Abstract
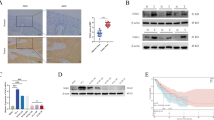
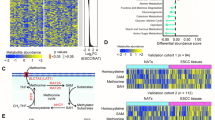
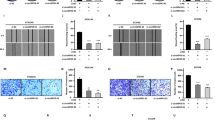
Esophageal squamous cell carcinoma (ESCC) is a common malignant tumor with a poor prognosis due to a lack of early detection. Indeed, the mechanisms underlying ESCC progression remain unclear. Here, we discovered that abnormal arginine metabolism contributes to ESCC progression. Based on transcriptomic and metabolomic analyses, we found that argininosuccinate synthetase 1 (ASS1) and argininosuccinate lyase (ASL) levels were increased in primary tumor tissues but decreased in lymph-metastatic tumor tissues. Intriguingly, FOXO3a was inversely correlated with ASS1 and ASL in primary and metastatic tumor tissues, suggesting that FOXO3a dissimilarly regulates ASS1 and ASL at different stages of ESCC. Silencing ASS1/ASL inhibited primary tumor growth and promoted metastasis. Conversely, overexpression of ASS1/ASL or increased arginine supply promoted tumor proliferation but suppressed metastasis. In addition, FOXO3a activation inhibited primary tumor growth by repressing ASS1 and ASL transcription, whereas inactivation of FOXO3a impeded metastasis by releasing ASS1 and ASL transcription. Together, the finding sheds light on metastatic reprogramming in ESCC.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
di Pietro M, Canto MI, Fitzgerald RC. Endoscopic management of early adenocarcinoma and squamous cell carcinoma of the esophagus: screening, diagnosis, and therapy. Gastroenterology. 2018;154:421–36.
Ohashi S, Miyamoto S, Kikuchi O, Goto T, Amanuma Y, Muto M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology. 2015;149:1700–15.
Zhang W, Hong R, Li L, Wang Y, Du P, Ou Y, et al. The chromosome 11q13.3 amplification associated lymph node metastasis is driven by miR-548k through modulating tumor microenvironment. Mol Cancer. 2018;17:125S.
Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154:360–73.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Morris SM Jr. Arginine: beyond protein. Am J Clin Nutr. 2006;83:508S–512S.
Patil MD, Bhaumik J, Babykutty S, Banerjee UC, Fukumura D. Arginine dependence of tumor cells: targeting a chink in cancer’s armor. Oncogene. 2016;35:4957–72.
Hall PE, Lewis R, Syed N, Shaffer R, Evanson J, Ellis S, et al. A phase I study of pegylated arginine deiminase (pegargiminase), csplatin, and pemetrexed in argininosuccinate synthetase 1-deficient recurrent high-grade glioma. Clin Cancer Res. 2019;25:2708–16.
Szlosarek PW, Steele JP, Nolan L, Gilligan D, Taylor P, Spicer J, et al. Arginine deprivation with pegylated arginine deiminase in patients with argininosuccinate synthetase 1-deficient malignant pleural mesothelioma: a randomized clinical trial. JAMA Oncol. 2017;3:58–66.
Cai J, Fang L, Hang Y, Li R, Yuan J, Yang Y, et al. miR-205 targets PTEN and PHLPP2 to augment AKT signaling and drive malignant phenotypes in non-small cell lung cancer. Cancer Res. 2013;73:5402–15.
Cai J, Li R, Xu X, Zhang L, Lian R, Fang L, et al. CK1α suppresses lung tumour growth by stabilizing PTEN and inducing autophagy. Nat Cell Biol. 2018;20:465–78.
Luo H, Yang Y, Duan J, Wu P, Jiang Q, Xu C. PTEN-regulated AKT/FoxO3a/Bim signaling contributes to reactive oxygen species-mediated apoptosis in selenite-treated colorectal cancer cells. Cell Death Dis. 2013;4:e481.
Lemos H, Huang L, Prendergast GC, Mellor AL. Immune control by amino acid catabolism during tumorigenesis and therapy. Nat Rev Cancer. 2019;19:162–75.
Bean GR, Kremer JC, Prudner BC, Schenone AD, Yao JC, Schultze MB, et al. A metabolic synthetic lethal strategy with arginine deprivation and chloroquine leads to cell death in ASS1-deficient sarcomas. Cell Death Dis. 2016;7:e2406.
Gaude E, Frezza C. Tissue-specific and convergent metabolic transformation of cancer correlates with metastatic potential and patient survival. Nat Commun. 2016;7:13041.
Ouimet M, Koster S, Sakowski E, Ramkhelawon B, van Solingen C, Oldebeken S, et al. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat Immunol. 2016;17:677–86.
Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, et al. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–21.
Chu Z, Huo N, Zhu X, Liu H, Cong R, Ma L, et al. FOXO3A-induced LINC00926 suppresses breast tumor growth and metastasis through inhibition of PGK1-mediated Warburg effect. Mol Ther. 2021;9:2737–53.
Chang X, Liu X, Wang H, Yang X, Gu Y. Glycolysis in the progression of pancreatic cancer. Am J Cancer Res. 2022;12:861–72.
Zhang X, Liu Q, Zhang X, Guo K, Zhang X, Zhou Z. FOXO3a regulates lipid accumulation and adipocyte inflammation in adipocytes through autophagy: role of FOXO3a in obesity. Inflamm Res. 2021;70:591–603.
Chen H, Wang SH, Chen C, Yu XY, Zhu JN, Mansell T, et al. A novel role of FoxO3a in the migration and invasion of trophoblast cells: from metabolic remodeling to transcriptional reprogramming. Mol Med. 2022;28:92.
Liu Y, Ao X, Ding W, Ponnusamy M, Wu W, Hao X, et al. Critical role of FOXO3a in carcinogenesis. Mol Cancer. 2018;17:104.
Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68:7661–9.
Fitzwalter BE, Towers CG, Sullivan KD, Andrysik Z, Hoh M, Ludwig M, et al. Autophagy inhibition mediates apoptosis sensitization in cancer therapy by relieving FOXO3a turnover. Dev Cell. 2018;44:555–65.
Zhang W, Zhang S, Yan P, Ren J, Song M, Li J, et al. A single-cell transcriptomic landscape of primate arterial aging. Nat Commun. 2020;11:2202.
Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–71.
Funding
This study was supported by the Jiangsu Provincial Key Research Development Program (BE 2017759) to QZ, the National Natural Science Foundation of China Research Grants (81972742, 81572742, 81372199) and the National Program on Key Research Project of China (No. 2016YFC0905900) to YX.
Author information
Authors and Affiliations
Contributions
WS contributed to the methodology development, omics analysis, investigation, data curation, formal analysis, and writing draft. HK contributed to methodology, investigation, data curation, formal analysis, and validation. contributed to, and omics analysis. YF, FX, ZX, and XW contributed to methodology, collection of patient samples data curation and formal analysis. RY contributed to the resources and project support. QZ contributed to the resources, supervision and funding acquisition. QJ contributed to resources, technical and facility support, and administration. YX contributed to project conception and design, investigation, data interpretation, supervision, funding acquisition, and manuscript writing, review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sun, W., Kou, H., Fang, Y. et al. FOXO3a-regulated arginine metabolic plasticity adaptively promotes esophageal cancer proliferation and metastasis. Oncogene 43, 216–223 (2024). https://doi.org/10.1038/s41388-023-02906-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02906-0