Abstract
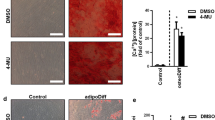
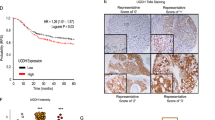
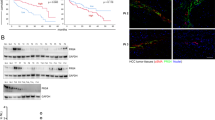
Chemotherapy resistance represents a major cause of therapeutic failure and mortality in cancer patients. Mesenchymal stromal cells (MSCs), an integral component of tumor microenvironment, are known to promote drug resistance. However, the detailed mechanisms remain to be elucidated. Here, we found that MSCs confer breast cancer resistance to doxorubicin by diminishing its intratumoral accumulation. Hyaluronan (HA), a major extracellular matrix (ECM) product of MSCs, was found to mediate the chemoresistant effect. The chemoresistant effect of MSCs was abrogated when hyaluronic acid synthase 2 (HAS2) was depleted or inhibited. Exogenous HA also protected tumor grafts from doxorubicin. Molecular dynamics simulation analysis indicates that HA can bind with doxorubicin, mainly via hydrophobic and hydrogen bonds, and thus reduce its entry into breast cancer cells. This mechanism is distinct from the reported chemoresistant effect of HA via its receptor on cell surface. High HA serum levels were also found to be positively associated with chemoresistance in breast cancer patients. Our findings indicate that the HA-doxorubicin binding dynamics can confer cancer cells chemoresistance. Reducing HA may enhance chemotherapy efficacy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All original data are available from the authors under request
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389:1134–50.
Ostman A. The tumor microenvironment controls drug sensitivity. Nat Med. 2012;18:1332–4.
Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat Rev Cancer. 2009;9:665–74.
Muerkoster S, Wegehenkel K, Arlt A, Witt M, Sipos B, Kruse ML, et al. Tumor stroma interactions induce chemoresistance in pancreatic ductal carcinoma cells involving increased secretion and paracrine effects of nitric oxide and in terleukin-1 beta. Cancer Res. 2004;64:1331–7.
Wang W, Kryczek I, Dostál L, Lin H, Tan L, Zhao L, et al. Effector T cells abrogate stroma-mediated chemoresistance in ovarian. Cancer Cell. 2016;165:1092–105.
Su S, Chen J, Yao H, Liu J, Yu S, Lao L, et al. CD10(+)GPR77(+) cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell. 2018;172:841–56.e816.
Biffi G, Tuveson DA. Diversity and biology of cancer-associated fibroblasts. Physiol Rev. 2021;101:147–76.
Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18:99–115.
Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16:582–98.
Shi Y, Du L, Lin L, Wang Y. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov. 2016;16:35–52.
Roodhart Jeanine ML, Daenen Laura GM, Stigter Edwin CA, Prins H-J, Gerrits J, Houthuijzen Julia M, et al. Mesenchymal stem cells induce resistance to chemotherapy through the release of platinum-induced fatty acids. Cancer Cell. 2011;20:370–83.
Zheng Z, Li YN, Jia S, Zhu M, Cao L, Tao M, et al. Lung mesenchymal stromal cells influenced by Th2 cytokines mobilize neutrophils and facilitate metastasis by producing complement C3. Nat Commun. 2021;12:6202.
Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72.
Coffman LG, Pearson AT, Frisbie LG, Freeman Z, Christie E, Bowtell DD, et al. Ovarian carcinoma-associated mesenchymal stem cells arise from tissue-specific normal stroma. Stem Cells. 2019;37:257–69.
Weber CE, Kothari AN, Wai PY, Li NY, Driver J, Zapf MA, et al. Osteopontin mediates an MZF1-TGF-beta1-dependent transformation of mesenchymal stem cells into cancer-associated fibroblasts in breast cancer. Oncogene. 2015;34:4821–33.
Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFalpha. Cell Stem Cell. 2012;11:812–24.
Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72:5130–40.
Ippolito L, Morandi A, Taddei ML, Parri M, Comito G, Iscaro A, et al. Cancer-associated fibroblasts promote prostate cancer malignancy via metabolic rewiring and mitochondrial transfer. Oncogene. 2019;38:5339–55.
Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229.
Revet I, Feeney L, Bruguera S, Wilson W, Dong TK, Oh DH, et al. Functional relevance of the histone gamma H2Ax in the response to DNA damaging agents. Proc Natl Acad Sci USA. 2011;108:8663–7.
