in vitro synergistic effect translates to tumor growth inhibition in vivo. Cell Cycle. 2012;11:2507–17.
Magnussen GI, Emilsen E, Giller Fleten K, Engesaeter B, Nahse-Kumpf V, Fjaer R, et al. Combined inhibition of the cell cycle related proteins Wee1 and Chk1/2 induces synergistic anti-cancer effect in melanoma. BMC Cancer. 2015;15:462.
Koh SB, Wallez Y, Dunlop CR, Bernaldo de Quiros Fernandez S, Bapiro TE, Richards FM, et al. Mechanistic distinctions between CHK1 and WEE1 inhibition guide the scheduling of triple therapy with gemcitabine. Cancer Res. 2018;78:3054–66.
Guertin AD, Martin MM, Roberts B, Hurd M, Qu X, Miselis NR, et al. Unique functions of CHK1 and WEE1 underlie synergistic anti-tumor activity upon pharmacologic inhibition. Cancer Cell Int. 2012;12:45.
Sausville E, Lorusso P, Carducci M, Carter J, Quinn MF, Malburg L, et al. Phase I dose-escalation study of AZD7762, a checkpoint kinase inhibitor, in combination with gemcitabine in US patients with advanced solid tumors. Cancer Chemother Pharm. 2014;73:539–49.
Walton MI, Eve PD, Hayes A, Henley AT, Valenti MR, De Haven Brandon AK, et al. The clinical development candidate CCT245737 is an orally active CHK1 inhibitor with preclinical activity in RAS mutant NSCLC and Emicro-MYC driven B-cell lymphoma. Oncotarget. 2016;7:2329–42.
Osborne JD, Matthews TP, McHardy T, Proisy N, Cheung KM, Lainchbury M, et al. Multiparameter lead optimization to give an oral checkpoint kinase 1 (CHK1) inhibitor clinical candidate: (R)-5-((4-((Morpholin-2-ylmethyl)amino)-5-(trifluoromethyl)pyridin-2-yl)amino)pyr azine-2-carbonitrile (CCT245737). J Med Chem. 2016;59:5221–37.
Jones R, Plummer R, Moreno V, Carter L, Roda D, Garralda E, et al. A phase I/II trial of oral SRA737 (a Chk1 Inhibitor) given in combination with low-dose gemcitabine in patients with advanced cancer. Clin Cancer Res. 2023;29:331–40.
Abida W, Cyrta J, Heller G, Prandi D, Armenia J, Coleman I, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci USA. 2019;116:11428–36.
Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56:337–44.
Liu Q, Yin X, Languino LR, Altieri DC. Evaluation of drug combination effect using a Bliss independence dose-response surface model. Stat Biopharm Res. 2018;10:112–22.
Gingrich JR, Barrios RJ, Morton RA, Boyce BF, DeMayo FJ, Finegold MJ, et al. Metastatic prostate cancer in a transgenic mouse. Cancer Res. 1996;56:4096–102.
Roy A, Veroli MV, Prasad S, Wang QJ. Protein kinase D2 modulates cell cycle by stabilizing Aurora A kinase at centrosomes. Mol Cancer Res. 2018;16:1785–97.
Acknowledgements
This work was supported in part by the National Institutes of Health grant R01CA229431 (Wang). It also used the UPMC Hillman Cancer Center and Tissue and Research Pathology/Pitt Biospecimen Core shared resource which is supported in part by the award P30CA047904.
Author information
Authors and Affiliations
Contributions
QJW conceived and presented the ideas. YaC, YuC, WZ, YL, and JC performed the experiments and conducted data analysis. QJW wrote the manuscript with the support of YaC, YuC, and JC. KD and HW performed the statistical analyses of the animal data, and QJW supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chao, Y., Chen, Y., Zheng, W. et al. Synthetic lethal combination of CHK1 and WEE1 inhibition for treatment of castration-resistant prostate cancer. Oncogene 43, 789–803 (2024). https://doi.org/10.1038/s41388-024-02939-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-024-02939-z