Bellamy WT, Dalton WS, Kailey JM, Gleason MC, Mccloskey TM, Dorr RT, et al. Verapamil reversal of doxorubicin resistance in multidrug-resistant human myeloma cells and association with drug accumulation and DNA damage. Cancer Res. 1988;48:6365–70.
Kauffman MK, Kauffman ME, Zhu H, Jia Z, Li YR. Fluorescence-based assays for measuring doxorubicin in biological systems. React Oxyg Species. 2016;2:432–9.
Triller N, Korosec P, Kern I, Kosnik M, Debeljak A. Multidrug resistance, in small cell lung cancer: expression of P-glycoprotein, multidrug resistance protein 1 and lung resistance protein in chemo-naive patients and in relapsed disease. Lung Cancer. 2006;54:235–40.
Nooter K, delaRiviere GB, Look MP, vanWingerden KE, HenzenLogmans SC, Scheper RJ, et al. The prognostic significance of expression of the multidrug resistance associated protein (MRP) in primary breast cancer. Br J Cancer. 1997;76:486–93.
Doyle LA, Yang WD, Abruzzo LV, Krogmann T, Gao YM, Rishi AK, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA. 1998;95:15665–70.
Qu C, Rilla K, Tammi R, Tammi M, Kroger H, Lammi MJ. Extensive CD44-dependent hyaluronan coats on human bone marrow-derived mesenchymal stem cells produced by hyaluronan synthases HAS1, HAS2 and HAS3. Int J Biochem Cell Biol. 2014;48:45–54.
Arasu UT, Karna R, Harkonen K, Oikari S, Koistinen A, Kroger H, et al. Human mesenchymal stem cells secrete hyaluronan-coated extracellular vesicles. Matrix Biol. 2017;64:54–68.
Jha AK, Xu XA, Duncan RL, Jia XQ. Controlling the adhesion and differentiation of mesenchymal stem cells using hyaluronic acid-based, doubly crosslinked networks. Biomaterials. 2011;32:2466–78.
Chen J, Meng JL, Jin C, Mo F, Ding Y, Gao XM, et al. 4-Methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy for chronic prostatitis. Prostate. 2021;81:1078–90.
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63.
Raz Y, Cohen N, Shani O, Bell RE, Novitskiy SV, Abramovitz L, et al. Bone marrow-derived fibroblasts are a functionally distinct stromal cell population in breast cancer. J Exp Med. 2018;215:3075–93.
Lankelma J, Dekker H, Luque FR, Luykx S, Hoekman K, van der Valk P, et al. Doxorubicin gradients in human breast cancer. Clin Cancer Res. 1999;5:1703–7.
Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharm. 2013;65:157–70.
Klemm F, Joyce JA. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213.
Velaei K, Samadi N, Barazvan B, Rad JS. Tumor microenvironment-mediated chemoresistance in breast cancer. Breast. 2016;30:92–100.
Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, Becette V, et al. A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med. 2009;15:68–74.
Tsujino T, Seshimo I, Yamamoto H, Ngan CY, Ezumi K, Takemasa I, et al. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin Cancer Res. 2007;13:2082–90.
Cox TR. The matrix in cancer. Nat Rev Cancer. 2021;21:217–38.
Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801.
Bohaumilitzky L, Huber AK, Stork EM, Wengert S, Woelfl F, Boehm H. A trickster in disguise: Hyaluronan’s ambivalent roles in the matrix. Front Oncol. 2017;7:242.
Tavianatou AG, Caon I, Franchi M, Piperigkou Z, Galesso D, Karamanos NK. Hyaluronan: molecular size-dependent signaling and biological functions in inflammation and cancer. FEBS J. 2019;286:2883–908.
Price ZK, Lokman NA, Ricciardelli C. Differing roles of hyaluronan molecular weight on cancer cell behavior and chemotherapy resistance. Cancers. 2018;10:482–502.
Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, et al. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499:346–9.
Dominguez-Gutierrez PR, Kwenda EP, Donelan W, O’Malley P, Crispen PL, Kusmartsev S. Hyal2 expression in tumor-associated myeloid cells mediates cancer-related inflammation in bladder cancer. Cancer Res. 2021;81:648–57.
Schmaus A, Klusmeier S, Rothley M, Dimmler A, Sipos B, Faller G, et al. Accumulation of small hyaluronan oligosaccharides in tumour interstitial fluid correlates with lymphatic invasion and lymph node metastasis. Br J Cancer. 2014;111:559–67.
Liu D, Pearlman E, Diaconu E, Guo K, Mori H, Haqqi T, et al. Expression of hyaluronidase by tumor cells induces angiogenesis in vivo. Proc Natl Acad Sci USA. 1996;93:7832–7.
Carvalho AM, Reis RL, Pashkuleva I. Hyaluronan receptors as mediators and modulators of the tumor microenvironment. Adv Healthcare Mater. 2023;12:e2202118.
Tammi MI, Oikari S, Pasonen-Seppanen S, Rilla K, Auvinen P, Tammi RH. Activated hyaluronan metabolism in the tumor matrix—causes and consequences. Matrix Biol. 2019;78-79:147–64.
Toole BP, Slomiany MG. Hyaluronan, CD44 and emmprin: partners in cancer cell chemoresistance. Drug Resist Updat. 2008;11:110–21.
Ohashi R, Takahashi F, Cui R, Yoshioka M, Gu T, Sasaki S, et al. Interaction between CD44 and hyaluronate induces chemoresistance in non-small cell lung cancer cell. Cancer Lett. 2007;252:225–34.
Gilg AG, Tye SL, Tolliver LB, Wheeler WG, Visconti RP, Duncan JD, et al. Targeting hyaluronan interactions in malignant gliomas and their drug-resistant multipotent progenitors. Clin Cancer Res. 2008;14:1804–13.
Compagnone M, Gatti V, Presutti D, Ruberti G, Fierro C, Markert EK, et al. DeltaNp63-mediated regulation of hyaluronic acid metabolism and signaling supports HNSCC tumorigenesis. Proc Natl Acad Sci USA. 2017;114:13254–9.
Provenzano PP, Hingorani SR. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br J Cancer. 2013;108:1–8.
Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–20.
Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–29.
Hingorani SR, Harris WP, Beck JT, Berdov BA, Wagner SA, Pshevlotsky EM, et al. Phase Ib study of pegylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res. 2016;22:2848–54.
Tammi RH, Passi AG, Rilla K, Karousou E, Vigetti D, Makkonen K, et al. Transcriptional and post-translational regulation of hyaluronan synthesis. FEBS J. 2011;278:1419–28.
Jokela TA, Karna R, Makkonen KM, Laitinen JT, Tammi RH, Tammi MI. Extracellular UDP-glucose activates P2Y14 receptor and induces signal transducer and activator of transcription 3 (STAT3) Tyr705 phosphorylation and binding to hyaluronan synthase 2 (HAS2) promoter, stimulating hyaluronan synthesis of keratinocytes. J Biol Chem. 2014;289:18569–81.
Jokela T, Karna R, Rauhala L, Bart G, Pasonen-Seppanen S, Oikari S, et al. Human keratinocytes respond to extracellular UTP by induction of hyaluronan synthase 2 expression and increased hyaluronan synthesis. J Biol Chem. 2017;292:4861–72.
Rauhala L, Jokela T, Karna R, Bart G, Takabe P, Oikari S, et al. Extracellular ATP activates hyaluronan synthase 2 (HAS2) in epidermal keratinocytes via P2Y2, Ca(2+) signaling, and MAPK pathways. Biochem J. 2018;475:1755–72.
Wang G, Cao K, Liu K, Xue Y, Roberts AI, Li F, et al. Kynurenic acid, an IDO metabolite, controls TSG-6-mediated immunosuppression of human mesenchymal stem cells. Cell Death Differ. 2017;25:1209–23.
Acknowledgements
This study was supported by grants from National Natural Science Foundation of China (81930085, 31961133024, 32150710523, 31900635, 82202032), the National Key R&D Program of China (2021YFA110060), Jiangsu Province International Joint Laboratory for Regenerative Medicine Fund, State Key Laboratory of Radiation Medicine and Protection, Soochow University (GZN1201902) and Suzhou Foreign Academician Workstation Fund (SWY20220). We especially thank Dr. Jian Jin for providing expert technical assistance in molecular dynamics simulation.
Author information
Authors and Affiliations
Contributions
ZHL: conception and design, collection and assembly of data, animal experiments, data analysis and interpretation, and manuscript writing. PBH, JKF: conception and design, data analysis and interpretation. JY Zhu: molecular dynamics simulations, data analysis and interpretation. JM Zha and YYD: contribution of key reagents. RL, MQZ, LJC and CF: animal experiments. PSL and GM: data analysis and interpretation. CSS and YFS: conception and design, manuscript writing, administrative support and financial support. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Z., Hou, P., Fang, J. et al. Mesenchymal stromal cells confer breast cancer doxorubicin resistance by producing hyaluronan. Oncogene 42, 3221–3235 (2023). https://doi.org/10.1038/s41388-023-02837-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02837-w